Abstract
High-dose melphalan (MEL200) followed by autologous stem cell transplantation (ASCT) remains a standard of care for multiple myeloma (MM). Bendamustine induces responses in MM resistant to other alkylators. Our prior Phase I trial adding bendamustine to MEL200 transplant conditioning resulted in no additional toxicity. We now report a single-arm, phase II study that evaluated the efficacy of bendamustine 225 mg/m2 with MEL200 conditioning for ASCT in 18 patients with newly diagnosed MM (NDMM) and 17 with relapsed or refractory MM (RRMM). The primary end point was the complete response (CR/sCR) rate at day+ 100. Sample size was determined according to Simon’s two-stage design. At stage 1, sixteen patients entered the study. As there were eight patients with CR/sCR, enrollment increased to 28 patients. Sixteen out of the first 28 evaluable patients achieved CR/sCR, meeting the design criteria. Enrollment was then expanded to a total of 35 patients. 51% achieved a CR/sCR. After a median follow-up of 65 months, 21 patients progressed, including 7 deaths. The median PFS for NDMM and RRMM was 48 and 45 months, respectively. Bendamustine/MEL200 conditioning resulted in excellent overall and depth of response as well as PFS, particularly in the RRMM patients, and is worthy of further investigation (NCT00916058).
Similar content being viewed by others
Introduction
High-dose chemotherapy followed by autologous stem cell transplantation (ASCT) remains a standard of care for Multiple Myeloma (MM) [1,2,3,4,5]. The conditioning regimen used for ASCT has not changed since the Intergroupe Francophone du Myélome demonstrated superior survival for melphalan 200 mg/m2 (MEL200) versus 8-Gy TBI with melphalan 140 mg/m2 (MEL140) in 2002 [6]. Since then, a number of agents [i.e., alkylators, proteasome inhibitors (PI), immunomodulatory drugs (IMiDs)] and other strategies have been tested in combination with MEL200 [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. With the exception of a sole phase III study showing improved progression-free survival (PFS) for conditioning with busulfan and MEL140 compared to MEL200, no other regimen has shown superiority to the standard MEL200 conditioning regimen in a randomized controlled trial (RCT) [24]. It is now well established that achievement of deeper responses post-transplant, as measured by sensitive measurable residual disease detection technologies (MRD), correlates highly with extended survival [25,26,27,28]. Therefore, augmenting transplant conditioning strategies with the purpose of increasing the depth of response after transplant, may result in further improvement in duration of remission and potentially improved survival.
Bendamustine is a synthetic agent that combines the alkylating properties of a mustard-group with the antimetabolic activity of a purine analog [29]. Similar to other alkylators, bendamustine is a DNA cross-linking agent that causes DNA breaks. However, DNA single- and double-strand breaks caused by bendamustine are more extensive and significantly more durable than those induced by other alkylators such as melphalan [30, 31]. Its antimetabolite activity includes induction of apoptosis and inhibition of mitosis [31, 32]. With this distinctive dual mechanism of action, bendamustine has proven to induce responses in MM resistant to other alkylators and is therefore a promising agent to test synergy in conditioning regimens [31,32,33,34,35,36,37,38,39]. Our group previously reported a phase I study of high-dose bendamustine up to 225 mg/m2 in combination with MEL200 in transplant-naive MM and showed that this regimen was well tolerated and a maximum tolerated dose of bendamustine was not reached [23]. We now report the results of the phase II study of chemotherapy conditioning comprised of bendamustine and melphalan in patients undergoing first ASCT.
Patients and Methods
Study design
This is a phase II, single-arm study using bendamustine 225 mg/m2 with MEL200 as determined in the prior phase I trial [23]. The primary objective was to determine the efficacy of bendamustine in combination with melphalan conditioning in patients with MM that would warrant further investigation. The IRB of Weill Cornell Medicine, in accordance with federal regulations and the ethical principles provided in the Helsinki Declaration, approved the protocol. All patients provided informed consent. Patients were enrolled between 05/2011 and 12/2015, and the trial is registered at ClinicalTrials.gov with identifier NCT00916058.
