Abstract
We evaluated the potential correlation of the hematopoietic cell transplantation comorbidity index (HCT-CI) with the risk of developing post-transplant invasive fungal infections (IFIs). Between January 2009 and March 2015, 312 consecutive patients who received a first allograft entered the study. Low/intermediate HCT-CI risk score (0–2) was observed in 172/312 (55%), whereas high HCT-CI score (≥3) was seen in 140/312 (45%). Overall, 51/312 (16%) patients experienced IFI, defined as possible in 19 (6%), probable in 27 (9%), and proven in 5 (2%). Cumulative incidence of probable-proven IFI at 1 year was 8.5% with a significant higher incidence in patients with high HCT-CI (12%) vs. those with low-intermediate HCT-CI (5%; p = 0.006). There was a strong trend for a higher incidence of baseline severe pulmonary comorbidity in patients who developed probable-proven IFI (p = 0.051). One-year cumulative incidence of non-relapse mortality was higher in patients with IFI vs. those without, 49 and 16% (p < 0.001). By multivariate analysis, disease status at transplant and high HCT-CI, when combined with acute GVHD, were independently associated with the risk of post-transplant IFI. This study shows that a high HCT-CI predicts the risk of developing IFI and may indicate the need of mold-active antifungal prophylaxis in high-risk patients.
Similar content being viewed by others
Introduction
Despite improvements in diagnostic strategies and antifungal treatments, invasive fungal infections (IFIs) remain a major life-threatening complication in patients who undergo allogeneic hematopoietic stem cell transplantation (HSCT) [1, 2]. Several variables have been associated with a higher risk of developing IFI. They include HSCT from alternative donors, active hematologic malignancy at the time of transplant, older age, and presence of graft-versus-host disease (GVHD), CMV infection, and iron overload [1,2,3,4]. Appropriate assessment of these variables is essential to identify patients who may require more aggressive prophylaxis and/or antifungal therapies. Moreover, risk scores based on pre-transplant clinical parameters with the aim of predicting HSCT outcomes have been developed in recent years [5,6,7]. In 2005, Sorror et al. [8] introduced the hematopoietic cell transplantation-comorbidity index (HCT-CI) that evaluated the impact of 17 comorbidities on non-relapse mortality (NRM) in a cohort of 1055 patients with various hematologic malignancies. A thorough pre-transplant assessment of comorbidities was shown to play a pivotal role in predicting the risk of NRM [8]. More recently, other clinical parameters have been added to the HCT-CI [9,10,11]. Of note, the application of the HCT-CI also showed its efficacy in predicting severity of acute GVHD and subsequent mortality [12]. The aim of the present study was to investigate whether the HCT-CI could correlate with the risk of developing IFI after HSCT, and help to select patients who may most benefit from mold-active prophylaxis and/or more intensive diagnostic workup.
Patients and methods
Study design and data collection
This is a retrospective study including all consecutive patients with hematologic malignancies who received a first allogeneic HSCT at the Bone Marrow Transplant Unit of the Department of Oncology at AOU Citta della Salute e della Scienza Hospital of Torino, Torino, Italy, between January 2009 and March 2015. The HCT-CI was retrospectively calculated from medical charts and electronic records for patients transplanted between 2009 and 2010 as originally described by Sorror et al. [8], whereas it was part of the routine pre-transplant workup since January 2011. Patients were divided into three groups: low (score = 0), intermediate (score = 1–2), and high (score ≥3) HCT-CI. For the purpose of the present analysis, patients with low and intermediate scores (0–2) were combined to increase group size. Disease status at transplant was stratified as early or advanced as follows: acute leukemia in first complete remission, chronic myeloid leukemia in chronic phase, myelodysplastic syndrome (MDS)-refractory anemia and severe aplastic anemia were defined as low risk; all other conditions were defined as high risk. The study was approved by the Institutional Review Board of the Città della Salute e della Scienza Hospital of Torino, Torino, Italy, according to the Declaration of Helsinki.
