Abstract
Donor lymphocyte infusion (DLI) might be used prophylactically to reduce relapse after allogeneic hematopoietic stem cell transplantation for very high-risk leukemia/lymphoma without effective targeted therapy. To compare the safety and efficacy of prophylactic DLI for prevention of relapse after allogeneic peripheral blood stem cell transplantation from haploidentical donors (HID-SCT) and matched-sibling donors (MSD-SCT) in patients with very high-risk acute myeloid leukemia (AML), we performed a retrospective analysis in a cohort of 21 HID-SCT and 13 MSD-SCT recipients, displaying similar baseline characteristics except for donor’s gender distribution. Grade 2–4 acute graft-versus-host disease (GVHD) at 100-day post-DLI was higher in HID-SCT group than that in MSD-SCT group (59.5% vs. 30.8%, p = 0.05). The grade 3–4 acute GVHD (17.5% vs. 7.7%), 1-year chronic GVHD (36.6% vs. 33.2%), and severe chronic GVHD (15.3% vs. 27.3%) were not statistically significant different between groups. One-year non-relapse mortality was higher in HID-SCT group than that in MSD-SCT group with marginal significance (27.9% vs. 0.0%, p = 0.061). One-year relapse rate was not statistically significant different between HID-SCT group and MSD-SCT group (21.6% vs. 36.5%, p = 0.543). For HID-SCT recipients, 1-year relapse rate was lower in patients receiving prophylactic DLI than that in a control cohort of eight patients with same very high-risk features but not receiving prophylactic DLI (62.5% vs. 28.3%, p = 0.037). No statistically significant difference was observed in 1-year overall survival (OS, 55.1% vs. 83.9%, p = 0.325) and relapse-free survival (RFS, 50.1% vs. 74.0%, p = 0.419) rates between HID-SCT group and MSD-SCT group. In multivariate analyses, non-remission status prior to transplant, poor-risk gene mutations, and donor’s age ≥ 48 years predicted a higher risk of relapse after DLI. Non-remission status prior to transplant predicted inferior OS and RFS. Patient’s age ≥ 40 years also predicted an inferior OS. In conclusion, prophylactic DLI was very safe and efficient for reducing relapse in patients with very high-risk AML receiving MSD-SCT. In the recipients of HID-SCT, the application of prophylactic DLI could reduce the risk of relapse, although with a higher incidence of DLI-associated acute GVHD than those of MSD-SCT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allogeneic hematopoietic stem cell transplantation (SCT) is a well-established and effective therapy for high-risk leukemia. Patients with relapsed/refractory acute myeloid leukemia (AML) have a very poor prognosis [1]. Even after allogeneic SCT, the rate of leukemia relapse is above 40% for acute leukemia, and the rate of 3-year overall survival (OS) is less than 20% [2]. Leukemia-associated gene mutations, such as TP53, ten-eleven translocation-2 (TET2), DNA-methyltransferase-3a (DNMT3a), and FMS-like tyrosine kinase 3 (FLT3)-internal tandem duplication (ITD), also predict extremely low relapse-free survival (RFS) after allogeneic SCT [3,4,5,6]. Therefore, leukemia relapse remains the leading cause of treatment failure after transplantation for patients with these very high-risk features.
Donor lymphocyte infusion (DLI) has shown efficacy for treating and preventing leukemia relapse after allogeneic SCT by exploiting the graft-versus-leukemia effect of donor-derived T cells. Other strategies with potential efficacy to reduce relapse after transplantation are included in the application of intensified conditioning regimens and/or targeted drugs. However, relapsed/refractory leukemia is usually resistant to chemotherapy [7]. Intensified conditioning regimens may lead to an increased transplant-related mortality and therefore offset part of the advantage of the reduced relapse rate. We have known that the median time of relapse after SCT is 4.5 months. During this relatively short time interval, relapse prevention methods are included in early application of targeted drugs (if any, attention should be paid to their inhibition of early hematopoiesis) and early reduction of immunosuppressive agents. However, the resulting risk of this strategy is graft-versus-host disease (GVHD), which limits the feasibility of reducing immunosuppressive agents within 3 months after SCT in this pretreatment model. Thus, DLI might be used prophylactically to reduce relapse after SCT for leukemia without effective targeted therapy. Conventional DLI has invariably been associated with high rates of severe GVHD and GVHD-related non-relapse mortality (NRM) [8]. In our previous studies, the DLI procedure has been modified to use granulocyte colony-stimulating factor (G-CSF)-primed donor peripheral blood stem cells (PBSCs) instead of steady-state lymphocytes [9,10,11]. We observed that this modified prophylactic DLI procedure was tolerable and could reduce the risk of relapse post-transplantation in patients with very high-risk leukemia/lymphoma, either in the setting of G-CSF-primed bone marrow as grafts (BMT) [9, 10] or in the setting of peripheral blood as grafts (PBSCT) [11].