Study eligibility patient population
Patients eligible for entry were 18–75 years with confirmed MM undergoing first ASCT. Patients could be screened for inclusion either i) post-induction for newly diagnosed MM (NDMM), or ii) after relapsed or progressive disease for relapsed or refractory MM (RRMM). Eligibility for ASCT was assessed by adequate collection of ≥2 × 106 of CD34+/kg stem cells, Karnofsky performance status ≥70% and adequate organ function defined as ejection fraction >40%, lung diffusion capacity >45%, creatinine clearance (CrCl) >25 ml/min, liver enzymes ≤5× and total bilirubin ≤1.5×. Major exclusion criteria included previous ASCT, pregnancy, or uncontrolled medical conditions. The Revised International Staging System (R-ISS) at the time of the initial diagnosis was used for prognostication [40]. High-risk cytogenetics were defined as any of the following: del(17p), t(4;14), or t(14;16). Any percentage positive signal on FISH testing was considered a positive result. Prior lines of therapy, defined by the International Myeloma Working Group (IMWG), were obtained for each patient [41].
Conditioning Regimen and Supportive care
The highest dose assessed from the phase I study of bendamustine (125 mg/m2 on day-2 and 100 mg/m2 on day-1) in combination with melphalan (100 mg/m2 daily on day-2 and day-1) were chosen for the study. Melphalan was dose-reduced to 70 mg/m2 per day for CrCl <70 ml/min. At the time of melphalan infusion, each patient was offered oral cryotherapy for 6 h [42]. Stem cell products were infused 24–48 h after the last dose of melphalan. All patients received G-CSF support starting on day + 1 until absolute neutrophil count (ANC) was ≥1000 neutrophils/mm3 for 48 h. Patients received levofloxacin, acyclovir or valacyclovir, and fluconazole prophylaxis starting day + 1, red blood cell and platelet transfusions to support a hemoglobin >7 g/dL and platelet count >10,000/mm3, and other supportive care as an in-patient until ANC engraftment.
Response determination
Engraftment was defined as per CIBMTR criteria as ANC >500/mm3 and platelet count of 20,000/mm3 without platelet support for at least 3 days [43]. Serum and urine protein electrophoresis with immunofixation as well as free light chain determinations were done before transplantation, day + 30, day + 60, day + 100, day + 180 and thereafter every 6 months to assess disease response as per IMWG criteria [44]. Bone marrow biopsy was performed before ASCT and at day + 100. Karyotyping and FISH with CD138-cell selection were performed on all samples. PFS was defined as time elapsed between the stem cell infusion (day + 0) and disease progression or death. OS was defined as time elapsed between day + 0 and death from any cause. The response assessment at day + 100 was independently reviewed by two investigators.
Safety Assessment
Patients were followed prospectively for adverse events (AEs) from day-2 through day + 100. Transplant-related mortality (TRM) was defined as death within the first 100 days due to any cause other than disease progression. All AEs were first documented by the trial physicians, and thereafter collated and graded by two independent reviewers according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAEv4.0). The Cornell Data and Safety Monitoring Board oversaw the monitoring plan.
Maintenance Therapy
At the time of the study design, maintenance therapy was not a standard of care and it was therefore not part of the initial protocol. As the practice evolved, the use and type of maintenance or continuous therapy post-ASCT was left to the discretion of the treating physician starting after day + 100.
Statistical Analyses
The primary end point was to evaluate the proportion of patients with complete response (CR) or better at day + 100. Sample size was determined according to Simon’s two-stage minimax design assuming 10% level of significance and 80% power [45]. Previous reports with induction followed by ASCT have shown a CR rate of ~20 to 60% depending on the induction regimen [5, 46]. Based on the objectives of this early phase II study, and the expected heterogeneity of the study population, a CR proportion of ≤40% was defined as unacceptable and >60% as worthy of further exploration. The null hypothesis that the CR proportion is ≤40% was tested against the alternative hypothesis that the CR proportion is ≥60%. In the first stage, 16 patients entered the study and 7 or more patients needed to respond to enter the second stage. At Stage 2, the treatment would be declared effective and worthy of further testing if 15 or more patients respond among 28 patients. This design yielded a ≥0.80 probability of a positive result if the true percentage of complete responders was ≥60%. It yielded a ≥0.90 probability of a negative result if the true percentage of complete responders was ≤40%. Assuming that approximately 10% of patients were declared unavailable/ineligible, we calculated that a total 32 patients were needed for enrollment.
