Abstract
We conducted two parallel prospective, multicenter, phase II studies to evaluate the safety and efficacy of HLA-haploidentical peripheral blood stem cell transplantation using post-transplant cyclophosphamide (PTCy-haploPBSCT) following myeloablative conditioning (MAC, n = 50) and reduced-intensity conditioning (RIC, n = 77). Event-free survival (EFS) at 1-year as for primary endpoint was 64% and 43% in the MAC and RIC groups, respectively. Neutrophil engraftment was achieved in 98% and 94% in the MAC and RIC groups, respectively. The incidences of grades II–IV and III–IV acute graft-versus-host disease (GVHD) were 18% and 8% in the MAC group, and 14% and 5% in the RIC group, respectively. Those of all grade and moderate to severe chronic GVHD at 2-year were 36% and 20% in the MAC group, and 27% and 20% in the RIC group, respectively. Overall survival (OS), EFS, nonrelapse mortality, and relapse rate at 2-year were 68%, 54%, 10%, and 36% in the MAC group, and 44%, 35%, 20%, and 45% in the RIC group, respectively. Notably, 83% and 86% of patients who survived without relapse stopped immunosuppressant at 2-year in the MAC and RIC groups, respectively. Our results indicate that both MAC and RIC are valid options for PTCy-haploPBSCT for adults with hematological malignancies.
Similar content being viewed by others
Introduction
Allogeneic hematopoietic stem cell transplantation (SCT) is a potentially curative treatment for patients with hematological malignancies. However, HLA-matched related or unrelated donors are not always available. Haploidentical related donors are alternative donors for patients in the absence of a HLA-matched donor and in an urgent need of transplantation. Over the last few decades, several strategies have been developed to overcome HLA barriers in HLA-haploidentical SCT, such as use of post-transplant cyclophosphamide (PTCy) [1, 2]. Rationale of this strategy is assumed to be selective and cytotoxic depletion of alloreactive T cells [3].
HLA-haploidentical SCT using PTCy was initially developed in the setting of bone marrow transplantation (BMT) following nonmyeloablative conditioning (NMC) [1, 2]. A series of previous studies demonstrated that this strategy was feasible and safe with low incidences of acute and chronic graft-versus-host disease (GVHD) and non-relapse mortality (NRM); however, relapse remained a major cause of treatment failure [1, 2]. Therefore, PTCy based HLA-haploidentical SCT programs using myeloablative conditioning (MAC) and reduced-intensity conditioning (RIC) have been developed to reduce relapse [4,5,6,7]. However, HLA-mismatch (vs. HLA-match), MAC regimen (vs. RIC or NMC regimen), and peripheral blood stem cell transplantation (PBSCT, vs. BMT) are risk factors for GVHD [8]. It thus remains to be investigated whether MAC could increase risks of GVHD and NRM compared to RIC in the setting of PTCy based HLA-haploidentical PBSCT (PTCy-haploPBSCT). Therefore, we conducted two parallel prospective multicenter phase II studies to evaluate the safety and efficacy of MAC and RIC based PTCy-haploPBSCT, and herein we reported outcomes.
Methods
Study design
Two parallel prospective, multicenter, phase II studies were conducted by the Japan Study Group for Cell Therapy and Transplantation (JSCT) to evaluate the safety and efficacy of PTCyhaploPBSCT following MAC (JSCT Haplo14 MAC, UMIN000014406) and RIC (JSCT Haplo14 RIC, UMIN000014408). The institutional review board of each participating institution approved study protocols and written informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Major inclusion criteria were as follows: age 15 to 60 years for the MAC study, age 15 to 65 years for the RIC study, diagnosis of hematological malignancies, a good performance status (ECOG PS 0–2), and adequate organ functions (bilirubin <2.0 mg/dl; AST/ALT, <3 × upper limit of normal; creatinine clearance, >30 ml/min, cardiac ejection fraction, >50%; and SpO2 at room air, >95%). Patients who had antibodies targeting mismatched donor HLAs (donor-specific antibodies, DSA) were excluded from the study. Patients who had a prior history of allogeneic SCT were excluded from the MAC study, but not from the RIC study.