Here, we performed a cohort study in a consecutive series of patients with very high-risk AML who received prophylactic DLI after unmanipulated allogeneic PBSCT from HLA-haploidentical sibling donors (HID-SCT) and HLA-matched-sibling donors (MSD-SCT) in our center and evaluated the tolerability and efficacy of this prophylactic strategy.
Methods
Study design
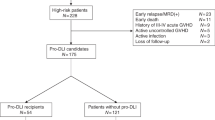
This is a retrospective, observational cohort study enrolled in a total of 106 patients with AML hospitalized at Chinese PLA General Hospital and received unmanipulated allogeneic PBSCT consecutively between March 2014 and November 2017 at our center (Fig. 1 ). Of them, 48 patients met at least one of the following criteria of very high-risk features: (i) in the non-remission (NR) state prior to transplantation, including primary induction failure, relapse untreated, or refractory to reinduction chemotherapy; (ii) achieving CR1 with ≥ 3 cycles of induction of chemotherapy; (iii) carrying TP53, DNMT3a, TET2, or FLT3-ITD gene mutation. Two patients with untreated AML evolution from MDS, one carrying t(3;3) chromosomal translocation and another carrying U2AF1 and CBL gene mutations, were enrolled in this study. Prophylactic G-CSF-primed DLI after transplantation was carried out in 34 patients, including 21 HID-SCT recipients and 13 MSD-SCT recipients (Fig. 1 ). AML patients without above very high-risk features and those experienced early relapse, either molecular relapse or hematological relapse, who received preemptive or therapeutic DLI were not considered. Fifteen of the 34 DLI patients enrolled in this study have been reported in our previous study [11], who were further followed in this study. There were a total of 11 consecutive HID-SCT recipients not receiving prophylactic DLI, who were transplanted during the same time period at our center and fulfilling the criteria of very high-risk AML (Fig. 1 ). Of them, two patients had early relapse at day + 74 and day + 39, respectively, and one patient had grade 4 acute GVHD at day + 46. Another eight consecutive patients not receiving prophylactic DLI, who were fulfilling the criteria for both very high-risk AML and prophylactic DLI (being alive in CR at day + 90 and no history of > grade 2 acute GVHD), were selected as a control to compare the outcomes of HID-SCT patients receiving prophylactic DLI and those not receiving prophylactic DLI. The clinical characteristics of patients and donors were described in Table 1 . This study was approved by the Ethics Committee of Chinese PLA General Hospital, and signed informed consents were obtained from all patients prior to transplantation in accordance with principles of Declaration of Helsinki.
Conditioning regimen and GVHD prophylaxis
The modified Bu/Cy regimen consisted of busulfan, carmustine, cytarabine, and cyclophosphamide was used for all recipients without organ dysfunction, and cyclophosphamide was substituted with fludarabine for those with organ dysfunction during previous chemotherapy, as previously reported [11]. ATG (thymoglobulin, rabbit; Genzyme Europe BV; 2.5 mg/kg/day, days − 5 to − 2) was used in all recipients of HID-SCT. For recipients of MSD-SCT, ATG (2.5 mg/kg/day, days − 5 to − 4) was used in case of either the donor or the recipient was older than 40 years of age. G-CSF-mobilized unmanipulated PBSCs were collected and infused into the recipients on the day of collection. The target mononuclear cell (MNC) count was 5 × 108/kg recipient body weight, and the CD34+ cell count was 2 × 106/kg recipient body weight. Cyclosporine A (CsA), mycophenolate mofetil, and short-term methotrexate were used for GVHD prophylaxis for all recipients as previously reported [12].