Secondary end points included OS, PFS, and rate of toxicities. PFS and OS were assessed by Kaplan–Meier survival analysis. Patients without OS/PFS events were censored at the last follow-up for OS and at the last disease evaluation for PFS. Post-hoc subgroup comparative analyses for overall response rate (ORR) and depth of response between NDMM and RRMM cohorts was performed by Χ2 test and Fisher-exact test. Comparisons of OS/PFS between groups of interest were evaluated by the log-rank test. A Cox proportional-hazards model was used to evaluate the impact of tumor/patient characteristics on the outcomes of interest. Ninety-five percent confidence intervals (95% CI) for all OS/PFS proportions were calculated to assess the precision of the obtained estimates. All analyses were performed in Stata version 15.0 (StataCorp, College Station, TX).
Results
Study population
At stage 1, 16 patients entered the study. As there were eight patients with a complete response/stringent complete response (CR/sCR), enrollment then increased to a total of 28 patients at stage 2. Sixteen out of the first 28 evaluable patients achieved a CR/sCR, meeting the bar for further exploration as prespecified in the statistical design. The study enrollment was then expanded to a total of 35 patients to improve the precision of the 95%CI. The baseline characteristics of the patients are summarized in Table 1.
ASCT was performed as part of the upfront therapy in 18 patients with NDMM (51%) and in 17 patients with RRMM (49%). Induction regimens for the NDMM included VRd (61%), BiRd [clarithromycin-lenalidomide-dexamethasone] (6%), Ixazomib/Rd (6%), CyBord (3%), V-DCEP (3%), Rd (3%). Of the RRMM group, 3 patients had relapsed disease, 4 had primary refractory disease, 7 had refractory disease to the last line of therapy, and 3 patients had received more than two lines of induction based on poor responses and were transplanted more than 12 months after initial diagnoses (for the purpose of this subgroup analysis, these patients were included in the RRMM group). Median lines of therapy prior to ASCT for the RRMM group was 2 (range: 1–4). Therapy exposure history is described in Table 1. All patients had received either an IMiD or a PI at some point during treatment, most commonly lenalidomide (16/17) and bortezomib (16/17). Four patients had received a newer-generation proteasome inhibitor or IMiD. Seven patients were refractory to lenalidomide, seven were refractory to bortezomib, three of them double-refractory. One patient was double-refractory to pomalidomide and carfilzomib. There was no statistical difference seen between the depth of response pre-transplant in the NDMM and RRMM groups (P = 0.63).
Stem Cell Infusion and Engraftment
Our algorithm for mobilization, based on preharvest HPC values and preemptive use of plerixafor, was followed [47]. Plerixafor was added based on CD34+ count on Day + 4 of G-CSF. Table 2 summarizes information for mobilization, melphalan dosing, CD34+ collection and engraftment.
Toxicity
Incidence of grade 3 AEs that occurred in at least 2 cases and all grade 4 AEs are listed in Table 3. There was no transplant-related mortality (TRM). The most common AEs (reported for more than 40% of the patients) were nausea (94%), fatigue (94%), hypocalcemia (94%), anorexia (91%), diarrhea (91%), hypoalbuminemia (91%), dry mouth (63%), dyspnea (60%), mucositis (57%), abdominal pain (51%), peripheral edema (49%), bloating (49%), constipation (49%) and febrile neutropenia (46%). The most frequently observed grade 3 AEs were febrile neutropenia (46%) and hypokalemia (20%). There was only one grade 4 AE, hyperbilirubinemia deemed to be secondary to a drug reaction to piperacillin/tazobactam. The patient had neutropenic fever on day + 5 and was started on piperacillin/tazobactam. The next day, the patient developed new bilirubinemia. Cultures came back positive on day + 6 (later identified as S.hominis). Piperacillin/tazobactam was discontinued after day + 7 and alternative antibiotic therapy was provided. Bilirubin peaked at day + 13 and thereafter resolved. Liver biopsy was performed and consistent with a drug reaction.
Disease Response
Day + 100 evaluation with bone marrow examination was performed at a median of 99 days (range 83–135). 51% of the 35 patients enrolled achieved a CR/sCR (95%CI 36%-67%). This included 8 patients (44%) transplanted with NDMM and 10 patients (59%) with RRMM (p = 0.31). The ORR for the cohort was 97%; 100% for the NDMM and 94% for the RRMM setting (p = 0.49). Figure 1 shows all response changes with respect to initial disease status prior to ASCT. Maximum CR/sCR response rate for the cohort was 54% as one additional patient achieved a CR during maintenance therapy (7 months after ASCT). Only 1 patient progressed before day + 100 and one additional patient progressed before Day + 180 (both with RRMM). Individual responses and outcomes for patients with dose-reduction of melphalan based on impaired renal function are described in the Supplementary Information.