Conditioning regimens and GVHD prophylaxis
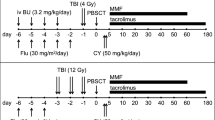
MAC regimen was either fludarabine (Flu, 90 mg/m2) plus total body irradiation (TBI, 12 Gy), or a combination of Flu (150 mg/m2), BU (12.8 mg/kg), and TBI (4 Gy). RIC regimen was a combination of Flu (150 mg/m2), BU (6.4 mg/kg), and TBI (4 Gy). Regimen was selected at physician’s discretion. GVHD prophylaxis consisted of cyclophosphamide (50 mg/kg/day on days 3 and 4) and tacrolimus plus mycophenolate mofetil starting on day 5. Peripheral blood stem cells (PBSCs) mobilized with granulocyte-colony stimulating factor were only accepted as stem cell grafts.
Endpoints
Endpoints of the 2 parallel studies were the same. The primary endpoint was event-free survival (EFS) at 1-year. EFS was defined as the time interval from transplantation to first event (either relapse or death in complete remission, whichever occurred first). Secondary endpoints included overall survival (OS), the incidence of engraftment, acute GVHD, chronic GVHD, relapse, NRM, and noninfectious fever early after transplantation. OS was defined as the time between transplantation and death. Neutrophil engraftment was defined as the absolute neutrophil count exceeded 0.5 × 109/L for 3 consecutive days after PBSCT. Platelet engraftment was defined as the absolute platelet count exceeded 20 × 109/L for 7 consecutive days without platelet transfusion. Acute and chronic GVHD were diagnosed and graded based on the traditional criteria [9, 10]. Relapse was defined based on morphologic evidence of malignant cells in the bone marrow, or other extra-medullary organs. Hematopoietic cell transplantation specific comorbidity index (HCT-CI) was determined as described by Sorror et al. [11]. Disease risk of the patients was determined according to the refined disease risk index (DRI) [12].
Statistical analysis
In the MAC study, expected 1-year EFS was estimated to be 50% and the threshold 1-year EFS was set to be 30% according to previous studies [1, 4]. The required sample size was 35 eligible patients for an 80% power to detect a 20% difference in 1-year EFS at from the threshold with a one-sided type I error of 0.05. The planned sample size was 38 with the expectation that 10% would be ineligible.
In the RIC study, expected 1-year EFS was estimated to be 40% and the threshold 1-year EFS was set to be 25% [1, 13]. The required sample size was 57 eligible patients for an 80% power to detect a 15% difference in 1-year EFS from the threshold with a one-sided type I error of 0.05. The planned sample size was 62 with the expectation that 10% would be ineligible.
The probabilities of EFS and OS and their confidence intervals were estimated using the Kaplan–Meier method, and the groups were compared using the log-rank test. The probabilities of neutrophil and platelet engraftment, acute and chronic GVHD, relapse, and NRM and their confidence were estimated based on cumulative incidence methods [14]. Competing events were death without engraftment for neutrophil and platelet engraftment, death or relapse without GVHD for acute and chronic GVHD, death without relapse for relapse, and relapse for NRM. The groups were compared using a Gray’s test [15].
A value of P < .05 was used to determine statistical significance. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphic user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions that are frequently used in biostatistics [16].
Results
Patient characteristics
Fifty-three patients were enrolled in the MAC study, and 81 patients were enrolled in the RIC study between 2014 and 2016. After excluding 7 patients who did not meet the inclusion criteria, 50 patients with a median age of 36 (range, 17 to 60) and 77 patients with a median age of 58 (range, 22 to 65) in the MAC and RIC studies, respectively, were analyzed (Table 1). Diagnoses included acute myeloid leukemia (AML; MAC, n = 23; RIC, n = 34), acute lymphoblastic leukemia (ALL; MAC, n = 11; RIC, n = 14), myelodysplastic syndrome/myeloproliferative neoplasms (MDS/MPN; MAC, n = 6; RIC, n = 12), lymphoma (MAC, n = 6; RIC, n = 14), and others (MAC, n = 4; RIC, n = 3). Twenty-four patients (48%) and 45 patients (58%) in the MAC and RIC studies, respectively, were not in remission at the time of transplantation. According to the refined DRI, patients were classified as low risk (MAC, n = 2; RIC, n = 3), intermediate risk (MAC, n = 22; RIC, n = 2), high risk (MAC, n = 14; RIC, n = 25), and very high risk (MAC, n = 12; RIC, n = 27). No patient had a history of prior allo-SCT in the MAC group, while 30 (39%) of 77 patients in the RIC group had a history of prior alloSCT. The median follow-up periods for survivors were 763 days (range, 380–1119) and 740.5 days (range, 365–1247) in the MAC and RIC groups, respectively.