Conditionings and HLA typing
Patients were prepared for transplant either with myeloablative or reduced-intensity/non-myeloablative conditionings (Table 1) in the light of their clinical conditions and HCT-CI. Reduced-intensity/non-myeloablative regimens were usually preferred in patients over 55 years of age and/or in presence of pre-transplant comorbidities. By the EBMT (European Blood and Marrow Transplant Group) criteria, myeloablative conditionings had to contain a total busulfan dose >6.4 mg/kg i.v.; or a cyclophosphamide dose >120 mg/kg (or >60 mg/kg if in combination with other drugs); or a melphalan dose >140 mg/m2; or a total body irradiation dose >6 Gy. Regimens with lower doses were defined as reduced-intensity/non-myeloablative conditionings. HLA typing was carried out at HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1 by high-resolution molecular methods.
GVHD prophylaxis and supportive care
GVHD prophylaxis included cyclosporin (CSA) and short-course methotrexate or CSA combined with micophenolate mofetil (MMF). In vivo T-cell depletion with thymoglobulin (5–7 mg/kg divided into two doses) was used in transplants from unrelated donors. Patients transplanted from haploidentical donors received tacrolimus, MMF, and post-transplant cyclophosphamide as described by Luznik et al. [13]. Both acute GVHD and chronic GVHD were diagnosed on the basis of clinical symptoms and/or biopsies according to standard criteria [14, 15]. Severity of chronic GVHD was assessed according to National Institutes of Health criteria, and graded as mild, moderate, or severe [16]. All patients received prophylactic quinolones and acyclovir. Antifungal prophylaxis included fluconazole in 252 (84%) patients, echinocandins in 29 (10%) (micafungin in 28; caspofungin in 1), and mold-active azoles in 3 (1%) (itraconazole in 2; posaconazole in 1). Seventeen (5%) patients received secondary antifungal prophylaxis with mold-active triazoles (n = 12) or liposomal amphotericin-B (L-AmB) (n = 5). Fever workup (≥38 °C) routinely included urine and blood cultures, blood chemistry including procalcitonin, and chest X-ray. Chest computed tomography scan was scheduled for persistent unexplained fever or as clinically indicated. In case of radiological chest abnormalities, bronchoscopy with bronchoalveolar lavage (BAL) was performed whenever possible for microbiological testing including galactomannan (GM) antigen detection. Surveillance serum Aspergillus GM antigen was tested twice a week post transplant by using the double-sandwich ELISA Platelia Aspergillus (Platelia Aspergillus Assay—Bio-Rad Laboratories, Marnes, La Couquette, France). Diagnosis of IFI was made according to the revised European Organization for Research and Treatment of Cancer/Mycoses study group (EORTC/MSG) definitions [17]. Patients were evaluated for the occurrence of IFI from the start of conditioning regimen until last follow-up or the date of disease recurrence. All patients were started on broad spectrum antibiotic treatment on the first day of neutropenic fever. Antifungal treatment was started on the basis of the diagnostic workup or as empiric treatment after 48–72 h of persistent unexplained fever without any microbiologically documented infection at the discretion of the attending physician.
Study end points and statistical analysis
Primary end point was the impact of HCT-CI on the risk of developing post-HSCT IFI. Secondary end points were cumulative incidence of IFI, overall survival (OS), NRM, and infection-related mortality (IRM). OS was defined as the time from diagnosis to death from any cause. OS was calculated by the Kaplan–Meier method [18] and compared with the log-rank test. OS was also analyzed by the Cox proportional hazards model, comparing the two risk factors by the Wald test and calculating 95% confidence intervals. Risk factors included: age at transplant (≥56 vs. ≤55 years), primary disease (acute leukemia vs. others), donor type (MUD/other vs. HLA-identical sibling), source of hematopoietic stem cells (peripheral blood progenitor cells vs. bone marrow), conditioning regimen (reduced-intensity/non-myeloablative conditioning vs. myeloablative), Cytomegalovirus (CMV) serology, HCT-CI (≥3 vs. 0–2), occurrence of IFI (probable/proven IFI vs. possible/none), acute and chronic GVHD (any vs. none). Cumulative incidences of NRM, invasive fungal disease, and IRM were estimated by the cumulative incidence function, comparing the curves of the main event (death without previous relapse for NRM; invasive fungal infection for IFI; death from any infection for IRM) in the presence of a competing event (relapse for NRM, relapse/death without infections for IFI and IRM) by the Gray test [19]. Patient characteristics were tested using the Fisher’s exact test for categorical variables and the Mann–Whitney test for continuous ones. All results for continuous variables were expressed as median (range). All reported p values were two-sided at the conventional 5% significance level. Data were analyzed as of October 2016 by R 3.3.1 package cmprsk (The R Foundation for Statistical Computing, Vienna-A; www.R-project.org).