Maintenance
Maintenance was given in 12 out of 18 patients transplanted in the NDMM setting (67%) and in 10 out of 16 eligible patients (63%) transplanted in the RRMM setting. Maintenance was continued until progression or unacceptable toxicity. Seventeen patients started lenalidomide 21/28 days (13 at 10 mg and 4 at 5 mg). Five patients received PI-based regimens (1 carfilzomib and 4 bortezomib). Six patients with NDMM and six with RRMM did not receive maintenance. Reasons included patient refusal [4], recurrent infections [1], physician recommendation [7].
Clinical Outcomes
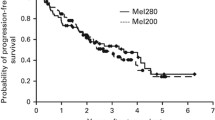
After a median follow-up of 65 months (range: 9–86 months), 21 patients had progressed (60%): 10 (56%) transplanted with NDMM and 11 (65%) with RRMM. Seven patients (20%) had died from MM progression (3 NDMM and 4 RRMM). Median OS was not reached. The 3-year OS was 88% (95% CI: 72–95%); 94% for NDMM and 81% for RRMM. The median PFS was 47 months (95% CI: 34 months- NE); 48 months for NDMM and 45 months for RRMM. The 3-year PFS was 78% (95% CI:51–91%) for NDMM and 57% (95%CI:30–77%) for RRMM. (Fig. 2). For RRMM patients, maintenance had a significant impact on the median PFS [49 months vs 9 months (p = 0.02)]. For NDMM, the use of maintenance had a clear trend but no statistically significant impact on PFS [median PFS 61 months vs 40 months (p = 0.38)]. Depth of pre-transplant response, categorized as CR/sCR, VGPR, PR and SD or PD, was significantly associated with PFS (p = 0.03). Multivariable analysis was not possible due to the small size of the patient cohorts.
Discussion
In this single-institution phase II interventional study, we confirmed that high-dose bendamustine in combination with standard high-dose melphalan was safe and effective in first transplant in both NDMM and RRMM. The observed complete response rate of the 35 patients enrolled was 51% (95% CI: 36–67%). High response rates resulted in excellent long-term outcomes, both in the NDMM setting with a median PFS of 48 months, and more remarkably in the RRMM setting, with a similar median PFS of 45 months.
A number of different agents have been tested in combination with melphalan conditioning. With acknowledgment that cross trial comparisons do not carry the same weight of evidence as a controlled study, the results of bendamustine/MEL200 conditioning can be put into context with previously performed trials. Table 4 summarizes the main conditioning studies for ASCT in MM with characteristics and outcomes reported. Initial combination of melphalan with oral/IV busulfan, cyclophosphamide alone or with idarubicin, showed a trend towards increased TRM or unacceptable toxicity [8, 11, 12, 15]. Combination with cyclophosphamide-topotecan-melphalan and mitoxantrone-melphalan showed response rates comparable to historical data [14, 17]. The most promising interventions in the RRMM setting have been with carmustine- bortezomib-thalidomide with median PFS of 28 months [18], high-dose lenalidomide with median PFS of 10 months [20], and with infusional gemcitabine with targeted-busulfan and melphalan with median PFS of 15 months [19]. Costa el al. published the results of carfilzomib with melphalan in the relapsed-setting with a 1-year PFS of 67% with carfilzomib maintenance [22]. For NDMM, a RCT of bortezomib-melphalan failed to show improved CR rates compared to MEL200, but mature data for PFS are pending [10]. A recently published RCT compared infusional busulfan-melphalan vs melphalan alone in NDMM and showed clear superiority of the combination arm with a median PFS of 64.7 vs 34.4 months [24]. Maintenance was used in both arms. The combination regimen had increased rates of non-hematological toxicity (febrile neutropenia, ALT elevation and mucositis) but there was no increase in TRM or SOS/VOD.