The most common donor was a sibling in the MAC group and a child in the RIC group (Table 2). The median numbers of CD34+ cells of PBSCs were 4.0 × 106/kg (range, 2.0–11.4 × 106/kg), and 4.2 × 106/kg (range, 1.4–11.1 × 106/kg), in the MAC and RIC groups, respectively.
Noninfectious fever early after transplantation
Forty-eight (96%) of 50 patients in the MAC group and 72 (94%) of 77 patients in the RIC group developed noninfectious fever with a peak at day 3, which was quickly relieved after administration of PTCy.
Engraftment
Neutrophil engraftment was achieved in 98% of patients with a median of 17 days (range, 12–39 days) and 94% with a median of 18 days (range, 13–50 days) in the MAC and RIC groups, respectively. In the RIC group, neutrophil engraftment was achieved in 96% patients without a history of prior allo-SCT and in 90% patients with it. Platelet engraftment was achieved in 84% of patients with a median of 31 days (range, 11–284 days) and 74% with a median of 37 days (range, 10–195 days) in the MAC and RIC groups, respectively. Peripheral blood or bone marrow samples were collected on day 30 after transplantation to evaluate donor chimerism. Complete donor chimerism defined by >95% donor chimerism was achieved in all the engrafted patients.
Acute and chronic GVHD
Figure 1 shows the incidences of acute and chronic GVHD. The cumulative incidences of grades II–IV and III–IV acute GVHD at 100 days were 18% (95% CI, 9–30%) and 8% (95% CI, 3–18%) in the MAC group, and 14% (95%CI, 8–23%) and 5% (95%CI, 2–12%) in the RIC group, respectively. The cumulative incidences of all grade and moderate to severe chronic GVHD at 2-year were 36% (95% CI, 23–49%) and 20% (95% CI, 10–32%) in the MAC group, and 27% (95%CI, 17–37%) and 20% (95%CI, 12–30%) in the RIC group, respectively.
Acute and chronic GVHD cumulative incidences are shown for (a) II–IV acute GHVD (solid line), and III–IV acute GVHD (dashed line) following MAC, b all grade chronic GVHD (solid line) and moderate to severe chronic GVHD (dashed line) following MAC, c II–IV acute GHVD (solid line), and III–IV acute GVHD (dashed line) following RIC, and d all grade chronic GVHD (solid line) and moderate to severe chronic GVHD (dashed line) following RIC
NRM and relapse
The cumulative incidences of NRM and relapse rate at 2-year were 10% (95% CI, 4–20%) and 36% (95%CI, 23–50%) in the MAC group, and 20% (95%CI, 12–30%) and 45% (95%CI, 33–56%) in the RIC groups, respectively. To elucidate the reason why NRM was higher in the RIC group, we have performed an additional analysis of NRM in patients aged 60 and under without a history of prior allo-SCT in the RIC group (n = 24, low-risk RIC group) and patients aged over 60 or with a history of prior allo-SCT in the RIC group (n = 53, high-risk RIC group). NRM was 4% (95%CI, 0–18%) and 27% (95%CI, 16–39%) in the low-risk and high-risk RIC groups, respectively (P = .07).
EFS and OS
The primary endpoint EFS at 1-year was 64% (95% CI, 49–76%) and 43% (95%CI, 32–54%) in the MAC and RIC groups, respectively (Fig. 2); they were clearly over the pre-defined thresholds 30% and 25%, respectively. In the median follow-up periods of 763 days (range, 380–1119) and 740.5 days (range, 365–1247) in the MAC and RIC groups, OS and EFS at 2-year was 68% (95% CI, 53–79%) and 54% (95% CI, 39–66%) in the MAC group, 44% (95%CI 33–55%) and 35% (95%CI 25–46%) in the RIC group, respectively.
