Abstract
The D-index assesses neutropenia dynamics. Prolonged neutropenia is a major risk for invasive fungal infection (IFI); we hypothesized that D-index is predictive of IFI risk. We retrospectively reviewed 789 adults who underwent allogeneic hematopoietic transplant (HSCT) from 1/1/2005 to 9/30/2015. Medical records were reviewed from transplant (D0) through Day 100. The D-index was calculated as area over the neutrophil curve until engraftment. 714 patients were included for analysis. Sixteen (2%) developed probable (11) or proven (5) IFI. Median time to IFI was 40 days (range 8–98) after HSCT. Groups with and without IFI did not differ significantly in duration of mild or profound neutropenia. Median D-index of those with IFI was 4293 days neutrophil/µl compared to 3590 days neutrophil/µl for those without IFI (P = 0.17). Patients who were neutropenic on D0 showed higher rates of IFI than those who were not (10/123 [8%] vs 6/591 [1%]; P < 0.001). Only 2% developed IFI, likely due to mold-active antifungal prophylaxis. The D-index was not significantly higher in those with IFI. Duration of profound neutropenia and neutropenia at D0 may be better markers for IFI among HSCT recipients during the first 30 and 100 days after transplant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infection remains the leading cause of morbidity and mortality in the early period after hematopoietic stem cell transplantation (HSCT) [1,2,3,4,5]. One of the major risk factors for infection includes the prolonged, severe state of neutropenia prior to transplant engraftment. This increases the risk for invasive fungal infections (IFI) particularly those caused by molds, such as Aspergillus sp., Fusarium sp. and the Zygomycetes [6]. Among patients with neutropenia, IFI occurs almost exclusively in those with profound neutropenia (ANC < 100/uL) lasting more than 10 days [7,8,9]. Clinicians rely on this prolonged duration of neutropenia above which the empiric use of an antifungal—for prophylaxis or early empiric treatment—is recommended.
Portugal et al. developed a tool called the D-index to predict an association between proven or probable IFI and severity of neutropenia in patients receiving induction therapy for acute myeloid leukemia (AML) [6]. Results from this small study showed that the receiver operating curve (ROC) showed better sensitivity and specificity for the D-index compared to simple duration of neutropenia. Using a cut-off point of 6200 days neutrophil/µl for the D-index, the sensitivity and specificity were 100 and 58%, respectively. The authors concluded that the D-index performed better than duration of neutropenia.
We aimed to validate findings from this earlier work and hypothesized that the D-index could be used in conjunction with imaging findings and other laboratory markers to help predict IFI and guide decisions regarding initiation or modification of anti-fungal therapy within the first 30 and 100 days of allogeneic HSCT.
Patients and methods
Patient selection
We retrospectively reviewed all adults who underwent allogeneic HSCT (allo-HSCT) at our institution from January 1, 2005 to September 30, 2015. The study proceeded after approval from the hospital Institutional Review Board. The hospital transplant database, an institutional computerized record search tool (Advanced Cohort Explorer [ACE]), and electronic health records were reviewed for relevant clinical and laboratory data from the date of transplant until day 100 post-transplant. We recorded co-morbid conditions such as diabetes mellitus (DM), chronic kidney disease (CKD) and chronic obstructive pulmonary disease (COPD). Co-morbid conditions were included if they were indicated in the patients electronic health as an international classification of disease (ICD) code. The total dose of corticosteroids equivalent to prednisone was calculated from day of transplant until day of engraftment. IFI was defined according to the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group (EORTC) criteria for IFI [10]. The primary endpoint was the occurrence of proven or probable IFI. Patients with a previous allogeneic transplant, or a diagnosis of probable or proven IFI prior to transplantation, or who were undergoing treatment for an IFI were excluded from the study.
Description of institutional policies
The HSCT unit
The blood and bone marrow transplant unit at Mayo Clinic is located at Rochester Methodist Hospital. There is a large hospital-based outpatient unit combined with an inpatient unit, which are geographically approximate. Each room is equipped with a laminar airflow (LAF) system with high efficiency particulate air (hepatoid) filters. Allogeneic transplant recipients are typically managed as outpatient until admission is required based on clinical status. For allogeneic transplants, myeloablative conditioning regimens are typically administered as an inpatient whereas reduced intensity conditioning regimens are given as outpatient. Hospital based out-patients live locally within 10–15 min of the transplant unit until engraftment and clinical stability occurs, typically close to day 100 for allogeneic transplant recipients.
Fungal prophylaxis
Fluconazole 400 mg by mouth or IV once daily from the start of conditioning regimen is the preferred anti-fungal prophylaxis. The dose of fluconazole is dose adjusted depending on creatinine clearance. For allogeneic patients, the dose of fluconazole is reduced to 100 mg by mouth once daily following neutrophil engraftment for prophylaxis of esophageal candidiasis. This is continued until the patient is off all immunosuppressive therapy. However, in patients with either a prior history, or deemed at high risk for fungal infection, the use of prophylactic voriconazole or posaconazole is advised and left upon the discretion of the clinician provider.