Our results should be carefully interpreted in the context ofhistorical results of melphalan conditioning. Specifically, for NDMM, median PFS of 48 months (61 months with maintenance) seems similar to the PFS of the transplant group of the IFM2009 study with VRd induction-consolidation (50 months from randomization prior to induction) [5], and to the PETHEMA/GEM Study with VTD induction (56 months from start of induction and with tandem-ASCT allowed) [48]. The limited number of patients, variable induction regimens and maintenance regimens used, and differences in study design preclude formal comparison. For example, it is notable that the control arm of the infusional busulfan-melphalan vs melphalan had a particularly short PFS of 34.4 months [24]. On the other hand, in RRMM with ASCT performed in the delayed setting, median PFS of 45 months seems promising when comparing to other intensified conditioning studies, as well as when compared to the reported PFS for newer therapies (i.e daratumumab, carfilzomib, pomalidomide), also listed in Table 4 [49,50,51,52,53,54,55,56,57,58,59,60,61,62,63]. Bendamustine/melphalan conditioning followed by maintenance may become a particularly cost-effective strategy for the management of MM at relapse for patients who did not receive ASCT upfront.
The efficacy of this regimen supports prior observations that bendamustine has anti-myeloma activity in patients with disease refractory to conventional alkylator chemotherapy. Single agent activity was initially shown in patients with progressive disease after ASCT [33]. Bendamustine-prednisone was then found to have higher potency than melphalan-prednisone as an induction regimen in NDMM where CR rates were doubled (32% vs 13%) and longer PFS were reached [64]. Different combination therapies of bendamustine have also shown clinical activity in heavily treated patients [35,36,37,38,39]. The efficacy and distinct toxicity profile of bendamustine provided the rationale for the combination with high-dose melphalan in multiple myeloma patients undergoing ASCT and explains the promising outcomes observed.
Limitations of this study include the relatively small cohort size, heterogeneity in patient characteristics, treatment history, variability in maintenance approaches and low number of advanced-stage patients. Furthermore, this early-phase study started in 2011 and was conducted during a time of rapid change in myeloma treatment. Nevertheless, we believe the excellent tolerance, encouraging response rates and duration of response justify a prospective randomized trial in more selected populations with a defined timing of transplantation and post-transplant approaches.
Conclusion
In summary, high-dose bendamustine with melphalan showed favorable safety and encouraging efficacy as an ASCT conditioning regimen in MM. CR/sCR was achieved in 51% of the patients at day + 100. This resulted in excellent long-term outcomes both in the NDMM setting, with a median PFS of 48 months, and most impressively in the RRMM setting, with a median PFS of 45 months. Based on our findings, further investigations of the role of this conditioning strategy are warranted. A RCT in the RRMM setting with MRD negativity as a surrogate outcome is currently planned.
References
Mikhael J, Ismaila N, Cheung MC, Costello C, Dhodapkar MV, Kumar S, et al. Treatment of multiple myeloma: ASCO and CCO Joint Clinical Practice Guideline Summary. J Oncol Pract. 2019;37:1228–63.
Gay F, Engelhardt M, Terpos E, Wasch R, Giaccone L, Auner HW, et al. From transplant to novel cellular therapies in multiple myeloma: European Myeloma Network guidelines and future perspectives. Haematologica. 2018;103:197–211.
Dhakal B, Szabo A, Chhabra S, Hamadani M, D’Souza A, Usmani SZ, et al. Autologous transplantation for newly diagnosed multiple myeloma in the era of novel agent induction: a systematic review and meta-analysis. JAMA Oncol. 2018;4:343–50.
Moreau P, San Miguel J, Sonneveld P, Mateos MV, Zamagni E, Avet-Loiseau H, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv52–iv61.
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–20.
Moreau P. Comparison of 200 mg/m2 melphalan and 8 Gy total body irradiation plus 140 mg/m2 melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002;99:731–5.
Maybury B, Cook G, Pratt G, Yong K, Ramasamy K. Augmenting autologous stem cell transplantation to improve outcomes in myeloma. Biol Blood Marrow Transplant. 2016;22:1926–37.
Lahuerta JJ, Mateos MV, Martinez-Lopez J, Grande C, de la Rubia J, Rosinol L, et al. Busulfan 12 mg/kg plus melphalan 140 mg/m2 versus melphalan 200 mg/m2 as conditioning regimens for autologous transplantation in newly diagnosed multiple myeloma patients included in the PETHEMA/GEM2000 study. Haematologica. 2010;95:1913–20.
Roussel M, Moreau P, Huynh A, Mary JY, Danho C, Caillot D, et al. Bortezomib and high-dose melphalan as conditioning regimen before autologous stem cell transplantation in patients with de novo multiple myeloma: a phase 2 study of the Intergroupe Francophone du Myelome (IFM). Blood. 2010;115:32–7.