Results
Patients
Overall, 360 patients underwent an allograft during the study period. Twenty patients who received a second or third transplant, 11 patients with a diagnosis of non-malignant disorders and 3 patients who underwent cord blood transplantation were excluded from the analysis. Comorbidity data were incomplete in 14 patients, whereas 312 patients had complete HCT-CI assessment and were included in the final analysis. Table 1 summarizes patient characteristics by low-intermediate or high HCT-CI.
Cumulative incidence, classification, and diagnosis of IFI
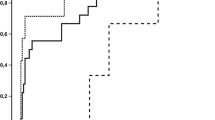
Overall, 51/312 (16%) patients experienced at least one IFI, defined as possible in 19 (6%), probable in 27 (9%), and proven in 5 (2%) by the EORTC/MSG criteria. Median time from transplant to diagnosis of IFI was day +70 (range 1–1122 days). Cumulative incidence of probable-proven IFI at 1 year was 8.5% with a significant higher IFI incidence in patients with high HCT-CI (12%) as compared with those with low-intermediate HCT-CI (5%; p = 0.006) (Fig. 1). In patients with high HCT-CI, 84/140 (60%) had severe pulmonary comorbidity (weighted HCT-CI score = 3). There was a remarkable trend for a higher incidence of baseline severe pulmonary comorbidity in patients who developed probable-proven IFI, 47% (15/32), as compared with those patients who did not develop IFI, 23% (60/261) (p = 0.051). All cases of probable IFI were diagnosed by the combination of typical lung infiltrates with positive GM antigen test (optical density index >0.5) from either blood samples (n = 7), BAL (n = 12), or both (n = 6). Two patients had positive beta-d-Glucan test (≥60 pg/ml). Proven IFI were caused by Aspergillus strains in three patients, by a zygomycete in 1 and by Trichosporon in 1.
Overall survival and non-relapse mortality
Median OS from the allograft for entire patient cohort was 41 (range 3–2421 days) months, whereas median OS for patients with and without IFI was 16 (range 15–1833 days) and 74 (range 3–2421) months, respectively, (p < 0.001). Kaplan–Meier estimates of 2-year OS for the entire patient cohort was 54%. Significant better OS was observed in patients who received a transplant from a sibling (72/102, 65%) as compared with those who received a transplant from alternative donors (matched unrelated donor [MUD] or haploidentical, 104/110, 49% (p = 0.012), in patients who did not develop IFI (167/280, 60%) vs. those who did (9/32, 27%) (p < 0.001) and in patients with low-intermediate HCT-CI (110/172, 64%) vs. those with high HCT-CI (66/140, 47%) (p < 0.001). Overall, 136/312 (43%) patients died, 71/136 (52%) of progressive disease, and 65/136 (48%) of transplant-related complications. Causes of death included IFI or other infectious complications in 9/65 (14%) and 23/65 (35%) patients, respectively. One-year cumulative incidence of NRM was higher in patients with high HCT-CI vs. low HCT-CI, 30 and 12% (p < 0.001), and in those with IFI as compared with those without, 49 and 16% (p < 0.001).
Predictors of overall survival, non-relapse mortality, and infectious-related mortality
Logistic regression models (Table 2) showed that an advanced disease phase at the time of transplant, acute grade II–IV GVHD, and a comorbidity score ≥3 were factors significantly associated in univariate analysis to an higher risk of proven/probable IFI. By multivariate analysis, disease status and the interaction of high HCT-CI with acute GVHD were factors significantly increasing the risk of IFI.