OS, EFS, NRM, and relapse rate stratified by the refined DRI
We then analyzed OS stratified by refined DRI (Fig. 3). In the MAC group, OS at 2-year was 87% (95%CI, 66–96%) in patients with low/intermediate risk and 50% (95%CI, 30–67%) in those with high/very high risk (P < .01). EFS at 2-year was 70% (95%CI, 47–85%) in patients with low/intermediate risk and 39% (95%CI, 20–57%) in those with high/very high risk (P < .01). Relapse rate at 2-year was 17% (95%CI, 5–35%) in patients with low/intermediate risk and 54% (95%CI, 33–71%) in those with high/very high risk (P < .01). There was no significant difference in the incidence of NRM (95%CI, 13% vs. 8%, P = .61) between patients with low/intermediate risk and those with high/very high risk. In the RIC group, it was 67% (95%CI, 45–82%) in patients with low/intermediate risk and 33% (95%CI, 20–46%) in those with high/very high risk (P < .01). EFS at 2-year was 59% (95%CI, 37–76%) in patients with low/intermediate risk and 24% (95%CI, 13–36%) in those with high/very high risk (P < .01). Relapse rate at 2-year was 25% (95%CI, 10–43%) in patients with low/intermediate risk and 55% (95%CI, 40–68%) in those with high/very high risk (P = .02). There was no significant difference in the incidence of NRM (17% vs. 21%, P = .51). Patients with high/very high risk had significantly worse OS, EFS, and relapse rate in both MAC and RIC groups.
OS stratified by refined DRI Probabilities stratified by refined DRI are shown for a OS in patients with low/intermediate risk (solid line), and high/very high risk (dashed line) following MAC, and b OS in patients with low/intermediate risk (solid line), and high/very high risk (dashed line) following RIC
OS, EFS, NRM, and relapse rate stratified by the presence of a history of prior allo-SCT
Since the RIC group include substantial number of patients having a history of prior allo-SCT, we performed a subgroup analysis of patients with or without a history of prior alloSCT in the RIC group. OS at 2-year was 31% (95%CI, 15–49%) and 52% (95%CI, 37–66%) in patients with a history of prior allo-SCT and those without it (P = .04, Fig. 4a). EFS at 2-year was 21% (95%CI, 9–38%) and 44% (95%CI, 29–57%) in patients with a history of prior allo-SCT and those without it, respectively (P = .02, Fig. 4b). Relapse rate at 2-year was 62% (95%CI, 40–78%) and 35% (95%CI, 21–49%) in these patients, respectively (P = .01). Although there was no significant difference in the incidences of NRM (17% vs. 22%, P = .67) between these patients, patients with a history of prior allo-SCT had significantly worse OS, EFS, and relapse rate.
OS and EFS stratified by the presence of a history of prior allo-SCT Probabilities stratified by the presence of a history of prior allo-SCT are shown for a OS and b EFS following RIC. The probabilities in patients without a history of prior allo-SCT and those with a history of prior allo-SCT are shown by solid line and dashed line, respectively
We performed a subgroup analysis of patients aged 50–60 without a history of prior allo-SCT. In the MAC group, 9 patients, including 3 patients with low/intermediate risk and 6 patients with high/very high risk, were analyzed. OS, EFS, NRM, and relapse rate at 2-year were 33% (95%CI, 8–62%), 22% (95%CI, 3–51%), 33% (95%CI, 6–65%), and 44% (95%CI, 11–75%), respectively. In the RIC group, 18 patients including 10 patients with low/intermediate risk and 8 patients with high/very high risk were analyzed. OS, EFS, NRM, and relapse rate at 2-year were 56% (95%CI, 31–75%), 44% (95%CI, 21–65%), 6% (95%CI, 0–23%), and 51% (95%CI, 25–72%) in the RIC group, respectively.