Roussel M, Hebraud B, Lauwers-Cances V, Macro M, Leleu X, Hulin C, et al. Bortezomib and high-dose melphalan vs. high-dose melphalan as conditioning regimen before autologous stem cell transplantation in de novo multiple myeloma patients: a phase 3 study of the Intergroupe Francophone Du Myelome (IFM 2014-02). Blood. 2017;130(Suppl 1):398.
Blanes M, Lahuerta JJ, González JD, Ribas P, Solano C, Alegre A, et al. Intravenous busulfan and melphalan as a conditioning regimen for autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: a matched comparison to a melphalan-only approach. Biol Blood Marrow Transplant. 2013;19:69–74.
Knop S, Bauer K, Hebart H, Wandt H, Trumper L, Liebisch P, et al. A randomized comparison of total-marrow irradiation, Busulfan and cyclophosphamide with tandem high-dose melphalan in patients with multiple myeloma. Blood. 2007;110:223a–4.
Park SS, Kim K, Kim SJ, Lee JH, Yoon SS, Mun YC, et al. A phase I/II, open-label, prospective, multicenter study to evaluate the efficacy and safety of lower doses of bortezomib plus busulfan and melphalan as a conditioning regimen in patients with multiple myeloma undergoing autologous peripheral blood stem cell transplantation: The KMM103 study. Biology of blood and marrow transplantation: Biol Blood and Marrow Transplant. 2019.
Beaven AW, Moore DT, Sharf A, Serody JS, Shea TC, Gabriel DA. Infusional mitoxantrone plus bolus melphalan as a stem cell transplant conditioning regimen for multiple myeloma. Cancer Invest. 2011;29:214–9.
Fenk R, Schneider P, Kropff M, Huenerlituerkoglu AN, Steidl U, Aul C, et al. High-dose idarubicin, cyclophosphamide and melphalan as conditioning for autologous stem cell transplantation increases treatment-related mortality in patients with multiple myeloma: results of a randomised study. Br J Haematol. 2005;130:588–94.
Donato ML, Aleman A, Champlin RE, Weber D, Alexanian R, Ippoliti CM, et al. High-dose topotecan, melphalan and cyclophosphamide (TMC) with stem cell support: a new regimen for the treatment of multiple myeloma. Leuk Lymphoma. 2004;45:755–9.
Kazmi SM, Saliba RM, Donato M, Wang M, Hosing C, Qureshi S, et al. Phase II trial of high-dose topotecan, melphalan and CY with autologous stem cell support for multiple myeloma. Bone Marrow Transplant. 2011;46:510–5.
Comenzo RL, Hassoun H, Kewalramani T, Klimek V, Dhodapkar M, Reich L, et al. Results of a phase I/II trial adding carmustine (300 mg/m2) to melphalan (200 mg/m2) in multiple myeloma patients undergoing autologous stem cell transplantation. Leukemia. 2006;20:345–9.
Nieto Y, Valdez BC, Pingali SR, Bassett R, Delgado R, Nguyen J, et al. High-dose gemcitabine, busulfan, and melphalan for autologous stem-cell transplant in patients with relapsed or refractory myeloma: a phase 2 trial and matched-pair comparison with melphalan. Lancet Haematol. 2017;4:e283–92.
Mark TM, Guarneri D, Forsberg P, Rossi A, Pearse R, Perry A, et al. A Phase I trial of high-dose lenalidomide and melphalan as conditioning for autologous stem cell transplantation in relapsed or refractory multiple myeloma. Biol Blood Marrow Transplant. 2017;23:930–7.
Patel P, Oh AL, Koshy M, Sweiss K, Saraf SL, Quigley JG, et al. A phase 1 trial of autologous stem cell transplantation conditioned with melphalan 200 mg/m(2) and total marrow irradiation (TMI) in patients with relapsed/refractory multiple myeloma. Leuk Lymphoma. 2018;59:1666–71.
Costa LJ, Landau HJ, Chhabra S, Hari P, Innis-Shelton R, Godby KN, et al. Phase 1/2 trial of carfilzomib plus high-dose melphalan preparative regimen for salvage autologous hematopoietic cell transplantation followed by maintenance carfilzomib in patients with relapsed/refractory multiple myeloma. Biol Blood Marrow Transplant. 2018;24:1379–85.
