Abstract
Posterior reversible encephalopathy syndrome (PRES) is a gradually recognised neurological complication of allogenic haematopoietic stem cell transplantation (allo-HSCT). However, there is a paucity of information on PRES after haploidentical HSCT (haplo-HSCT). We performed a retrospective nested case-control study in patients following haplo-HSCT for malignant and nonmalignant haematologic diseases between January 2009 and December 2018 in our centre. A total of 45 patients were diagnosed with PRES after transplant, accounting for an incidence of 1.17%. Grades II to IV acute graft-versus-host disease (aGVHD) (HR 2.370, 95% CI 1.277–4.397, p = 0.006) and hypertension (HR 14.466, 95% CI 7.107–29.443, p < 0.001) were identified as risk factors for developing PRES after haplo-HSCT. There was no difference in overall survival (OS), disease-free survival (DFS), the cumulative incidence of relapse or nonrelapse mortality (NRM) between patients with PRES and controls without PRES following haplo-HSCT in either adults or children. All but one patient with PRES showed nearly complete clinical and neurologic recovery. In conclusion, PRES is a rare condition with benign outcomes following haplo-HSCT. Further multicentre prospective studies are needed to confirm the results and help to establish the standard therapy for posttransplant PRES.
Similar content being viewed by others
Introduction
Posterior reversible encephalopathy syndrome (PRES) is an increasingly recognised neurological complication in patients receiving allogeneic haematopoietic stem cell transplantation (allo-HSCT). PRES is a clinical syndrome characterised by various neurological manifestations including drowsiness, emesis, headache, confusion, visual abnormality and seizures, associated with typical transient white matter oedema on magnetic resonance imaging (MRI) [1, 2]. The incidence of PRES after HSCT has been reported to be 1.1–22% [3,4,5,6,7,8], which varies widely in different studies. Several studies have tried to explore the risk factors and influence of PRES on posttransplant survival, but due to the differences in definition of PRES, types of diseases, donor types of transplant, conditioning protocols and other characteristics in various studies, it is difficult to interpret the existing conclusions [3,4,5,6,7,8].
Allo-HSCT has shown promising potential in curing various malignant and nonmalignant disorders of haematopoiesis. However, there is only a one-in-four chance for each patient to have a human leucocyte antigen (HLA)-matched sibling (MSD). Haploidentical HSCT (haplo-HSCT) has therefore become the largest donor source in comparison to MSD-HSCT since 2013 and now accounts for almost 59.4% of allo-HSCTs in China [9]. During past decades, Huang et al have successfully established a protocol for unmanipulated haplo-HSCT using a myeloablative conditioning (MAC) regimen with granulocyte colony-stimulating factor mobilised/primed grafts [10]. A series of prospective studies have demonstrated comparable outcomes of haplo-HSCT following this protocol to MSD-HSCT for acute myeloid leukaemia, acute lymphoblastic leukaemia, myelodysplastic syndrome and severe aplastic anaemia (SAA) [11,12,13,14]. However, haplo-HSCT still brings some neurological problems. As a common neurological complication after allo-HSCT, the incidence and outcomes of PRES following haplo-HSCT have remained poorly understood.
Therefore, given the paucity of information on PRES after haplo-HSCT, we retrospectively analysed the incidence and outcomes and evaluated risk factors and clinical features of PRES in patients receiving haplo-HSCT in our centre.
Methods
Patients
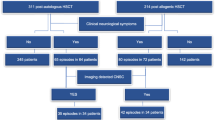
Between January 2009 and December 2018, 3832 patients received haplo-HSCT at the Institute of Hamatology, Peking University People’s Hospital. Among them, there were 2825 adults and 1007 children. A total of 45 patients were diagnosed with PRES. We performed a nested case-control study, and three controls were matched for each case according to age (±5 years) and time of transplant (±30 days).
Written informed consent in accordance with the Declaration of Helsinki was approved by the Ethics Committee of Peking University People’s Hospital. All patients and guardians of children signed written informed consent forms.
