Abstract
Although cytogenetic abnormalities at diagnosis are recognized as an important prognostic factor in patients with Philadelphia chromosome (Ph)-negative acute lymphoblastic leukemia (ALL), the prognostic impact has not been evaluated in allogeneic stem cell transplant (allo-SCT) recipients. Thus, we assessed 373 Ph-negative ALL patients who underwent allo-SCT. The high-risk (HR) group included those with t(4;11), t(8;14), low hypodiploidy, and complex karyotype, and the standard risk (SR) group included all other karyotypes. Among the 204 patients who underwent a transplant during the first remission (167 in the SR group and 37 in the HR group), the overall survival (OS) rates were similar between these groups (64.1% vs. 80.0% at 5 years, respectively; p = 0.12). Conversely, among the 106 patients who underwent a transplant while not in remission (84 in the SR group and 22 in the HR group), patients in the SR group showed a significantly superior OS rate compared to the HR group (15.4% vs. 4.5% at 5 years, respectively; p = 0.022). These results suggested that treatment outcomes of Ph-negative ALL patients with HR cytogenetic abnormalities may improve following allo-SCT, especially in the first remission. Innovative transplant approaches are warranted in patients who are not in remission.
Similar content being viewed by others
Introduction
Adult Philadelphia chromosome (Ph)-negative acute lymphoblastic leukemia (ALL) is an aggressive hematological malignancy that accounts for approximately 10–20% of adult acute leukemia. Allogeneic stem cell transplant (allo-SCT) has held a prominent position in treatment strategies for adult Ph-negative ALL patients due to a more potent anti-leukemia effect than chemotherapy [1, 2]. However, the higher rate of non-relapse mortality (NRM) hampers the survival benefit of allo-SCT, and the indication for allo-SCT in these patients is controversial, especially during the first complete remission (CR1) [3, 4]. Moreover, treatment outcomes have improved with introduction of pediatric-type chemotherapy regimens [5,6,7] and targeted therapy such as rituximab [8, 9] and nelalabine [10, 11]. Thus, identification of patients who will potentially obtain a survival benefit from allo-SCT is an emergent issue in this field.
Cytogenetic abnormality at diagnosis is one of the most important prognostic factors in acute leukemia. In adult acute myeloid leukemia patients, categorization of patients into cytogenetic risk groups is a useful tool for determining prognosis after chemotherapy [12, 13] and after allo-SCT [14, 15]. Moorman et al. [16] showed that overall survival (OS) rates were clearly stratified according to cytogenetic risk groups in ALL patients, and t(4;11), t(8;14), low hypodiploidy, and complex karyotype (five or more abnormalities) are associated with poor survival rates in adult Ph-negative ALL patients.
However, very few data are available regarding whether the prognostic impact of cytogenetic abnormalities is applicable to patients with Ph-negative ALL even after allo-SCT. Aldoss et al. [17] evaluated the prognostic impact of cytogenetic abnormalities on transplant outcomes in 202 adult ALL patients who underwent a transplant during the first remission. Although no significant difference in survival rates was seen between the good/intermediate and poor risk group, nearly half of patients included in their study were Ph-positive ALL patients. Nishiwaki et al. [18] identified t(4;11) and t(8;14) as unfavorable prognostic factors in Ph-negative ALL patients undergoing allo-SCT from related or unrelated donors during the subsequent CR and non-CR. As their study was performed using Japanese registry data, which could not provide complete cytogenetic data, a complex karyotype was categorized as other abnormalities. Therefore, a clinical study with more precise cytogenetic data was warranted to clarify this important issue.
To this end, we performed this large-scale retrospective study, which compared transplant outcomes of Ph-negative ALL patients between two cytogenetic risk groups.
Patients and methods
Study population
Of the 513 adult Ph-negative ALL patients over 15 years of age who underwent allo-SCT for the first time between January 2001 and December 2012 at the 23 institutions participating in the Kanto Study Group for Cell Therapy (KSGCT), 373 patients whose cytogenetic data at diagnosis were available were included in this study. The study included both B cell and T cell lineage ALL patients. The Institutional Review Board of Gunma University approved the protocol of this study.
Definition
Cytogenetic risk groups were defined according to the suggestion from Moorman’s study. The high-risk (HR) group included patients with t(4;11), t(8;14), low hypodiploidy, and complex karyotype (≥5e abnormalities), and the standard-risk (SR) group included all other karyotypes [16]. CR was determined only with morphological manner. Conditioning intensity was categorized as myeloablative conditioning (MAC) and reduced-intensity conditioning (RIC) according to published criteria [19].