Mark TM, Reid W, Niesvizky R, Gergis U, Pearse R, Mayer S, et al. A phase 1 study of bendamustine and melphalan conditioning for autologous stem cell transplantation in multiple myeloma. Biol Blood Marrow Transplant. 2013;19:831–7.
Bashir Q, Thall PF, Milton DR, Fox PS, Kawedia JD, Kebriaei P, et al. Conditioning with busulfan plus melphalan versus melphalan alone before autologous haemopoietic cell transplantation for multiple myeloma: an open-label, randomised, phase 3 trial. Lancet Haematol. 2019;6:E266–75.
Perrot A, Lauwers-Cances V, Corre J, Robillard N, Hulin C, Chretien ML, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018;132:2456–64.
Lahuerta JJ, Paiva B, Vidriales MB, Cordon L, Cedena MT, Puig N, et al. Depth of response in multiple myeloma: a pooled analysis of three PETHEMA/GEM clinical trials. J Clin Oncol. 2017;35:2900–10.
Munshi NC, Avet-Loiseau H, Rawstron AC, Owen RG, Child JA, Thakurta A, et al. Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: a meta-analysis. JAMA Oncol. 2017;3:28–35.
Rawstron AC, Child JA, de Tute RM, Davies FE, Gregory WM, Bell SE, et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol. 2013;31:2540–7.
Gandhi V, Burger JA. Bendamustine in B-cell malignancies: the new 46-year-old kid on the block. Clin Cancer Res. 2009;15:7456–61.
Strumberg D, Harstrick A, Doll K, Hoffmann B, Seeber S. Bendamustine hydrochloride activity against doxorubicin-resistant human breast carcinoma cell lines. Anticancer Drugs. 1996;7:415–21.
Leoni LM, Bailey B, Reifert J, Bendall HH, Zeller RW, Corbeil J, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008;14:309–17.
Leoni LM, Hartley JA. Mechanism of action: the unique pattern of bendamustine-induced cytotoxicity. Semin Hematol. 2011;48(Suppl 1):S12–23.
Knop S, Straka C, Haen M, Schwedes R, Hebart H, Einsele H. The efficacy and toxicity of bendamustine in recurrent multiple myeloma after high-dose chemotherapy. Haematologica. 2005;90:1287–8.
Ponisch W, Holzvogt B, Plotze M, Andrea M, Bourgeois M, Heyn S, et al. Bendamustine and prednisone in combination with bortezomib (BPV) in the treatment of patients with newly diagnosed/untreated multiple myeloma. J Cancer Res Clin Oncol. 2014;140:1947–56.
Fenk R, Michael M, Zohren F, Graef T, Czibere A, Bruns I, et al. Escalation therapy with bortezomib, dexamethasone and bendamustine for patients with relapsed or refractory multiple myeloma. Leuk Lymphoma. 2007;48:2345–51.
Ludwig H, Kasparu H, Leitgeb C, Rauch E, Linkesch W, Zojer N, et al. Bendamustine-bortezomib-dexamethasone is an active and well-tolerated regimen in patients with relapsed or refractory multiple myeloma. Blood. 2014;123:985–91.
Lentzsch S, O’Sullivan A, Kennedy RC, Abbas M, Dai L, Pregja SL, et al. Combination of bendamustine, lenalidomide, and dexamethasone (BLD) in patients with relapsed or refractory multiple myeloma is feasible and highly effective: results of phase 1/2 open-label, dose escalation study. Blood. 2012;119:4608–13.
Sivaraj D, Green MM, Kang Y, Long GD, Rizzieri DA, Li Z, et al. Bendamustine, pomalidomide, and dexamethasone for relapsed and/or refractory multiple myeloma. Blood Cancer J. 2018;8:71.
Gramatzki M, Günther A, Offidani M, Engelhardt M, Corradini P, Gentili S, et al. Carfilzomib in combination with bendamustine and dexamethasone (CBd) in relapsed and/or refractory patients with multiple myeloma: the phase I/II EMN09 Study. Blood. 2016;128:3334.
Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33:2863–9.
Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–5.
Lilleby K, Garcia P, Gooley T, McDonnnell P, Taber R, Holmberg L, et al. A prospective, randomized study of cryotherapy during administration of high-dose melphalan to decrease the severity and duration of oral mucositis in patients with multiple myeloma undergoing autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 2006;37:1031–5.