By univariate analysis, main risk factors for OS were age (<55 vs. ≥55 years, p= 0.042); transplant from alternative vs. matched sibling donors (p 0.013); development of IFI (p < 0.001), high HCT-CI (≥3, p 0.001), and absence of chronic GVHD (p= 0.042). Older age (p< 0.001), transplant from alternative donors (p =0.005), development of IFI (p < 0.001), high-risk CMV serology (defined as recipient-positive/donor-negative by pre-transplant CMV serology) (p= 0.006); and chronic GVHD (p< 0.001); high HCT-CI (p < 0.001) were significantly associated with higher risk of 1-year NRM. A transplant from an alternative donor (p= 0.009), reduced-intensity preparative regimens (p= 0.03), development of IFI (p < 0.001), high HCT-CI (p= 0.033), and chronic GVHD (p= 0.003) were significantly correlated with higher risk of IRM.
By multivariate analyses, the development of IFI (HR 3.45, 2.01–5.93; p < 0.001) and the absence of chronic GVHD (HR 0.41, 0.19–0.93; p= 0.032) were significant predictors of worse OS; the development of IFI and mild to moderate cGVHD (p< 0.001) were associated with significantly higher risk of NRM (HR 9.39, 5.98–22.17; p < 0.001 and HR 4.61, 1.83–11.65; p< 0.001, respectively) and IRM (HR 11.19, 3.59–34.89; p < 0.001 and HR 4.8, 1.56–14.83; p =0.006, respectively), while older age was a significant predictor of NRM (HR 3.29, 1.35–8.0; p < 0.001).
Discussion
Among patients with hematologic malignancies, recipients of an allograft represent one of the categories at higher risk of developing IFI [1, 2]. Disease status at the time of transplant, HSCT from alternative donors, and presence of GVHD have clearly been described as major independent variables for both the risk of developing IFI and their clinical outcomes [2, 4]. The HCT-CI was developed to predict HSCT-related morbidity and mortality. A consistent number of studies showed that HCT-CI is associated with NRM, GVHD, and OS [12, 20,21,22]. In the present study, we investigated if the HCT-CI could be predictive of the development of IFI and associated mortality.
In keeping with reports by other groups, our study showed an incidence of proven and probable IFI of 8.5% [2, 4, 23]. Notably, patients with HCT-CI ≥3 had a significantly higher incidence of IFI as compared with those with HCT-CI 0–2 (12% vs. 5%, respectively). Univariate analysis confirmed that high HCT-CI was associated with IFI. Two factors may have particularly influenced the risk of developing IFI in our patient cohort: firstly, advanced disease at the time of transplant in more than two-thirds of the patients that was correlated with increased risk of IFI in a study by Gruppo Italiano Trapianti di Midollo [2]; secondly, severe pulmonary comorbidities that frequently contributed to high HCT-CI scores and may have predisposed to IFI. Several studies clearly documented that chronic obstructive pulmonary disease should be regarded as a predisposing condition for the development of IFI [5, 24, 25]. Interestingly, our multivariate analysis showed that, in patients who had both, the interaction of high HCT-CI and acute grade II–IV GVHD had a significant impact on the risk of IFI. This finding further confirms the well-documented role of GVHD as a factor influencing the risk of IFI in HSCT recipients [2, 23, 26].
In our study, other possible confounding factors could be ruled out. Fluconazole-based antifungal prophylaxis was largely employed in both HCT-CI cohorts (87% of patients in HCT-CI 0–2 group vs. 78% in HCT-CI ≥3 group) and only 17 patients (n = 8 in HCT-CI 0–2 group; n = 9 in HCT-CI ≥3 group) with a history of IFI prior to transplant received secondary prophylaxis. A reliable pre-transplant scoring system to assess the risk of developing IFI would be of great value in clinical practice. Liu et al. [23] demonstrated that an EBMT risk score >2 was an independent risk factor for IFI. The Bologna group developed an IFI risk prediction model in patients with hematological malignancies [27]. Neutropenia, lymphocytopenia, disease status, and prior IFI were factors that could discriminate between patients at low (risk score <6) or at high risk of IFI [27]. To our knowledge, our report is the first to show a significant association between HCT-CI and the risk of developing IFI. This finding may clinically be highly relevant as it identifies patients who may benefit from prompt and aggressive diagnostic workup. Furthermore, current guidelines recommend mold-active antifungal prophylaxis for patients who develop moderate-to-severe GVHD [28, 29]. Our results suggest that also the HCT-CI may identify patients who may benefit from this prophylaxis. Of note, the recommendation for mold-active prophylaxis in HSCT recipients with high HCT-CI is reinforced by our observation that all IFI but one was mold infections.