D-index calculation
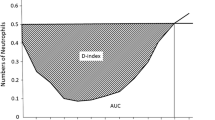
The D-index (Ae – A0) was calculated by the trapezoidal method as the difference between the observed area under the curve (A0), and the expected neutrophil area (Ae; 500/µL × days with neutropenia) if the patient did not develop neutropenia (Fig. 1) [11]. The D-index was calculated from day 0 until engraftment, and was assigned a value of 0 if free of neutropenia during this period.
where Ae = expected area = 500 × number of days with neutropenia < 500/µL. A0 = area under the curve. (ti − ti−1) is the time interval (days) between two consecutive neutrophil counts. Ni and Ni−1 are the respective neutrophil counts (per microliter) at times ti and ti−1. n = total number of neutrophil counts.
Statistical methods
Values of baseline descriptors are reported as median and range or as number and percentage, as appropriate. To quantify indices for the severity of neutropenia, we calculated D-index, duration of neutropenia (<500/µl), and duration of profound neutropenia (<100/µl) from transplantation (day 0) to engraftment. As the main study endpoint, IFI was analyzed primarily as a binary outcome within 100 days of transplant and secondarily within 30 days of transplant. Comparisons between the two groups with and without IFI were evaluated with the nonparametric Wilcoxon rank sum test for continuous variables and with Pearson’s Chi-squared test or Fisher’s exact test for categorical variables, as indicated. Time to IFI was analyzed by the Kaplan–Meier product limit method and by the log-rank test. The ability of each neutropenia measure to separate subjects with and without IFI was illustrated with Receiver-Operating Characteristic (ROC) curves and quantified based on the total area under the ROC curve (i.e., concordance index). Difference in discriminative ability between D-index and duration of neutropenia was determined based on the non-parametric approach of DeLong, DeLong, and Clarke–Pearson for comparing areas under the ROC curve. Additionally, we identified an optimal threshold for D-index by selecting from the range of possible thresholds along its ROC curve the value that maximized the sum of sensitivity and specificity. Data analysis was conducted using SAS statistical software (Version 9.4, SAS Institute Inc., Cary, NC). For all results, P < 0.05 was considered statistically significant.
Results
A total of 789 patients who received an allogeneic HSCT transplant within the study period were identified and reviewed for study eligibility. Of these, 714 patients fulfilled the inclusion criteria and were retained in the final analysis (Fig. 2).
Characteristics of the study cohort
Of the 714 patients in the study, 42% were female and median age was 53 years (range 18–75 years) at the time of transplantation. Most of the patients in the cohort were Caucasian (96%), and the number who had comorbid conditions was fairly low: chronic obstructive pulmonary disease (2%), diabetes mellitus (7%), and chronic renal insufficiency (4%). The underlying reason for transplant in nearly two-thirds of the cohort (63%) was a myeloid malignancy–acute myeloid leukemia (37%), myelodysplastic syndrome/myeloproliferative disorder (22%), or chronic myeloid leukemia (4%). This was the first HSCT for most patients, with only 11% having undergone a prior autologous SCT. The characteristics of patients and details regarding their transplantation are summarized in Table 1.
Overall indices of neutropenia
Nearly all patients (705/714, 99%) developed neutropenia (<500 cells/ µl) lasting a median of 14 (range 2–90) days. Most patients (613/714, 86%) also developed profound neutropenia (<100 cells/ µl) lasting a median of 6 (1–60) days; of these patients, 85% (518/613) remained severely neutropenic for ≤ 10 days, 12% (76/613) for 11–20 days, and 3% (19/613) for over 20 days. Neutrophil engraftment occurred in 94% of patients. The overall median D-index was 3594 (0–25,160) days neutrophil/µl.
Patients with invasive fungal infection
Only 16 (2.2%) patients in our study cohort developed probable or proven IFI within 100 days of transplant, of whom 7 developed IFI within 30 days. Profound neutropenia was noted in 15 of the 16 patients with IFI (median 7 days with ANC < 100) and in all 7 patients with 30-day IFI (median 12 days with ANC < 100). A detailed listing of the 16 patients who developed IFI is presented in Table 2.
Based on comparisons between groups with and without IFI, positive CMV recipient status (P = 0.04) and higher number of packed red blood cell transfusions (P < 0.001) were associated with developing IFI. There was also a borderline significant association between having COPD at time of transplant and IFI (P = 0.05). As expected, 100-day mortality was significantly higher in those who developed IFI than those who did not (56% vs. 8%; P < 0.001). All but one of the 9 deaths (8/9, 89%) in the IFI group was related to infection, compared to half (27/54, 50%) of those with an infectious cause of death in the non-IFI group (P = 0.03).