Prophylactic DLI protocol
The scheduled timing of the first prophylactic DLI was + 30~+ 60 days after transplantation for MSD-SCT recipients and + 60~+ 90 days after transplantation for HID-SCT recipients. The reasons for delay of DLI included GVHD or infection occurred before the scheduled timing of prophylactic DLI. The G-CSF-mobilized PBSCs from cryopreserved cells of the graft were infused to the recipient at a dose of 2 × 107-CD3+ cells/kg recipient body weight. After prophylactic DLI, minimal residual disease was evaluated every 4 weeks by flow cytometry analysis and/or quantitative real-time PCR. It was not mandatory to stop CsA prior to prophylactic DLI. CsA was given at 2 mg/kg b.i.d. from day − 3 to day + 90 (HID-SCT) or to day + 60 (MSD-SCT) and then tapered at 33% per month to be discontinued on day + 150~+ 180 (HID-SCT) or on day + 120~+ 150 (MSD-SCT) unless GVHD developed. If the patients received prophylactic DLI before day + 90 (HID-SCT) or day + 60 (MSD-SCT), CsA was given 8 weeks after DLI in HID group and 4 weeks in MSD group at a though concentration of 150–250 ng/ml for DLI-associated GVHD prophylaxis and then tapered and discontinued within 2 weeks unless GVHD developed. If GVHD occurred before the scheduled timing of prophylactic DLI, it would be delayed for 8 weeks when GVHD was well controlled. Patients with positive MRD or hematologic relapse before the scheduled timing of prophylactic DLI received chemotherapy followed by preemptive or therapeutic DLI, which were not evaluated in this study.
Endpoints and definitions
The primary endpoints were the cumulative incidence of post-DLI acute GVHD and chronic GVHD, which were assessed as previously defined [13]. The secondary endpoints included the cumulative incidence of relapse and NRM, OS, and RFS. Relapse was defined as the hematologic recurrence of leukemia. NRM was defined as death from any cause without disease relapse. The time points after transplantation are represented by “+” signs.
Statistical analyses
Clinical features between groups were compared using the two-sided Fisher’s exact test for categorical data and non-parametric Mann-Whitney U test for continuous variables. The cumulative incidence of GVHD, relapse, and NRM was estimated considering the competing risks. Univariate analysis for acute GVHD, chronic GVHD, relapse, and NRM with competing events was performed using Gray’s method [14]. Fine and Gray semiparametric proportional hazards regression model was used for multivariate analysis to confirm the factors associated with the risks of GVHD, relapse, and NRM [15]. OS and RFS were estimated by the Kaplan-Meier method with log-rank test for univariate analysis. The Cox proportional hazards regression model with stepwise forward selection was used for multivariate analysis to confirm the factors associated with RFS or OS. Factors for univariate analysis and multivariate analysis of risk for GVHD, relapse, NRM, OS, or RFS were patient’s age (< 40 years vs. ≥ 40 years), high-risk gene mutations (no vs. yes), disease status at SCT (CR vs. NR), type of donor (HID vs. MSD), donor’s age (< 48 years vs. ≥48 years), the interval from diagnosis to transplant (< 6 months vs. ≥ 6 months), and prophylactic DLI (no vs. yes). Statistical analyses were performed using R statistical software and the cmprsk package (Comprehensive R Archive Network, TU Wien, Austria), Stata 14.0 software (Stata Corporation, College Station, TX, USA), and SPSS 20.0 software (IBM Corporation, Armonk, NY, USA). A p value < 0.05 was chosen as a threshold for significance. The end point of follow-up for all surviving subjects was April 30, 2018.
Results
Baseline characteristics of patients
Prophylactic DLI was administered at a median of 71 (34–240) days for HID-SCT recipients and 53 (35–97) days for MSD-SCT recipients (p = 0.008). The median number of CD3+ cells infused for HID-SCT and MSD-SCT recipients was 2.3 (0.4–7.3) × 107/kg and 2.3 (0.5–5.2) × 107/kg, respectively (p = 0.901). A total of 12 patients were in the NR state prior to transplantation, six patients achieved CR1 after ≥ 3 cycles of induction chemotherapy, and 19 patients carried gene mutations with a poor prognosis. There were no significant differences in the majority of baseline characteristics between HID-SCT group and MSD-SCT group except for no male donor to female recipient grafts in the MSD-SCT group (Table 1 ).