Transplantation regimens
All patients received a BU-based myeloablative conditioning regimen. The conditioning therapy of haematological malignancies consisted of cytarabine (ARA-C, 4 g/m2/day) administered i.v. on days −10 and −9, busulfan (BU, 3.2 mg/kg/d) administered i.v. on days −8 to −6, cyclophosphamide (CY, 1.8 g/m2/d) administered i.v. on days −5 and −4, methyl chloride hexamethylene urea nitrate (Me-CCNU, 250 mg/m2/d) orally on day −3 and anti-thymocyte globulin (ATG, rabbit (Sang Stat, Lyon, France), 2.5 mg/kg/d) administered i.v. on days −5 to −2. The conditioning therapy of SAA in haplo-HSCT consisted of the following: BU (0.8 mg/kg) administered i.v. on days −7 and −6; CY (50 mg/kg) administered i.v. on days −5 to −2; and ATG (2.5 mg/kg) administered i.v. for four consecutive days from days −5 to −2.
After haplo-HSCT, all patients were given cyclosporine (CSA), mycophenolate mofetil (MMF) and short-term methotrexate (MTX) for graft-versus-host disease (GVHD) prophylaxis. The trough level of CSA was targeted at 150–250 ng/mL within 40 days after transplant and then gradually tapered in the absence of GVHD. The therapeutic tacrolimus trough level was 5–20 ng/ml. The diagnoses of acute GVHD (aGVHD) and chronic GVHD (cGVHD) were made according to the widely acceptable criteria [11]. The management of GVHD included 1–2 mg/kg/day methylprednisolone (MP) and resumption of full-dose CSA. The alternative treatment for refractory aGVHD included tacrolimus, MMF and CD25 monoclonal antibody (daclizumab; Roche, Basel, Switzerland) or MTX [15].
Definitions
The diagnostic criteria of PRES were defined as (1) clinical manifestations of seizures, headache, altered mental status, or visual disturbances; (2) characteristic findings of cortical/subcortical signal abnormalities on brain X-ray computed tomography (CT) or MRI; (3) exclusion of other reasons for neurologic dysfunction, including infection, metabolic abnormalities, thrombotic microangiopathy, haemorrhage and tumour infiltration; and (4) reversible neurological symptoms and imaging findings. None of the patients had infections or fevers of unknown origin within the 72 h before the onset of PRES. The day of PRES onset was defined as the start date of neurologic symptoms in the clinical records. All patients with symptoms of seizures were evaluated by a neurologist. Status epilepticus was defined as 30 min of continuous seizures or without complete recovery of consciousness between a series of seizures [16]. Standard electroencephalogram (EEG) records were reviewed by a neurologist. Hypertension was defined as an average systolic or diastolic blood pressure higher than the 95th percentile for sex, age and height three or more times on different occasions [17]. Mean arterial blood pressure (MAP) was calculated as 2/3 diastolic pressure plus 1/3 systolic pressure. Hypokalaemia was defined as a serum potassium level < 3.5 mmol/l. Hyponatraemia was defined as a serum sodium level < 135 mmol/l. Serum electrolyte concentrations were measured within 24 h of PRES onset.
CSF examinations
All CSF samples were analysed for varicella-zoster virus, Epstein–Barr virus, cytomegalovirus, human herpes virus 6, herpes simplex virus and other viruses as our previous study reported [18]. All CSF samples also underwent histopathologic examination and fluorescence-activated cell sorting analysis for malignant cells.
Statistical analysis
The baseline characteristics were compared using the chi-square test for categorical variables and Student’s t test for continuous variables. The Kaplan–Meier method and log-rank test were used to estimate survival. Overall survival (OS) was defined as the time from transplant to death for any reason. Disease-free survival (DFS) was defined as survival without death, relapse or graft rejection. Nonrelapse mortality (NRM) was defined as death without evidence of disease recurrence after transplant. Relapse was defined as blasts ≥5% in the BM or reappearance of blasts in the peripheral blood or extramedullary site [11]. Univariate and multivariate analyses were performed to identify risk factors for PRES after haplo-HSTC using Cox proportional hazards models. Variables with p < 0.1 in univariate analysis were further included in the multivariate analysis. All tests were two-tailed, and p < 0.05 was considered to be statistically significant. The analyses were performed using SPSS 22.0 (SPSS Inc., USA).