Data collection
KSGCT is a clinical study group for SCT consisted of 23 institutions in Japan. Clinical data of the 373 patients assessed in this study were collected from the KSGCT database. The presence of a complex karyotype was confirmed with a secondary survey sent to each institution.
Statistical analysis
OS was defined as the interval from the date of transplantation to the date of death. NRM was defined as any death during continuous CR. Fisher’s exact test was used for comparison of binary variables. OS was estimated by the Kaplan–Meier method and compared using the log-rank test. Cumulative incidences (CIs) of relapse and NRM were compared using the stratified Gray test. Multivariate analysis for OS was performed with Cox proportional hazard model, and cytogenetic risk, age at transplant, white blood cell count at diagnosis, donor type, HLA disparity, intensity of conditioning regimen (MAC or RIC), and conditioning regime with or without total body irradiation (TBI) were included as explanatory variables. All calculations were performed using the EZR software package (http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmed.html), and p < 0.05 was considered statistically significant [20].
Results
Study population
Of the 373 transplant recipients included in this study, 199 patients were male and 174 were female. The median age was 34 years (range, 16–66 years). Karyotypes at diagnosis resulted in 308 patients being categorized into the SR group and 65 patients being categorized into the HR group (Table 1). The analysis was performed by stratifying the study population based on disease status at the time of transplant. Of the 373 patients, 204 underwent allo-SCT during CR1 (167 in the SR group and 37 in the HR group), 63 during the subsequent CR (57 in the SR group and 6 in the HR group), and 106 during non-CR (84 in the SR group and 22 in the HR group).
Patient characteristics and transplant procedures in CR1 patients
We found no significant difference in patient characteristics between the SR group and the HR group (Table 2). Of the 204 patients who underwent a transplant during CR1, 75, 91, and 38 patients received a graft from related donors, unrelated donors, and cord blood, respectively, and almost all patients were conditioned with TBI-containing MAC regimens. Transplant procedures also did not significantly differ between these groups.
Transplant outcomes in CR1 patients
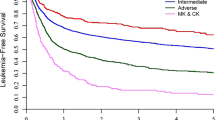
The OS rates were not significantly different between the SR group and the HR group in CR patients (64.1% vs. 80.0% at 5 years, respectively; p = 0.12) (Fig. 1a). Significant differences were also not found in the CI of relapse and NRM rates between the two groups (relapse: 21.1% vs. 18.0% at 5 years, respectively; p = 0.408, NRM: 18.5% vs. 8.4% at 5 years, respectively; p = 0.267) (Fig. 1b, c). Multivariate analysis of OS revealed that no factor showed independent prognostic significance including cytogenetic risk.
Transplant outcomes in 204 Philadelphia chromosome (Ph)-negative acute lymphoblastic leukemia (ALL) patients who underwent a transplant during first complete remission (CR1) stratified according to the cytogenetic risk group. Overall survival (a), cumulative incidence (CI) of relapse (b), and CI of non-relapse mortality (NRM) (c)
Background and transplant outcomes in subsequent CR patients
Among the 63 patients who underwent a transplant during the subsequent CR, we found no significant difference in patient characteristics or transplant procedures between the SR group and the HR group (Table 4). No significant difference was found in the OS rates between the two groups (49.7% vs. 40.0% at 5 years, respectively; p = 0.345). Likewise, the CI of relapse and NRM rates were similar between the two groups (relapse: 40.5% vs. 60.0% at 5 years, respectively; p = 0.575, NRM: 23.2% vs. 20.0% at 5 years, respectively; p = 0.771). No independent prognostic factor was identified with multivariate analysis of OS.
Background and transplant outcomes in non-CR patients
We found no significant difference in patient characteristics or transplant procedures between the SR group and the HR group, except for female predominance in the HR group, in the 106 patients who underwent a transplant during non-CR (Table 3). Patients in the SR group showed a significantly superior OS rate compared to the HR group (15.4% vs. 4.5% at 5 years, respectively; p = 0.022) (Fig. 2b). We found no significant difference in the CI of relapse between the SR and HR groups (60.3% vs. 50.0% at 5 years, respectively; p = 0.411), whereas the CI of NRM in the HR group was significantly higher than that in the SR group (24.8% vs. 45.5% at 5 years, respectively; p = 0.024). Multivariate analysis revealed that the cytogenetic risk group at diagnosis was a significant factor predicting poor OS (Table 4).