Comparison of transplantation outcomes between the HID-SCT and MSD-SCT group
No onset of DLI-associated pancytopenia was recorded in all of the prophylactic DLI recipients. A total of 17/34 patients (50.0%) developed acute GVHD at a median of 40 (14–97) days after prophylactic DLI. The cumulative incidence of grade 2–4 acute GVHD at 100-day post-DLI was 59.5% (95% CI, 32.5%–78.7%) in HID-SCT group, which was significantly higher compared with that in MSD-SCT group (30.8% (95% CI, 8.7%–56.6%), p = 0.050, Fig. 2 a). The cumulative incidence of grade 3–4 acute GVHD at 100-day post-DLI in HID-SCT group and MSD-SCT group was 17.5 (95% CI, 3.7–40.0%) and 7.7% (95% CI, 0.4–30.4%), respectively (p = 0.436, Fig. 2 b). The cumulative incidence of chronic GVHD at 1-year post-DLI in HID-SCT group and MSD-SCT group was 36.6 (95% CI, 15.3–58.4%) and 33.2% (95% CI, 8.8–60.7%), respectively (p = 0.982, Fig. 2 c). The cumulative incidence of severe chronic GVHD at 1-year post-DLI in HID-SCT group and MSD-SCT group was 15.3 (95% CI, 3.6–34.7%) and 27.3% (95% CI, 5.3–56.3%), respectively (p = 0.551, Fig. 2 d). No factors tested significantly correlated with the risk of occurrence of grade 3–4 acute GVHD, chronic GVHD, and severe chronic GVHD in univariate analyses (Table 2 ). No independent risk factors were found to be correlated with grade 2–4 or grade 3–4 acute GVHD, chronic GVHD, and severe chronic GVHD in multivariate analyses (data not shown).
The cumulative incidence of NRM at 1-year post-DLI was 27.9% (95% CI, 9.5–50.1%) in HID-SCT group, which appeared to be a trend of higher than that in MSD-SCT group (0.0%, p = 0.061, Fig. 3 a). Of the five patients in HID-SCT group who died of NRM, three died of GVHD on day + 145, + 249, and + 266 post-DLI, respectively, one died of hemorrhagic cystitis on day + 250 post-DLI, and one died of cerebral hemorrhage on day + 68 post-DLI. The first patient who died of GVHD was a 53-year-old woman with relapsed t(8;21) AML who exhibited NR to reinduction chemotherapy and received an salvage HID-PBSCT from her son followed by prophylactic DLI at day + 64 after transplantation. At day + 120 after transplantation (day + 56 after DLI), she developed a severe extensive chronic GVHD involving skin, oral mucosa, eyes, and liver. She died of GVHD with cachexia at day + 145 after DLI. The second patient who died of GVHD was a 46-year-old woman with FLT3-ITD and DNMT3a mutation AML who received a HID-PBSCT from her daughter followed by prophylactic DLI at day + 96 after transplantation. At day + 126 after transplantation (30 days after DLI), she developed a grade 2 acute GVHD involving skin and upper gastrointestinal tract and was cured. At day + 296 after transplantation (200 days after DLI), she developed a moderate GVHD involving gastrointestinal tract, but she abandoned of treatment and died of GVHD at + 345 after transplantation (249 days after DLI). The third patient who died of GVHD was a 19-year-old man with AML who achieved CR1 after three induction chemotherapies and received a HID-PBSCT from his father followed by prophylactic DLI at day + 86 after transplantation. He developed a grade 2 acute GVHD involving skin at day + 165 after transplantation (79 days after DLI), which was successfully treated by steroids. At day + 235 after transplantation (149 days after DLI), he developed a severe GVHD involving gastrointestinal tract. Although received treatment with CD25 monoclonal antibody and ruxolitinib, he finally abandoned of treatment and succumbed to his illness at day + 352 after transplantation (266 days after DLI). No other factors tested significantly correlated with the risk of NRM in univariate analyses (Table 2 ) and multivariate analyses (data not shown).
A total of eight (23.5%) patients relapsed at a median of 221 (74–447) days after prophylactic DLI. The cumulative incidences of relapse at 1 year after SCT in HID-SCT group were 21.6% (95% CI, 6.2–42.9%), which was not significantly different than that in MSD-SCT group (36.5% (95% CI, 9.5–65.1%), p = 0.543, Fig. 3 b). Disease in NR status prior to SCT (p = 0.001) and donor’s age ≥ 48 years (p = 0.028) correlated with a higher risk of relapse in univariate analyses (Table 2 ). In multivariate analysis, disease in NR status prior to SCT (hazard ratio (HR) = 26.5; p < 0.001), poor-risk gene mutations (HR = 51.4; p < 0.001), and donor’s age ≥ 48 years (HR = 7.6; p = 0.009) predicted higher risks of relapse (Table 3 ).