Results
Patient characteristics and Incidence of PRES
A total of 3832 patients received haplo-HSCT during the study period. Forty-five patients developed PRES following transplantation, with an incidence of 1.17%. The baseline characteristics of patients and controls are shown in Table 1. Among the patients with PRES, there were 23 adults and 22 children. The incidence of PRES following haplo-HSCT was 0.81% in adults and 2.18% in children.
Clinical characteristics
The median time to PRES onset was 67 days (range: 17–841) after transplant, with 64.4% patients developing within 100 days. The main clinical manifestations of PRES were epilepsy (n = 44), visual disturbances (n = 5), headaches (n = 3) and metal status change (n = 1). Among them, two patients presented with status epilepticus. All patients received MRI or CT scans within 72 h after PRES onset. Among them, 42 had MRI scans, 29 had CT scans and 26 had both CT and MRI scans. None of the patients had imaging features of haemorrhage, infection, or tumour infiltration. PRES was identified on CT scans in 10 of 29 patients. The radiologic characteristics of patients with PRES are shown in Table 2. Most patients presented with symmetrical lesions and abnormal signals consistent with PRES, most commonly seen in the parietal and occipital lobes (Fig. 1). Follow-up imaging records were available in 21 patients, and all showed nearly complete remission. All three EEG recordings available were abnormal. No signs of infection or tumour infiltration were found in the 19 patients who underwent CSF examination within 2 weeks of PRES onset.
None of the patients had hypertension before transplant. The average MAP at baseline was 85.67 and 110.08 mmHg at PRES onset, with an average increase of 29.8% from baseline. The median concentrations of serum creatinine, sodium and potassium were within the normal range. A minority of patients had renal dysfunction (n = 1), hypokalaemia (n = 10) and hyponatraemia (n = 10) at PRES onset. The median trough concentration of plasma CSA was 217.1 ng/mL (range: 40.9– 422.8), with 13 patients having supratherapeutic CSA levels on the day of or day prior to PRES onset. Four patients were treated with tacrolimus before PRES onset, and all of them presented therapeutic concentrations of plasma tacrolimus, ranging from 5.0 to 16.1 ng/mL.
Risk factors for PRES
Univariate analysis showed that only hypertension (p < 0.001) was significantly associated with PRES after transplant. Variables with p < 0.1 were further included in the Cox proportional hazards model to perform a multivariate analysis. The results showed acute grade II to IV GVHD (HR 2.370, 95% CI 1.277–4.397, p = 0.006) and hypertension (HR 14.466, 95% CI 7.107–29.443, p < 0.001) were independent risk factors for developing PRES after haplo-HSCT (Table 3).
Survival
There was no significant difference in OS (p = 0.405, Fig. 2a), DFS (p = 0.737, Fig. 2b), cumulative incidence of relapse (p = 0.864, Fig. 2c) or NRM (p = 0.531, Fig. 2d) between the patients with and without PRES following haplo-HSCT in either adults or children.
Management and clinical outcome
The basic management of patients with PRES included antiepileptic, antihypertensive and supportive care. All but one patient showed gradual clinical remission. The subsequent strategy for using calcineurin inhibitor was roughly divided into the following. Ten patients (22.2%) completely discontinued calcineurin inhibitor and continued anti-GVHD treatment with MP, MMF, CD25 monoclonal antibody or MTX. Among them, one patient died of severe irreversible PRES, one died of infection, and the remaining eight patients survived with this strategy to the final follow-up time. Eleven patients (24.4%) switched to another calcineurin inhibitor after a washout period. One of these 11 patients had repeated PRES after switching CSA to tacrolimus and subsequently completely discontinued the calcineurin inhibitor, with no recurrence of PRES. Two patients experienced exacerbations of GVHD during the washout period before switching CSA to tacrolimus and eventually died of severe GVHD. Twenty-four patients (53.3%) continued to use the same calcineurin inhibitor after a dose reduction or short pause, and none of them had repeated PRES. Among them, four patients experienced an exacerbation of GVHD after the initial dose reduction or discontinuation, and then, GVHD was controlled after continuing the medication. Among them, two patients died of infection. Of the 24 patients who continued to use the same calcineurin inhibitor, another two died of infection and disease progression.