Despite the availability of a wide spectrum of antifungal agents, the development of IFI remains associated with poor clinical outcome. Martino et al. [30] showed that patients who developed IFI had a 2.1- and a 2.5-fold higher risk of NRM and IRM, respectively. Similarly, we found that the cumulative incidence of NRM was 49% and 16% in patients with and without IFI, respectively. Moreover, multivariate analysis confirmed that development of IFI was significantly associated with increased NRM and IRM. However, definitive conclusions that apply to all HSCT recipients cannot be drawn from our study, given the retrospective single-center analysis including only first transplants, and the not very large patient cohorts.
In conclusion, our study indicates that the HCT-CI correlates with the probability of developing IFI and their clinical outcome is very poor in patients with high HCT-CI. A further understanding of risk factors associated with IFI would improve our ability to discriminate high-risk patients who may benefit from more aggressive diagnostic and therapeutic strategies.
References
Garcia-Vidal C, Upton A, Kirby KA, Mar KA. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis. 2008;47:1041–50.
Girmenia C, Raiola AM, Piciocchi A, Algarotti A, Stanzani M, Cudillo L, et al. Incidence and outcome of invasive fungal diseases after allogeneic stem cell transplantation: a prospective study of the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol Blood Marrow Transplant. 2014;20:872–80.
Atalla A, Garnica M, Maiolino A, Nucci M. Risk factors for invasive mold diseases in allogeneic hematopoietic cell transplant recipients. Transpl Infect Dis. 2015;17:7–13.
Sun Y, Meng F, Han M, Zhang X, Yu L, Huang H, et al. Epidemiology, management, and outcome of invasive fungal disease in patients undergoing hematopoietic stem cell transplantation in China: a multicenter prospective observational study. Biol Blood Marrow Transplant. 2015;21:1117–26.
Hemmati PG, Terwey TH, le Coutre P, Vuong LG, Massenkeil G, Dorken B, Arnold R. A modified EBMT risk score predicts the outcome of patients with acute myeloid leukemia receiving allogeneic stem cell transplants. Eur J Haematol. 2011;86:305–16.
Gratwohl A, Stern M, Brand R, Apperley J, Baldomero H, de Witte T, et al. Risk score for outcome after allogeneic hematopoietic stem cell transplantation. Cancer. 2009;115:4715–26.
Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, Storer B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9.
Vaughn JE, Storer BE, Armand P, Raimondi R, Gibson C, Rambaldi A, et al. Design and validation of an augmented hematopoietic cell transplantation-comorbidity index comprising pretransplant ferritin, albumin, and platelet count for prediction of outcomes after allogeneic transplantation. Biol Blood Marrow Transplant. 2015;21:1418–24.
Raimondi R, Tosetto A, Oneto R, Cavazzina R, Rodeghiero F, Bacigalupo A, et al. Validation of the hematopoietic cell transplantation-specific comorbidity index: a prospective, multicenter GITMO study. Blood. 2012;120:1327–33.
Sorror ML, Storb RF, Sandmaier BM, Maziarz RT, Pulsipher MA, Maris MB, et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol. 2014;32:3249–56.
Sorror ML, Martin PJ, Storb RF, Bathia S, Maziarz RT, Pulsipher MA, et al. Pretransplant comorbidities predict severity of acute graft-versus-host disease and subsequent mortality. Blood. 2014;124:287–95.
Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable engraftment of major histocompatibility complex–incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98:3456–64.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA-matched sibling donors. Transplantation. 1974;18:295–304.
Lee SJ, Klein JP, Barrett AJ, Ringden O, Antin JH, Cahn JY, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100:406–14.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–56.
De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Am Stat Assoc J. 1958;53:457–81.
Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54.
Sorror ML, Giralt S, Sandmaier BM, De Lima M, Shajahan M, Maloney DG, et al. Hematopoietic cell transplantation-specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007;110:4606–13.