OS, EFS, NRM, and relapse rate stratified by the conditioning regimen in patients without a history of prior allo-SCT
We performed a subgroup analysis of transplant outcomes stratified by the conditioning regimen in patients without a history of receiving all-SCT. In TBI based MAC group, 27 patients including 12 patients with low/intermediate risk and 15 patients with high/very high risk were analyzed, OS, EFS, NRM, and relapse rate at 2-year were 70% (95%CI, 49–84%), 67% (95%CI, 45–81%), 7% (95%CI, 1–21%), and 26% (95%CI, 11–44%), respectively. In BU based MAC group, 23 patients including 12 patients with low/intermediate risk and 11 patients with high/very high risk were analyzed, OS, EFS, NRM, and relapse rate at 2-year were 65% (95%CI, 42–81%), 39% (95%CI, 20–58%), 13% (95%CI, 3–30%), and 48% (95%CI, 26–67%). In BU based RIC group, 47 patients including 19 patients with low/intermediate risk and 28 patients with high/very high risk were analyzed, OS, EFS, NRM, and relapse rate at 2-year were 52% (95%CI, 37–66%), 44% (95%CI, 29–57%), 22% (95%CI, 11–35%), and 35% (95%CI, 21–49%).
Cause of death
Relapse was the most common cause of death in both groups, with 13 (46%) out of 28 deaths in the MAC group and 28 (65%) of 43 deaths in the RIC group. The causes of NRM include infection (MAC, n = 3; RIC; n = 6), graft failure (RIC, n = 3), GVHD (RIC, n = 2), sinusoidal obstruction syndrome (MAC, n = 1; RIC, n = 1), acute respiratory distress syndrome (RIC, n = 1), interstitial pneumonia (RIC, n = 1), and multiorgan failure (MAC, n = 1; RIC, n = 1).
Rates of off-immunosuppressants
Figure 5 shows rates of off-immunosuppressants in patients who survived without relapse (MAC, n = 26; RIC, n = 27). In the MAC group, 65% of patients stopped immunosuppressant at 1-year and 83% at 2-year. In the RIC group, 56% of patients were off immunosuppressant at 1-year and 86% at 2-year. In the MAC group, 6 (60%) of 10 patients who developed moderate to severe chronic GVHD stopped immunosuppressants. In the RIC group, 9 (60%) of 15 patients who developed moderate to severe chronic GVHD stopped immunosuppressants.
Discussion
We demonstrate outcomes of two parallel prospective, multicenter, phase II studies of MAC and RIC based PTCy-haploPBSCT. The primary endpoint, EFS at 1-year was 64% and 43% in the MAC and RIC groups, respectively, clearly meeting its primary objectives of demonstrating better results than predefined threshold based on prior studies.
As for secondary endpoints, rapid and stable engraftment was achieved in almost all patients and engraftment rates were comparable between the MAC and RIC groups. These results indicate that use of peripheral blood stem cells ensures engraftment in PTCy-based haploidentical SCT (PTCy-haploSCT) following MAC and RIC. In our previous phase 2 prospective study of PTCy-haploPBSCT after RIC (Haplo13 study), engraftment rate was only 65% in patients with a history of prior allo-SCT, although it was 100% in those without it [17]. We therefore increased TBI dose from 2 Gy to 4 Gy to improve engraftment in the Haplo14 RIC study; engraftment rate was 90% in patients with a history of prior allo-SCT. An increased dose of low-dose TBI may improve engraftment, which has been reported [18].
HLA mismatch, PBSCT, and MAC are well known risks for GVHD [8]. That is why PTCy-haploSCT was originally initiated by using bone marrow grafts and NMC [2, 3]. In our present study using PBSCT, the incidences of grade II–IV acute GVHD, III–IV acute GVHD, all grade chronic GVHD, and moderate to severe chronic GVHD were 18%, 8%, 36%, 20% in the MAC group, and 14%, 5%, 27%, and 20% in the RIC group, respectively. According to previous reports, the incidences of II–IV acute GVHD, III–IV acute GVHD, all grade chronic GVHD, and moderate to severe chronic GVHD were 30–43%, 10–23%, 35–56%, and 22% in PTCy-haploPBSCT [4, 5], and 12–24%, 6–10%, 20–26%, and 10% in PTCy-haploBMT [6, 7]. Therefore, the incidences of chronic GVHD in our study seem to be equivalent to those of other studies of haploPBSCT, but higher than those after haploBMT. Recently, Bashey et al. retrospectively reported that incidences of acute and chronic GVHD were higher after PTCy-haploPBSCT compared to PTCy-haploBMT [19]. Our results seem to be lower than those reported by Bashey et al. with 42% grade II–IV acute GVHD, and comparable to those with 41% chronic GVHD in PTCy-haploPBSCT [19]. It remains to be elucidated whether ethnicity could be responsible for this difference, as in HLA-matched SCT [20].