Median follow-up after SCT among surviving prophylactic DLI recipients was 425 days (111–1232). The estimated 1-year OS rate in HID-SCT group and MSD-SCT group was 55.1 (95% CI, 29.6–74.7%) and 83.9% (95% CI, 49.4–95.7%), respectively (p = 0.325, Fig. 3 c). The estimated 1-year RFS rate was 50.1% (95% CI, 25.5–70.5%) for HID-SCT group and 74.0% (95% CI, 38.2–91.0%) for MSD-SCT group (p = 0.419, Fig. 3 d). The quality of life of the prophylactic DLI recipients who survived without relapse (HID-SCT group, n = 12; MSD-SCT group, n = 9) was excellent with Karnofsky performance scores of 90–100%. In univariate analyses (Table 2 ), disease in NR status prior to SCT correlated with inferior OS (p = 0.018) and RFS (p = 0.005). In multivariate analyses, disease in NR status prior to SCT predicted an inferior OS (HR = 58.0; p = 0.035) and RFS (HR = 46.9; p = 0.045). Patient’s age ≥ 40 years (HR = 4.1; p = 0.045) predicted an inferior OS in multivariate analyses. There was a trend toward an inferior OS in patients who received HID-SCT (HR = 4.1; p = 0.056) (Table 3 ).
Comparison of outcomes of HID-SCT recipients receiving and not receiving prophylactic DLI
We further compared the outcomes of HID-SCT recipients who received prophylactic DLI with a control group of eight consecutive patients not receiving prophylactic DLI. There were no significant differences about baseline characteristics between two groups (Table 1 ). The cumulative incidence of NRM at 1 year after SCT was not statistically significant difference between non-DLI group and DLI group (0.0% vs. 27.9% (95% CI, 9.5–50.1%), p = 0.184). In both the univariate and multivariate analyses, there was no significant difference in the cumulative incidence of NRM depending on age, poor-risk gene mutations, disease status at transplantation, age of donor, time from diagnosis to transplantation, and prophylactic DLI (Supplementary Table 2). A total five (62.5%) patients relapsed at a median of 155 (99–351) days after transplantation in non-DLI group. Patients received prophylactic DLI had a significantly lower cumulative incidence of relapse (62.5% (95% CI, 18.4–87.8%)) than those not received prophylactic DLI (21.6% (95% CI, 6.2–42.9%), p = 0.049). Disease in NR status prior to SCT (p = 0.010), donor’s age ≥ 48 years (p = 0.018), and time from diagnosis to transplantation ≥ 6 months (p = 0.031) correlated with a higher risk of relapse in univariate analyses (Supplementary Table 1). No factors tested significantly correlated with the risk of relapse in multivariate analyses (Supplementary Table 2). There was no significant difference in the probability of OS (50.0% (95% CI, 15.2–77.5%) vs. 55.1% (95% CI, 29.6–74.7%), p = 0.999) and RFS (37.5% (95% CI, 8.7–67.4%) vs. 50.1% (95% CI, 25.5–70.5%), p = 0.570) between non-DLI group and DLI group. In univariate analyses (Supplementary Table 2), time from diagnosis to transplantation ≥ 6 months correlated with an inferior OS (p = 0.022) and RFS (p = 0.031). In multivariate analyses, there was a trend toward an inferior OS in patients who had disease in NR status prior to SCT (HR = 6.1; p = 0.061) and those who not undergoing transplantation within 6 months from diagnosis (HR = 4.4; p = 0.061) (Supplementary Table 2).
Discussion
Although prophylactic DLI has been used in allogeneic PBSCT setting for decades, its effectivity and toxicity remain unpredictable in many patients. Understanding the clinical and laboratory factors influencing the effectiveness and thereby abrogating the toxicity of prophylactic DLI would be benefit for further optimization of DLI procedure. In this study, we demonstrated the safety and efficacy of prophylactic DLI for the prevention of relapse in patients with very high-risk features received HID-SCT and those received MSD-SCT. The current results, representing an extension of our previously reported 15 HID-SCT recipients with identical inclusion criteria [11], were encouraging with a lower relapse rate after DLI of 21.6%, as compared with 62.5% in the control group of patients not receiving prophylactic DLI. In the HID-SCT group of patients who receiving prophylactic DLI, the cumulative incidences of grade 3–4 acute GVHD, chronic GVHD, severe chronic GVHD, risk of relapse, 1-year OS, and RFS after DLI were not statistically different from those of MSD-SCT group. Grade 2–4 acute GVHD and NRM in these HID-SCT recipients were higher than the MSD-SCT recipients. The differences about grade 2–4 acute GVHD and NRM between the HID-SCT and MSD-SCT recipients were not statistically significant anymore in multivariate analyses. Of course, the marginal significance for cumulative incidence of grade 2–4 acute GVHD (59.5% vs. 30.8%, p = 0.050) and NRM (27.9% vs. 0.0%, p = 0.061) between the HID-SCT group and MSD-SCT group might come from the small number of patients. Similarly, we speculated that the lack of difference in multivariate analyses might again result from the small number of patients.