Barba P, Martino R, Pérez-Simón JA, Fernandez-Aviles F, Castillo N, Pinana JL, et al. Combination of the hematopoietic cell transplantation comorbidity index and the European Group for Blood and Marrow Transplantation score allows a better stratification of high-risk patients undergoing reduced-toxicity allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20:66–72.
Sorror ML, Logan BR, Zhu X, Rizzo JD, Cooke KR, McCarthy PL, et al. Prospective validation of the predictive power of the hematopoietic cell transplantation comorbidity index: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant. 2015;21:1479–87.
Liu Y-C, Chien S-H, Fan N-W, Hu M-H, Gau J-P, Liu C-J, et al. Incidence and risk factors of probable and proven invasive fungal infection in adult patients receiving allogeneic hematopoietic stem cell transplantation. J Microbiol Immunol Infect. 2015;49:567–574.
Guinea J, Torres-Narbona M, Gijo´ P, Munoz P, Pozo F, Pelaez T, et al. Pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: incidence, risk factors, and outcome. Clin Microbiol Infect. 2010;16:870–7.
Caira M, Candoni A, Verga L, Busca A, Delia M, Nosari A, et al. Pre-chemotherapy risk factors for invasive fungal diseases: prospective analysis of 1192 patients with newly diagnosed acute myeloid leukemia (SEIFEM 2010-a multicenter study). Haematologica. 2015;100:284–92.
Parody R, Martino R, de la Cámara R, Garcia-Noblejas A, Esquirol A, Garcia-Cadenas I, et al. Fungal and viral infections after allogeneic hematopoietic transplantation from unrelated donors in adults: improving outcomes over time. Bone Marrow Transplant. 2015;50:274–81.
Stanzani M, Lewis RE, Fiacchini M, Ricci P, Tumietto F, Viale P, et al. A risk prediction score for invasive mold disease in patients with hematological malignancies. PLoS ONE. 2013;8:e75531.
Tacke D, Buchheidt D, Karthaus M, et al. Primary prophylaxis of invasive fungal infections in patients with haematologic malignancies. 2014 update of the recommendations of the Infectious Diseases Working Party of the German Society for Haematology and Oncology. Ann Hematol. 2014;93:1449–56.
Patterson TF, Thompson GR, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63:433–42.
Martino R, Kerguelen A, Valcarcel D, Sureda A, Fachini L, Pinana JL, et al. Reduction of infection-related mortality after allogeneic PBSCT from HLA-identical siblings: longitudinal analysis from 1994 to 2008 at a single institution. Bone Marrow Transplant. 2010;46:690–701.
Acknowledgements
This research was supported in part by Regione Piemonte: Ricerca Finalizzata 2008, 2009; Compagnia di San Paolo and Comitato Regionale Piemontese Gigi Ghirotti; Fondazione Neoplasie Sangue Onlus; Fondazione Cariplo (Grant per la Ricerca Biomedica 2015/0603 to BB). Our thanks to the nurses and medical staff for caring for the patients and to the data managers who collected the study and follow-up information.
Author contributions
AB and BB contributed to the initial conception and designed the study. AB, EM, MF, LB, CMD, SA, CF, SM, SB, GI, LG, and BB provided the study materials or patients. RS, MF, LB, and BB collected and assembled the data. RP performed statistical analyses. AB, BB, RP, MF, MS, FGDR (equally contributing author), and RS analyzed and interpreted the data. AB and BB wrote the manuscript. All authors gave the final approval to the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AB has received honoraria from Gilead Sciences, Merck, Pfizer Pharmaceuticals, Jazz Pharmaceuticals, and Basilea; he has been the speaker for Gilead Sciences, Merck, Pfizer Pharmaceuticals, Astellas Pharma, and Basilea. BB has received honoraria from Gilead, Pfizer, Celgene, Hospira, and research support form Celgene, Pierre Fabre, ADIENNE, Hospira Italia, MSD Italia. The The remaining authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the present study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Busca, A., Passera, R., Maffini, E. et al. Hematopoietic cell transplantation comorbidity index and risk of developing invasive fungal infections after allografting. Bone Marrow Transplant 53, 1304–1310 (2018). https://doi.org/10.1038/s41409-018-0161-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-018-0161-1
- Springer Nature Limited