In our study, 83% and 86% of patients who survived without relapse stopped immunosuppressant at 2-year in the MAC and RIC groups, respectively. Even in patients who developed moderate to severe chronic GVHD, immunosuppressants are off in 60% of patients. Remarkably, no patient in the MAC group and only 2 patients in the RIC group died of chronic GVHD in our study. These results suggest that chronic GVHD after PTCy-haploPBSCT was manageable. In HLA-matched SCT using standard GVHD prophylaxis, Mielcarek et al. reported that only 4 of 32 patients who underwent SCT following NMA conditioning and 1 of 46 patients who underwent SCT following MAC were off immunosuppressants [21]. Thus, rates of off-immunosuppression seem to be higher after PTCy-haploSCT than those after standard HLA-matched SCT. In addition, rates of off-immunosuppessants appear to be higher than those of 48–56% following HLA-matched related or unrelated BMT using PTCy as single-agent GVHD prophylaxis in a study by Kanakry et al. [22]. These data suggest that PTCy minimizes the immunosuppressive burden particularly in combination with calcineurin inhibitors.
NRM appears to be higher in the RIC group than MAC group. However, NRM was not higher in the RIC group compared to RIC group in patients aged 60 or less without a history of prior allo-SCT. Thus, inclusion of elderly patients and those with a prior history of allo-SCT may be associated with higher NRM in the RIC group.
The upper age limit was set at 65 years in our RIC study. Kasamon et al. reported safety of PTCy-haploBMT following NMA conditioning in patients aged 50 to 75 [23]. Recently, Bashey et al. reported the outcomes of older patients aged 60 and over in PTCy-haploSCT including 48% of PBSCT [24]. In their study, patients’ age (60–64 vs. ≥65) was not significantly associated with OS, DFS, NRM, or relapse in multivariate analysis. On the other hand, Slade et al. demonstrated that patients aged 65 and over had significantly inferior OS in PTCy-haploPBSCT compared with younger patients [25]. Therefore, upper age limit in PTCy-haploPBSCT following RIC is still controversial.
Our results suggest that both MAC and RIC can be safely applied to PTCy-haploPBSCT. However, there was no prospective study to compare the transplant outcomes between MAC and RIC in PTCy-haploSCT. In standard HLA-matched SCT, three recent randomized trial compared MAC and RIC [26, 27, 28], but optimal conditioning is still controversial. BMT CTN 0901 study demonstrated that relapse-free survival was significantly better in patients received MAC [26]; however, the other two studies failed to show difference in relapse and OS [27, 28]. In a subgroup analysis of patients aged 50–60 without a history of prior allo-SCT, NRM seemed to be higher in the MAC group and RIC regimen would be preferable in this patient population in this regard, although this subgroup analysis had limitation: small number of patients with heterogeneous disease risk indices. Prospective studies are needed to compare the impact of conditioning intensity on transplant outcomes after PTCy-haploSCT.
In a subgroup analysis of patients without a prior history of allo-SCT, there are no statistically significant differences in transplant outcome between the BU-based RIC and BU-based MAC group, but this analysis may not be powered enough to detect any significant differences due to small number of patients with heterogeneous disease risks.