Discussion
To the best of our knowledge, this is the largest study focusing on the prognostic impact of cytogenetic abnormalities at diagnosis in allo-SCT recipients with Ph-negative ALL. Although cytogenetic abnormalities are recognized as one of the most important prognostic factors among patients with Ph-negative ALL [16, 21, 22], its prognostic impact has not been precisely evaluated in transplant recipients to date. The OS rate of patients in the HR group who underwent a transplant during CR1 was similar to that of the SR group, showing a 5-year OS rate of up to 80%. As the equality of the CR rates between the two groups was described in several studies, the higher incidence of relapse was considered to contribute to unfavorable outcomes in the HR group [21, 22]. Therefore, if a suitable donor is available, allo-SCT in the early phase of the clinical course potentially improves the prognosis of the HR group, just like Ph-positive ALL in the pre-imatinib era [23, 24].
Improvement in treatment outcomes was recently described in younger adult patients with Ph-negative ALL treated with pediatric-type regimens, which contain increased doses of vincristine and l-asparaginase [5,6,7]. And now, it is aggressively investigated whether pediatric-type regimens is applicable to elderly patients. Thus, the indication of allo-SCT in patients treated with pediatric-type regimens should be reevaluated in the near future. Furthermore, the minimal residual disease (MRD) status clearly stratifies treatment outcomes in Ph-negative ALL patients [25, 26]. Although the status of MRD was not available for the analysis in our study, its impact should be considered on deciding the indication of allo-SCT.
Although the effectiveness of allo-SCT was shown even in patients in the HR group who underwent a transplant during CR1, four types of cytogenetic abnormalities were assessed together in this study. However, patients with each abnormality are considered to possess distinctive clinical courses. For instance, the majority of relapses reportedly occur within a year after diagnosis in patients with t(4;11) and t(8;14) [16]. Thus, patients with each abnormality should be assessed separately, but the small number of patients in this study with each abnormality hampered this important analysis. A further study with a larger number of subjects is warranted.
Conversely, transplant outcomes of patients who underwent a transplant during non-CR were extremely poor, especially those in the HR group who showed a 5-year OS rate of <5%. Current transplant procedures are considered unsatisfactory in this population. Thus, a tailored treatment strategy should be urgently developed that includes intensification of conditioning regimens, post-transplant maintenance therapies, and incorporation of molecular targeting strategies into allo-SCT, such as blinatumomab and chimeric antigen receptor T cells, which show a potent anti-leukemic effect in patients with CD19-positive ALL [27, 28].
In conclusion, our findings suggested that the transplant outcomes of adult Ph-negative ALL patients in the HR group were comparable to those of patients in the SR group when a transplant is performed during CR1. Based on our data, we strongly recommend performing a transplant for Ph-negative ALL patients with HR cytogenetic abnormalities early in the disease course, preferably during CR1, as the transplant outcome of patients not in remission was dismal. Innovative transplant approaches are clearly warranted to improve the transplant outcome of patients, especially HR patients with cytogenetic abnormalities who are not in remission.
References
Sebban C, Lepage E, Vernant JP, Gluckman E, Attal M, Reiffers J, et al. Allogeneic bone marrow transplantation in adult acute lymphoblastic leukemia in first complete remission: a comparative study. French Group of Therapy of Adult Acute Lymphoblastic Leukemia. J Clin Oncol. 1994;12:2580–7.
Hunault M, Harousseau JL, Delain M, Truchan-Graczyk M, Cahn JY, Witz F, et al. Better outcome of adult acute lymphoblastic leukemia after early genoidentical allogeneic bone marrow transplantation (BMT) than after late high-dose therapy and autologous BMT: a GOELAMS trial. Blood. 2004;104:3028–37.
Ram R, Gafter-Gvili A, Vidal L, Paul M, Ben-Bassat I, Shpilberg O, et al. Management of adult patients with acute lymphoblastic leukemia in first complete remission: systematic review and meta-analysis. Cancer. 2010;116:3447–57.
Yanada M, Matsuo K, Suzuki T, Naoe T. Allogeneic hematopoietic stem cell transplantation as part of postremission therapy improves survival for adult patients with high-risk acute lymphoblastic leukemia: a metaanalysis. Cancer. 2006;106:2657–63.
Ribera JM, Oriol A, Sanz MA, Tormo M, Fernández-Abellán P, del Potro E, et al. Comparison of the results of the treatment of adolescents and young adults with standard-risk acute lymphoblastic leukemia with the Programa Español de Tratamiento en Hematología pediatric-based protocol ALL-96. J Clin Oncol. 2008;26:1843–9.
Huguet F, Leguay T, Raffoux E, Thomas X, Beldjord K, Delabesse E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27:911–8.
Storring JM, Minden MD, Kao S, Gupta V, Schuh AC, Schimmer AD, et al. Treatment of adults with BCR-ABL negative acute lymphoblastic leukaemia with a modified paediatric regimen. Br J Haematol. 2009;146:76–85.