Discussion
PRES is a gradually recognised neurologic complication after allo-HSCT. Here, we report the first and largest study on PRES following haplo-HSCT. Several previous studies have focused on posttransplant PRES for either solid organs or haematologic diseases. However, there are still no comparative data available on PRES following haplo-HSCT. In this study, we observed an incidence of 1.17% of PRES following haplo-HSCT, which provided PRES incidence in patients receiving haplo-HSCT for the first time, and it was significantly lower than that reported in most previous studies [3, 7, 8, 17]. The discrepancy could be due to the differences in disease, other baseline characteristics of the included patients and different transplantation protocols.
The potential mechanism of PRES is usually considered to be endothelial damage, leading to decreased levels of endothelium-derived vasorelaxants and systemic vasoconstriction [19, 20]. This theory could explain the arterial border-zone distribution of PRES. Another potential mechanism for PRES is hypertension [21]. The continuous rise of blood pressure exceeds the limit of autoregulation of cerebral blood vessels, and then the blood–brain barrier is destroyed, causing vascular edema [22]. Abnormal regulation of cerebrovascular adrenergic sympathetic nervous system may also be involved in the occurrence of PRES [21, 23].
Calcineurin inhibitors, one of the most commonly used immunosuppressants to prevent GVHD after allo-HSCT, have been reported to be associated with posttransplant PRES. However, several studies have suggested that the occurrence of PRES was independent of the serum level of calcineurin inhibitors, and it could occur even within the therapeutic concentration [1, 5, 24, 25]. Similar results were also observed in our study, and the majority of patients had therapeutic serum concentrations of CSA or tacrolimus before PRES onset. Hypertension is a common side effect of calcineurin inhibitors. An average increase in MAP of 29.8% was observed in our study at PRES onset, which was similar with previous study [26]. Further multivariate analysis also demonstrated that hypertension was an independent risk factor for PRES following haplo-HSCT. Therefore, for patients with neurological symptoms after transplant, even if the calcineurin inhibitors are within the therapeutic concentration, physicians should be alert to the occurrence of PRES and improve the imaging examination in time. In addition, it is necessary to pay attention to the management of blood pressure during the use of calcineurin inhibitors.
Another potential risk factor reported for PRES is aGVHD [3, 5, 27,28,29]. Some scholars have suggested that GVHD induces vascular endothelial injury, which disrupts the blood–brain barrier, allowing calcineurin inhibitors to pass through the blood–brain barrier. In addition, GVHD led to increased release of TNF-α and IL-1, causing extensive vasoconstriction, including the CNS [27,28,29]. In our study, multivariate analysis also indicated that severe grade II–IV aGVHD was significantly associated with PRES in haplo-HSCT. A few previous studies have contributed the high incidence of PRES to more common occurrence of aGVHD in haplo-HSCT [29], but the incidence of PRES was not higher than previously reported when we restricted the study to patients receiving haplo-HSCT, which might need to be confirmed by further multicentre studies.
There are still no systematic guidelines for the treatment of posttransplant PRES. Basic treatments generally include stopping or reducing calcineurin inhibitors, controlling blood pressure, and preventing epilepsy. In our study, symptoms and imaging findings were reversible in most patients, and there was no significant difference in primary clinical outcomes. However, several studies have reported an inferior survival of patients with posttransplant PRES [3, 8]. The discrepancy might be the different donor types of transplant between our study and previous studies. Further multicentre studies may be needed to confirm the differences in survival of patients with PRES following haplo-HSCT and other donor types allo-HSCT.
There were some limitations in our study. First, it was a retrospective study design in a single centre. Furthermore, although we chose as representative a control as possible, there might still be some selection bias. However, it is the largest study on PRES following haplo-HSCT and is from one of the largest haplo-HSCT centres. The patients received similar and mature conditioning regimens and GVHD prophylaxis; therefore, the results could be well generalised.