OS, EFS, and relapse were 68%, 54%, and 36%, at 2-year, respectively, after PTCy-haploPBSCT following MAC. Solomon et al. reported that OS, disease-free survival (DFS), and relapse were 65%, 50%, and 40%, respectively, at 1-year after PTCy-haploPBSCT using BU-based MAC [4], and 78%, 73%, and 24%, respectively, at 2-year after PTCy-haploPBSCT using TBI-based MAC [5]. Bacigalupo et al. reported that 18-month OS and DFS were 62% and 51% after PTCy-haploBMT following MAC, respectively [7]. Thus, it seems transplant outcomes are similar between Caucasian and Japanese, although incidence of GVHD might differ.
DRI was developed to stratify heterogeneous patients undergoing allo-SCT by using disease type and disease status [12]. The refined DRI can prognosticate heterogeneous patients undergoing allo-SCT, regardless of conditioning intensity or graft source. McCurdy et al. reported that refined DRI was significantly associated with relapse, progression-free survival, and OS in NMC based PTCy-haploBMT [29]. In our study, patients with high/very high risk had significantly worse OS, EFS, and relapse rate, thus extending the predictive value of refined DRI to haplo-PBSCT following both MAC and RIC.
PTCy-haploSCT is one of the treatment options in patients who relapsed after allo-SCT because of rapid and high availability of donors [30,31,32]. In our study, 30 patients in the RIC group had a history of prior alloSCT. Two-year OS was 31%, which is comparable with previous several studies. OS, EFS, and relapse rate were significantly worse in patients with a history of prior allo-SCT compared to those without it.
In conclusion, we conducted two parallel prospective, multicenter, phase II studies of MAC and RIC based PTCy-haploPBSCT. Our results indicate that both MAC and RIC are valid options for PTCy-haploPBSCT for adults with hematological malignancies.
References
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–50.
McCurdy SR, Kanakry JA, Showel MM, Tsai H-L, Bolaños-Meade J, Rosner GL, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125:3024–31.
Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol. 2016;13:10–24.
Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK, et al. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transplant. 2012;18:1859–66.
Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK, et al. Total body irradiation-based myeloablative haploidentical stem cell transplantation is a safe and effective alternative to unrelated donor transplantation in patients without matched sibling donors. Biol Blood Marrow Transplant. 2015;21:1299–307.
Raiola AM, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant. 2013;19:117–22.
Bacigalupo A, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, et al. Unmanipulated haploidentical bone marrow transplantation and post-transplant cyclophosphamide for hematologic malignanices following a myeloablative conditioning: an update. Bone Marrow Transplant. 2015;50:S37–9.
Jagasia M, Arora M, Flowers MED, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows, J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8.
Filipovich AH, Weisdorf D, Pavletic S, Socié G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–56.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9.
Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71.
Tuve S, Gayoso J, Scheid C, Radke J, Kiani A, Serrano D, et al. Haploidentical bone marrow transplantation with post-grafting cyclophosphamide: multicenter experience with an alternative salvage strategy. Leukemia. 2011;25:880–3.
Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706.
RJ G. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Sugita J, Kawashima N, Fujisaki T, Kakihana K, Ota S, Matsuo K, et al. HLA-haploidentical peripheral blood stem cell transplantation with post-transplant cyclophosphamide after busulfan-containing reduced-intensity conditioning. Biol Blood Marrow Transplant. 2015;21:1646–52.
Fernandes JF, Bonfim C, Kerbauy FR, Rodrigues M, Esteves I, Silva NH, et al. Haploidentical bone marrow transplantation with post transplant cyclophosphamide for patients with X-linked adrenoleukodystrophy: a suitable choice in an urgent situation. Bone Marrow Transplant. 2018;53:392–9.
Bashey A, Zhang M-J, McCurdy SR, St Martin A, Argall T, Anasetti C, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35:3002–9.
Kanda J, Brazauskas R, Hu Z-H, Kuwatsuka Y, Nagafuji K, Kanamori H, et al. GVHD after HLA-matched sibling BMT or PBSCT: comparison of North American Caucasian and Japanese Populations. Biol Blood Marrow Transplant. 2016;22:744–51.
Mielcarek M, Martin PJ, Leisenring W, Flowers MED, Maloney DG, Sandmaier BM, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102:756–62.
Kanakry CG, Bolaños-Meade J, Kasamon YL, Zahurak M, Durakovic N, Furlong T, et al. Low immunosuppressive burden after HLA-matched related or unrelated BMT using posttransplantation cyclophosphamide. Blood. 2017;129:1389–93.