Both the cell dose and the timing of DLI after transplantation might influence the toxicity and response. The optimal cell dose and timing of prophylactic DLI have yet to be determined in the setting of SCT with PBSCs as the stem cell source. Compared with previous studies using similar G-CSF-primed peripheral blood DLI but in the setting of SCT with bone marrow combined with PBSCs as the graft [9, 10, 16, 17], the number of CD3+ cells infused in this study was reduced to approximately 2 × 107/kg, considering the higher incidence of GVHD after PBSCT than after BMT. Moreover, because we have observed a higher incidence of grade 2–4 acute GVHD in the HID-SCT than that in the MSD-SCT [12], the scheduled timing of prophylactic DLI in this study was 4 weeks later in HID-SCT than that in MSD-SCT to avoid the potential increased incidence of DLI-associated GVHD. In this study, we showed that the cumulative incidences of DLI-associated acute GVHD were 59.5% for grades 2–4 and 17.5% for grades 3–4 in HID-SCT and 30.8% for grade 2–4 and 7.7% for grade 3–4 in MSD-SCT. In above-mentioned previous studies in the setting of SCT with bone marrow combined with PBSCs as the graft [9, 10, 16, 17], the dose of CD3+ cells infused was 3–5 × 107/kg, and the cumulative incidences of DLI-associated acute GVHD were 35–55% for grades 2–4 and 13–28% for grades 3–4 in HID-SCT and 18% for grades 2–4 in MSD-SCT. In our previous study using similar DLI procedure and in the setting of SCT with PBSCs as the graft [11], the incidence of DLI-associated grade 2–4 and grade 3–4 acute GVHD was 55.3 and 10.2% in a group of 31 patients with very high-risk leukemia/lymphoma, receiving prophylactic DLI from G-CSF-primed PBSCs after HID-SCT. Therefore, the result in this study was comparable to previously reported DLI-associated GVHD incidence either in HID-SCT or in MSD-SCT.
The greatest caution from usage of prophylactic DLI is the potential toxicity of fatal GVHD, leading to an increased NRM. We found a marginal significance for cumulative incidence of NRM in patients receiving prophylactic DLI between HID-SCT group and MSD-SCT group (27.9% vs. 0.0%, p = 0.061). It is noted that there was no patient died of DLI-associated GVHD in MSD-SCT group in this study, suggesting its apparent low toxicity in terms of GVHD and NRM in MSD-SCT setting. However, despite prophylactic treatment with immunosuppressive agents after DLI, three patients died of DLI-associated GVHD in the 21 patients in HID-SCT group. Of the three patients, one died of not initiating treatment for GVHD timely and abandoned of treatment for economic reasons, and finally, another two died of treatment failure for GVHD. Therefore, DLI-associated GVHD remained to be the main toxicity for HID-SCT patients receiving prophylactic DLI.
In the current study, the relapse of leukemia was not statistically different between HID-SCT group and MSD-SCT group. The risk of relapse in the HID-SCT patients who received prophylactic DLI was lower than that in very high-risk patients who did not receive prophylactic DLI (21.6% vs. 62.5%), suggesting that the prophylactic DLI was effective in reducing early relapse. However, this did not transform an improved RFS and OS, suggesting that once the patients who received prophylactic DLI experienced relapse of leukemia, the time of survival would be very short, even shorter than those who did not received prophylactic DLI. We hypothesized that the leukemic cells survived the graft-versus-leukemia effect of lymphocyte from donor would be more resistance. Of course, this hypothesis need to be further verified. The decreased rate of relapse provides a possibility to take measures to prevent future relapse. A recent study found that patients who received 2–4 times of prophylactic DLI had significantly lower risks of relapse than those who received DLI once [18], suggesting that minimal residual disease monitoring-guided DLI in PBSCT might be used to further reduce the risk of late relapse. In addition, the applications of new drugs, such as tyrosine kinase inhibitor (sorafenib, midostaurin), histone deacetylase inhibitor (panobinostat), or DNA methyltransferase inhibitors (azacytidine, decitabine), may also benefit to reduce the long-term relapse.