In summary, this retrospective study is the first and largest review of PRES after haplo-HSCT, demonstrating an incidence of 1.17% of PRES in patients following haplo-HSCT, which is a rare but benign condition. However, for patients with aGVHD after transplant and those with hypertension due to the use of calcineurin inhibitors, there is a higher risk of developing PRES, which still requires close attention. Further multicentre prospective studies are needed to establish standardised prophylactic and therapeutic strategies for patients with posttransplant PRES.
References
Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500.
Cordelli DM, Masetti R, Zama D, Gueraldi D, Rondelli R, Cottone C, et al. Etiology, characteristics and outcome of seizures after pediatric hematopoietic stem cell transplantation. Seizure. 2014;23:140–5. https://doi.org/10.1016/j.seizure.2013.11.003.
Gaziev J, Marziali S, Paciaroni K, Isgrò A, Di Giuliano F, Rossi G, et al. Posterior reversible encephalopathy syndrome after hematopoietic cell transplantation in children with hemoglobinopathies. Biol Blood Marrow Transpl. 2017;23:1531–40. https://doi.org/10.1016/j.bbmt.2017.05.033.
Chaudhary RK, Dhakal P, Aryal A, Bhatt VR. Central nervous system complications after allogeneic hematopoietic stem cell transplantation. Future Oncol. 2017;13:2297–312. https://doi.org/10.2217/fon-2017-0274.
Hammerstrom AE, Howell J, Gulbis A, Rondon G, Champlin RE, Popat U. Tacrolimus-associated posterior reversible encephalopathy syndrome in hematopoietic allogeneic stem cell transplantation. Am J Hematol. 2013;88:301–5. https://doi.org/10.1002/ajh.23402.
Zama D, Masetti R, Cordelli DM, Vendemini F, Giordano L, Milito G, et al. Risk factor analysis of posterior reversible encephalopathy syndrome after allogeneic hematopoietic SCT in children. Bone Marrow Transpl. 2014;49:1538–40. https://doi.org/10.1038/bmt.2014.182.
Tavares M, Arantes M, Chacim S, Júnior AC, Pinto A, Mariz JM, et al. Posterior reversible encephalopathy syndrome in children with hematologic malignancies. J Child Neurol. 2015;30:1669–75. https://doi.org/10.1177/0883073815578525.
Siegal D, Keller A, Xu W, Bhuta S, Kim DH, Kuruvilla J, et al. Central nervous system complications after allogeneic hematopoietic stem cell transplantation: incidence, manifestations, and clinical significance. Biol Blood Marrow Transpl. 2007;13:1369–79.
Lv M, Chang Y-J, Huang X-J. Update of the “Beijing Protocol” haplo-identical hematopoietic stem cell transplantation. Bone Marrow Transpl. 2019;54(Suppl 2):703–7. https://doi.org/10.1038/s41409-019-0605-2.
Huang X-j, Han W, Xu L-p, Chen Y-h, Liu D-h, Lu J, et al. A novel approach to human leukocyte antigen-mismatched transplantation in patients with malignant hematological disease. Chin Med J. 2004;117:1778–85.
Wang Y, Liu Q-F, Xu L-P, Liu K-Y, Zhang X-H, Ma X, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125:3956–62. https://doi.org/10.1182/blood-2015-02-627786.
Wang Y, Liu Q-F, Xu L-P, Liu K-Y, Zhang X-H, Ma X, et al. Haploidentical versus matched-sibling transplant in adults with philadelphia-negative high-risk acute lymphoblastic leukemia: a biologically phase III randomized study. Clin Cancer Res. 2016;22:3467–76. https://doi.org/10.1158/1078-0432.CCR-15-2335.
Wang Y, Wang HX, Lai YR, Sun ZM, Wu DP, Jiang M, et al. Haploidentical transplant for myelodysplastic syndrome: registry-based comparison with identical sibling transplant. Leukemia. 2016;30:2055–63. https://doi.org/10.1038/leu.2016.110.
Xu L-P, Jin S, Wang S-Q, Xia L-H, Bai H, Gao S-J, et al. Upfront haploidentical transplant for acquired severe aplastic anemia: registry-based comparison with matched related transplant. J Hematol Oncol. 2017;10:25 https://doi.org/10.1186/s13045-017-0398-y.