Thomas DA, O’Brien S, Faderl S, Garcia-Manero G, Ferrajoli A, Wierda W. Ret al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia. J Clin Oncol. 2010;28:3880–9.
Hoelzer D, Gökbuget N. Chemoimmunotherapy in acute lymphoblastic leukemia. Blood Rev. 2012;26:25–32.
Jain P, Kantarjian H, Ravandi F, Thomas D, O’Brien S, Kadia T, et al. The combination of hyper-CVAD plus nelarabine as frontline therapy in adult T-cell acute lymphoblastic leukemia and T-lymphoblastic lymphoma: MD Anderson Cancer Center experience. Leukemia. 2014;28:973–5.
Dunsmore KP, Devidas M, Linda SB, Borowitz MJ, Winick N, Hunger SP, et al. Pilot study of nelarabine in combination with intensive chemotherapy in high-risk T-cell acute lymphoblastic leukemia: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:2753–9.
Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92:2322–33.
Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–83.
Ferrant A, Labopin M, Frassoni F, Prentice HG, Cahn JY, Blaise D, et al. Karyotype in acute myeloblastic leukemia: prognostic significance for bone marrow transplantation in first remission: a European Group for Blood and Marrow Transplantation study. Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Blood. 1997;90:2931–8.
Fang M, Storer B, Estey E, Othus M, Zhang L, Sandmaier BM, et al. Outcome of patients with acute myeloid leukemia with monosomal karyotype who undergo hematopoietic cell transplantation. Blood. 2011;118:1490–4.
Moorman AV, Harrison CJ, Buck GA, Richards SM, Secker-Walker LM, Martineau M, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109:3189–97.
Aldoss I, Tsai NC, Slovak ML, Palmer J, Alvarnas J, Marcucci G, et al. Cytogenetics does not impact outcomes in adult patients with acute lymphoblastic leukemia undergoing allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:1212–7.
Nishiwaki S, Miyamura K, Ohashi K, Kurokawa M, Taniguchi S, Fukuda T, et al. Impact of a donor source on adult Philadelphia chromosome-negative acute lymphoblastic leukemia: a retrospective analysis from the Adult Acute Lymphoblastic Leukemia Working Group of the Japan Society for Hematopoietic Cell Transplantation. Ann Oncol. 2013;24:1594–602.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–33.
Kanda Y. Investigation of the freely-available easy-to-use software “EZR” (Easy R) for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Pullarkat V, Slovak ML, Kopecky KJ, Forman SJ, Appelbaum FR. Impact of cytogenetics on the outcome of adult acute lymphoblastic leukemia: results of Southwest Oncology Group 9400 study. Blood . 2008;111:2563–72.
Wetzler M, Dodge RK, Mrózek K, Carroll AJ, Tantravahi R, Block AW, et al. Prospective karyotype analysis in adult acute lymphoblastic leukemia: the cancer and leukemia Group B experience. Blood. 1999;93:3983–93.
Fielding AK, Rowe JM, Richards SM, Buck G, Moorman AV, Durrant IJ, et al. Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993. Blood. 2009;113:4489–96.
Dombret H, Gabert J, Boiron JM, Rigal-Huguet F, Blaise D, Thomas X, et al. Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia–results of the prospective multicenter LALA-94 trial. Blood. 2002;100:2357–66.
Gökbuget N, Kneba M, Raff T, Trautmann H, Bartram CR, Arnold R, et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120:1868–76.
Ribera JM, Oriol A, Morgades M, Montesinos P, Sarrà J, González-Campos J, et al. Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: final results of the PETHEMA ALL-AR-03 trial. J Clin Oncol. 2014;32:1595–604.
Topp MS, Kufer P, Gökbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–8.
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18.
Acknowledgements
We wish to thank all of the staff in the participating institutions of the Kanto Study Group for Cell Therapy.
Author information
Authors and Affiliations
Consortia
Contributions
HS designed and performed the research, analyzed the data, and wrote the manuscript; ND, HK, TS, TM, SM, ST, CO, SF, SY, MH, YK, MO, MG, SK, JT, KU, NK, NA, and SO provided the patient data, reviewed, and confirmed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shimizu, H., Doki, N., Kanamori, H. et al. Prognostic impact of cytogenetic abnormalities in adult patients with Philadelphia chromosome-negative ALL who underwent an allogeneic transplant. Bone Marrow Transplant 54, 2020–2026 (2019). https://doi.org/10.1038/s41409-019-0585-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0585-2
- Springer Nature Limited
This article is cited by
-
A prognostic score system in adult T‐cell acute lymphoblastic leukemia after hematopoietic stem cell transplantation
Bone Marrow Transplantation (2024)