The major limitations of the current study are the relatively small patient numbers, the retrospective character of the analysis, and the lack of randomization. Because the high-risk patients with AML have a very short survival and are more likely to have comorbidities, poor performance status, and impaired organ function, not all of them have the opportunity to access to salvage transplantation. Even if they have the chance to undergo transplantation, some of them still cannot receive prophylactic DLI due to early relapse, severe GVHD, or other reasons. Therefore, the proportion of very high-risk patients with AML who can eventually receive prophylactic DLI is small. Nevertheless, the patients enrolled in this study were consecutive, and the protocol of prophylactic DLI was consistent, guaranteeing the objectivity of the conclusion. Due to the relatively small sample size in this study, there is a greater probability of false-negative results in the differences in grade 3–4 acute GVHD, chronic GVHD, OS, and RFS between HID-SCT and MSD-SCT groups. To further increase the power of our test, we would extend the follow-up period and recruit more patients in the future research.
In summary, this study demonstrated that the dose and timing of prophylactic DLI we used were very safe and the prophylactic DLI was efficient in reducing the incidence of relapse in patients with very high-risk AML receiving MSD-SCT. For those patients receiving HID-SCT, the application of prophylactic DLI could reduce the risk of relapse with acceptable toxicity, but we need to optimize the protocol of prophylactic DLI to reduce the DLI-associated fatal toxicity.
References
Craddock C, Tauro S, Moss P, Grimwade D (2005) Biology and management of relapsed acute myeloid leukaemia. Br J Haematol 129(1):18–34
Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM, Kamble R, Copelan E, de Lima M, Gupta V, Keating A, Lazarus HM, Litzow MR, Marks DI, Maziarz RT, Rizzieri DA, Schiller G, Schultz KR, Tallman MS, Weisdorf D (2010) Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol 28(23):3730–3738
Middeke JM, Herold S, Rücker-Braun E, Berdel WE, Stelljes M, Kaufmann M, Schäfer-Eckart K, Baldus CD, Stuhlmann R, Ho AD, Einsele H, Rösler W, Serve H, Hänel M, Sohlbach K, Klesse C, Mohr B, Heidenreich F, Stölzel F, Röllig C, Platzbecker U, Ehninger G, Bornhäuser M, Thiede C, Schetelig J, Study Alliance Leukaemia (SAL) (2016) TP53 mutation in patients with high-risk acute myeloid leukaemia treated with allogeneic haematopoietic stem cell transplantation. Br J Haematol 172(6):914–922
Metzeler KH, Maharry K, Radmacher MD, Mrózek K, Margeson D, Becker H, Curfman J, Holland KB, Schwind S, Whitman SP, Wu YZ, Blum W, Powell BL, Carter TH, Wetzler M, Moore JO, Kolitz JE, Baer MR, Carroll AJ, Larson RA, Caligiuri MA, Marcucci G, Bloomfield CD (2011) TET2 mutations improve the new European LeukemiaNet risk classification of acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol 29(10):1373–1381
Ahn JS, Kim HJ, Kim YK, Lee SS, Jung SH, Yang DH, Lee JJ, Kim NY, Choi SH, Jung CW, Jang JH, Kim HJ, Moon JH, Sohn SK, Won JH, Kim SH, Kim DD (2016) DNMT3A R882 mutation with FLT3-ITD positivity is an extremely poor prognostic factor in patients with normal-karyotype acute myeloid leukemia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 22(1):61–70
Schlenk RF, Kayser S, Bullinger L, Kobbe G, Casper J, Ringhoffer M, Held G, Brossart P, Lübbert M, Salih HR, Kindler T, Horst HA, Wulf G, Nachbaur D, Götze K, Lamparter A, Paschka P, Gaidzik VI, Teleanu V, Späth D, Benner A, Krauter J, Ganser A, Döhner H, Döhner K, German-Austrian AML Study Group (2014) Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood 124(23):3441–3449
Mawad R, Lionberger JM, Pagel JM (2013) Strategies to reduce relapse after allogeneic hematopoietic cell transplantation in acute myeloid leukemia. Curr Hematol Malig Rep 8(2):132–140