Lu D-P, Dong L, Wu T, Huang X-J, Zhang M-J, Han W, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 2006;107:3065–73.
Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med. 1998;338:970–6.
Zama D, Gasperini P, Berger M, Petris M, De Pasquale MD, Cesaro S, et al. A survey on hematology-oncology pediatric AIEOP centres: the challenge of posterior reversible encephalopathy syndrome. Eur J Haematol. 2018;100:75–82. https://doi.org/10.1111/ejh.12984.
Zhang X-H, Zhang J-M, Han W, Chen H, Chen Y-H, Wang F-R, et al. Viral encephalitis after haplo-identical hematopoietic stem cell transplantation: causative viral spectrum, characteristics, and risk factors. Eur J Haematol. 2017;98:450–8. https://doi.org/10.1111/ejh.12855.
Loscalzo J. Endothelial injury, vasoconstriction, and its prevention. Tex Heart Inst J. 1995;22:180–4.
Sandoo A, van Zanten JJCSV, Metsios GS, et al. The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J. 2010;4:302–12. https://doi.org/10.2174/1874192401004010302.
Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. Am J Neuroradiol. 2008;29:1043–9. https://doi.org/10.3174/ajnr.A0929.
Bartynski WS, Boardman JF. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. Am J Neuroradiol. 2007;28:1320–7.
Reddy P, Ferrara JLM. Immunobiology of acute graft-versus-host disease. Blood Rev. 2003;17:187–94.
Wong R, Beguelin GZ, de Lima M, Giralt SA, Hosing C, Ippoliti C, et al. Tacrolimus-associated posterior reversible encephalopathy syndrome after allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2003;122:128–34.
Erer B, Polchi P, Lucarelli G, Angelucci E, Baronciani D, Galimberti M, et al. CsA-associated neurotoxicity and ineffective prophylaxis with clonazepam in patients transplanted for thalassemia major: analysis of risk factors. Bone Marrow Transpl. 1996;18:157–62.
Tam CS, Galanos J, Seymour JF, Pitman AG, Stark RJ, Prince HM. Reversible posterior leukoencephalopathy syndrome complicating cytotoxic chemotherapy for hematologic malignancies. Am J Hematol. 2004;77:72–6.
Wu Q, Marescaux C, Wolff V, Jeung M-Y, Kessler R, Lauer V, et al. Tacrolimus-associated posterior reversible encephalopathy syndrome after solid organ transplantation. Eur Neurol. 2010;64:169–77. https://doi.org/10.1159/000319032.
Misawa A, Takeuchi Y, Hibi S, Todo S, Imashuku S, Sawada T. FK506-induced intractable leukoencephalopathy following allogeneic bone marrow transplantation. Bone Marrow Transpl×. 2000;25:331–4.
Kanekiyo T, Hara J, Matsuda-Hashii Y, Fujisaki H, Tokimasa S, Sawada A, et al. Tacrolimus-related encephalopathy following allogeneic stem cell transplantation in children. Int J Hematol. 2005;81:264–8.
Acknowledgements
The work was supported by the Beijing Municipal Science and Technology Commission (No. Z171100001017084), the Beijing Natural Science Foundation (H2018206423), the Key Program of the National Natural Science Foundation of China (No. 81730004), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (81621001), the Beijing Natural Science Foundation (No. 7171013), National Natural Science Foundation of China (No. 81670116) and the National Key Research and Development Program of China (No. 2017YFA0105500, No. 2017YFA0105503).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Q., Zhao, X., Fu, HX. et al. Posterior reversible encephalopathy syndrome (PRES) after haploidentical haematopoietic stem cell transplantation: incidence, risk factors and outcomes. Bone Marrow Transplant 55, 2035–2042 (2020). https://doi.org/10.1038/s41409-020-0894-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-0894-5
- Springer Nature Limited
This article is cited by
-
Posterior Reversible Encephalopathy Syndrome in Children and Adolescents
Current Hypertension Reports (2024)
-
Posterior Reversible Encephalopathy Syndrome
Current Pain and Headache Reports (2021)