CIBMTR. Instructions for Post-Transplant Essential Data (Post-TED) Form 2012. https://www.cibmtr.org/DataManagement/TrainingReference/Manuals/DataManagement/Documents/post-ted-instruction.pd.
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–46.
Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin trials. 1989;10:1–10.
Harousseau JL, Attal M, Avet-Loiseau H. The role of complete response in multiple myeloma. Blood. 2009;114:3139–46.
Storch E, Mark T, Avecilla S, Pagan C, Rhodes J, Shore T, et al. A novel hematopoietic progenitor cell mobilization and collection algorithm based on preemptive CD34 enumeration. Transfusion. 2015;55:2010–6.
Rosinol L, Oriol A, Teruel AI, Hernandez D, Lopez-Jimenez J, de la Rubia J, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012;120:1589–96.
Kumar SK, Lacy MQ, Dispenzieri A, Buadi FK, Hayman SR, Dingli D, et al. Early versus delayed autologous transplantation after immunomodulatory agents-based induction therapy in patients with newly diagnosed multiple myeloma. Cancer. 2012;118:1585–92.
Dunavin NC, Wei L, Elder P, Phillips GS, Benson DM Jr., Hofmeister CC, et al. Early versus delayed autologous stem cell transplant in patients receiving novel therapies for multiple myeloma. Leuk Lymphoma. 2013;54:1658–64.
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–31.
Bahlis N, Dimopoulos MA, White DJ, Benboubker L, Cook G, Leiba M, et al. Three-year follow up of the phase 3 pollux study of daratumumab plus lenalidomide and dexamethasone (d-rd) versus lenalidomide and dexamethasone (rd) alone in relapsed or refractory multiple myeloma (RRMM). Blood. 2018;132(Suppl 1):1996.
Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754–66.
Spencer A, Lentzsch S, Weisel K, Avet-Loiseau H, Mark TM, Spicka I, et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of CASTOR. Haematologica. 2018;103:2079–97.
Chari A, Suvannasankha A, Fay JW, Arnulf B, Kaufman JL, Ifthikharuddin JJ, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130:974–81.
Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Spicka I, Oriol A, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–52.
Siegel DS, Dimopoulos MA, Ludwig H, Facon T, Goldschmidt H, Jakubowiak A, et al. Improvement in overall survival with carfilzomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2018;36:728–34.
Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hájek R, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38.
Dimopoulos MA, Goldschmidt H, Niesvizky R, Joshua D, Chng WJ, Oriol A, et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:1327–37.
Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621–31.
Dimopoulos MA, Lonial S, Betts KA, Chen C, Zichlin ML, Brun A, et al. Elotuzumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended 4-year follow-up and analysis of relative progression-free survival from the randomized ELOQUENT-2 trial. Cancer. 2018;124:4032–43.
Dimopoulos MA, Dytfeld D, Grosicki S, Moreau P, Takezako N, Hori M, et al. Elotuzumab plus pomalidomide and dexamethasone for multiple myeloma. N Engl J Med. 2018;379:1811–22.
Cavo M, Hájek R, Pantani L, Beksac M, Oliva S, Dozza L, et al. Autologous stem cell transplantation versus bortezomib-melphalan-prednisone for newly diagnosed multiple myeloma: second interim analysis of the phase 3 EMN02/HO95 study. Blood. 2017;130(Suppl 1):397.
Ponisch W, Mitrou PS, Merkle K, Herold M, Assmann M, Wilhelm G, et al. Treatment of bendamustine and prednisone in patients with newly diagnosed multiple myeloma results in superior complete response rate, prolonged time to treatment failure and improved quality of life compared to treatment with melphalan and prednisone--a randomized phase III study of the East German Study Group of Hematology and Oncology (OSHO). J cancer Res Clin Oncol. 2006;132:205–12.
Funding
PC was partially supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (1-UL1-TR002384–01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gomez-Arteaga, A., Mark, T.M., Guarneri, D. et al. High-dose bendamustine and melphalan conditioning for autologous stem cell transplantation for patients with multiple myeloma. Bone Marrow Transplant 54, 2027–2038 (2019). https://doi.org/10.1038/s41409-019-0587-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0587-0
- Springer Nature Limited
This article is cited by
-
Adding bendamustine to melphalan before ASCT improves CR rate in myeloma vs. melphalan alone: A randomized phase-2 trial
Bone Marrow Transplantation (2022)
-
Initial Therapeutic Approaches to Patients with Multiple Myeloma
Advances in Therapy (2021)