Friedrichs B, Tichelli A, Bacigalupo A, Russell NH, Ruutu T, Shapira MY, Beksac M, Hasenclever D, Socié G, Schmitz N (2010) Long-term outcome and late effects in patients transplanted with mobilized blood or bone marrow: a randomised trial. Lancet Oncol 11(4):331–338
Wang Y, Liu DH, Fan ZP, Sun J, Wu XJ, Ma X, Xu LP, Liu KY, Liu QF, Wu DP, Huang XJ (2012) Prevention of relapse using DLI can increase survival following HLA-identical transplantation in patients with advanced-stage acute leukemia: a multi-center study. Clin Transpl 26(4):635–643
Wang Y, Liu DH, Xu LP, Liu KY, Chen H, Zhang XH, Chen YH, Han W, Wang FR, Wang JZ, Yan CH, Huang XJ (2012) Prevention of relapse using granulocyte CSF-primed PBPCs following HLA-mismatched/haploidentical, T-cell-replete hematopoietic SCT in patients with advanced-stage acute leukemia: a retrospective risk-factor analysis. Bone Marrow Transplant 47(8):1099–1104
Gao XN, Lin J, Wang SH, Huang WR, Li F, Li HH, Chen J, Wang LJ, Gao CJ, Yu L, Liu DH (2018) Donor lymphocyte infusion for prevention of relapse after unmanipulated haploidentical PBSCT for very high-risk hematologic malignancies. Ann Hematol 98:185–193. https://doi.org/10.1007/s00277-018-3482-7
Li HH, Li F, Gao CJ, Huang WR, Bo J, Dou LP, Wang LL, Jing Y, Wang L, Li WJ, Yu L, Liu DH (2017) Similar incidence of severe acute GVHD and less severe chronic GVHD in PBSCT from unmanipulated, haploidentical donors compared with that from matched sibling donors for patients with haematological malignancies. Br J Haematol 176(1):92–100
Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Henslee-Downey PJ, Cahn JY, Calderwood S, Gratwohl A, Socié G, Abecasis MM, Sobocinski KA, Zhang MJ, Horowitz MM (1997) IBMTR severity index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol 97(4):855–864
Scrucca L, Santucci A, Aversa F (2007) Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant 40(4):381–387
Scrucca L, Santucci A, Aversa F (2010) Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant 45(9):1388–1395
Yan CH, Liu DH, Xu LP, Liu KY, Zhao T, Wang Y, Chen H, Chen YH, Han W, Huang XJ (2012) Modified donor lymphocyte infusion-associated acute graft-versus-host disease after haploidentical T cell-replete hematopoietic stem cell transplantation: incidence and risk factors. Clin Transpl 26(6):868–876
Yan CH, Xu LP, Liu DH, Chen H, Wang Y, Wang JZ, Wang FR, Han W, Liu KY, Huang XJ (2015) Low-dose methotrexate may preserve a stronger antileukemic effect than that of cyclosporine after modified donor lymphocyte infusion in unmanipulated haploidentical SCT. Clin Transpl 29(7):594–605
Yan CH, Liu QF, Wu DP, Zhang X, Xu LP, Zhang XH, Wang Y, Huang H, Bai H, Huang F, Ma X, Huang XJ (2017) Prophylactic donor lymphocyte infusion (DLI) followed by minimal residual disease and graft-versus-host disease-guided multiple DLIs could improve outcomes after allogeneic hematopoietic stem cell transplantation in patients with refractory/relapsed acute leukemia. Biol Blood Marrow Transplant 23(8):1311–1319
Funding
This work was partially supported by grants from the National Natural Science Foundation of China (81770203 to D-H L, 81670135 and 81870109 to X-N G), the Natural Science Foundation of Beijing (7162174 to J L), and the National Clinical Specialist Focus on Military Construction Projects.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 60 kb)
Rights and permissions
About this article
Cite this article
Gao, XN., Lin, J., Wang, LJ. et al. Comparison of the safety and efficacy of prophylactic donor lymphocyte infusion after haploidentical versus matched-sibling PBSCT in very high-risk acute myeloid leukemia. Ann Hematol 98, 1267–1277 (2019). https://doi.org/10.1007/s00277-019-03636-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-019-03636-8