Kasamon YL, Bolaños-Meade J, Prince GT, Tsai H-L, McCurdy SR, Kanakry JA, et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol. 2015;33:3152–61.
Bashey ZA, Zhang X, Brown S, Jackson K, Morris LE, Holland HK, et al. Comparison of outcomes following transplantation with T-replete HLA-haploidentical donors using post-transplant cyclophosphamide to matched related and unrelated donors for patients with AML and MDS aged 60 years or older. Bone Marrow Transplant. 2018; https://doi.org/10.1038/s41409-018-0126-4
Slade M, DiPersio JF, Westervelt P, Vij R, Schroeder MA, Romee R. Haploidentical hematopoietic cell transplant with post-transplant cyclophosphamide and peripheral blood stem cell grafts in older adults with acute myeloid leukemia or myelodysplastic syndrome. Biol Blood Marrow Transplant. 2017;23:1736–43.
Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35:1154–61.
Kröger N, Iacobelli S, Franke G-N, Platzbecker U, Uddin R, Hübel K, et al. Dose-reduced versus standard conditioning followed by allogeneic stem-cell transplantation for patients with myelodysplastic syndrome: a prospective randomized phase III study of the EBMT (RICMAC Trial). J Clin Oncol. 2017;35:2157–64.
Bornhauser M, Kienast J, Trenschel R, Burchert A, Hegenbart U, Stadler M, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13:1035–1044.
McCurdy SR, Kanakry CG, Tsai H-L, Kasamon YL, Showel MM, Bolaños-Meade J, et al. Grade II acute graft-versus-host disease and higher nucleated cell graft dose improve progression-free survival after HLA-haploidentical transplant with post-transplant cyclophosphamide. Biol Blood Marrow Transplant. 2018;24:343–52.
Imus PH, Blackford AL, Bettinotti M, Iglehart B, Dietrich A, Tucker N, et al. Major histocompatibility mismatch and donor choice for second allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2017;23:1887–94.
Gorgeis J, Zhang X, Connor K, Brown S, Solomon SR, Morris LE, et al. T Cell-Replete HLA haploidentical donor transplantation with post-transplant cyclophosphamide is an effective salvage for patients relapsing after an HLA-matched related or matched unrelated donor transplantation. Biol Blood Marrow Transplant. 2016;22:1861–6.
Tischer J, Engel N, Fritsch S, Prevalsek D, Hubmann M, Schulz C, et al. Second haematopoietic SCT using HLA-haploidentical donors in patients with relapse of acute leukaemia after a first allogeneic transplantation. Bone Marrow Transplant. 2014;49:895–901.
Acknowledgements
This work was supported by the grant from Regional Medicine Research Foundation (Tochigi, Japan), North Japan Hematology Study Group (NJHSG), and Japan Agency for Medical Research and Development (AMED, JP17ek0510012).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sugita, J., Kagaya, Y., Miyamoto, T. et al. Myeloablative and reduced-intensity conditioning in HLA-haploidentical peripheral blood stem cell transplantation using post-transplant cyclophosphamide. Bone Marrow Transplant 54, 432–441 (2019). https://doi.org/10.1038/s41409-018-0279-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-018-0279-1
- Springer Nature Limited
This article is cited by
-
Effect of graft-versus-host disease on outcomes of HLA-haploidentical peripheral blood transplantation using post-transplant cyclophophamide
Bone Marrow Transplantation (2024)
-
Posttransplant cyclophosphamide in unrelated and related peripheral blood stem cell transplantation from HLA-matched and 1 allele mismatched donor
Bone Marrow Transplantation (2024)
-
Longitudinal outcome over four decades of allogeneic stem cell transplantation: a single center experience
Bone Marrow Transplantation (2024)
-
Allogeneic hematopoietic cell transplantation from alternative donors in acute myeloid leukemia
Annals of Hematology (2024)
-
Reduced intensity conditioning regimen of fludarabine, busulfan, ATG based haploidentical stem cell transplantation for older or unfit patients
Annals of Hematology (2024)