From primary analyses, groups with and without 100-day IFI did not differ significantly on duration of mild neutropenia (median, 15 vs. 13 days; P = 0.27) or of profound neutropenia (7 vs. 5 days; P = 0.06). Despite a higher median D-index of 4293 days neutrophil/µl in those with IFI compared to 3590 days neutrophil/µl; for those without IFI, the overall difference was not statistically significant P = 0.17). When the outcome was defined by the development of IFI by Day 30, median values of neutropenia duration and D-index were higher in the IFI group, but only the duration of severe neutropenia was significantly different between the 2 groups (median, 12 vs. 5 days; P = 0.045).(4440 vs. 3590 days neutrophil/µl; P = 0.19).
Figure 3 illustrates the predicted risk and discrimination statistics for separate logistic models of 100-day and 30-day IFI. As shown in Fig. 4, the predictive value of D-index for the development of IFI was weak (area under ROC = 0.60) and comparable (P = 0.451) to that of mild and profound neutropenia duration (area = 0.58 and 0.64, respectively). By comparison the model statistics in predicting 30-day IFI were higher, most notably for duration of profound neutropenia (area = 0.72), but the difference in predictive value between the 3 measures was not statistically significant (P = 0.114). Additional ROC analysis revealed an apparent optimal threshold for D-index of 4105 days neutrophil/µl in predicting IFI, yet dichotomizing patients as above or below this threshold would result in sensitivity and specificity of only 63% and 64% respectively.
Neutropenia at day 0
The D-index was calculated beginning from date of transplant (e.g. day 0) until neutrophil recovery in order to provide a uniform evaluation across all patients, since the D-index prior to transplant could not be calculated for patients who received chemotherapy elsewhere. Of the 714 patients, 123 (17%) were neutropenic at day 0. Ten of the 123 (9%) patients who were neutropenic at Day 0 had developed IFI, compared to 6 of 591 (1%) patients who were non-neutropenic (P < 0.001). The D-index differed between the patients who were and were not neutropenic on day 0 (median 5320 versus 3425 days neutrophil/µl; P < 0.001).
Discussion
Neutropenia is considered to be a critical risk factor for infectious complications in the pre-engraftment phase of HSCT. Prior studies [1, 4, 8] have reported that profound neutropenia is significantly associated with the risk of fungal infection among bone marrow transplant recipients. The D-index was proposed as a tool that could assess the dynamics of prolonged neutropenia.
Our study is the largest study to date to evaluate the D-index. We showed that the D-index was a fairly insensitive test, and does not correlate well with development of IFI within the early post-transplant time period. Unlike the D-index, the simple duration of profound neutropenia (<100 cells/μL) was marginally associated with IFI, showing modest discrimination in predicting 30-day IFI (ROC area of 0.72) but weak discrimination in predicting 100-day IFI (ROC area of 0.64). This is in contrast to prior studies [6, 12], although a direct comparison among studies is difficult, given the difference in patient populations and the period of D-index calculation. In the case–control study by Portugal [6] involving patients with acute myeloid leukemia (AML) undergoing induction chemotherapy, the authors showed that the D-index was predictive of IFI, and had a better sensitivity and specificity than duration of profound neutropenia (ROC area of 0.857 vs. ROC area of 0.811, respectively) until the first clinical manifestation of IFI. A later study by Aoki [12] involving 68 patients receiving reduced-intensity allo-HSCT had similar results and showed that the D-index was higher in patients who developed pulmonary infection. However, the study was limited by a non-specific definition of pulmonary infection, and these infections may not have been fungal in etiology.
The results of our study are more consistent with the findings of Kimura et al. [11] In their study, which evaluated the impact of neutropenia on bloodstream and pulmonary infections among allogeneic (n = 35) and autologous (n = 23) HSCT recipients, the D-index was equivalent to the duration of neutropenia for predicting early pulmonary infection in HSCT recipients and correlated well with total days of neutropenia. However, the definition of pulmonary infection was non-specific and based simply on radiographic findings, in contrast to proven/probable IFI definitions used in our study. It is worth mentioning, however, that median duration of profound neutropenia among patients with infection was longer (18 days) compared to our study (13 days), likely due to the differences in choice of underlying conditioning regimens. In the study by Kimura et al. [11] for example, 25 allo-HSCT recipients were given a fully myeloablative conditioning regimen as follows—Cyclophosphamide (Cy) and total body irradiation (TBI) (n = 16), Cy/Busulfan (Bu) (n = 2), high-dose cytarabine/Cy/TBI (n = 2), Flu/Cy/TBI (n = 2) or alemtuzumab-containing regimens (n = 3). In our cohort, of patients who underwent myeloablative conditioning (n = 374) a majority received Cy/TBI (n = 176), but a significant minority received Bu/Cy (n = 112), while the rest received varying regimens (n = 86). Duration of profound neutropenia and D index for Cy/TBI and Bu/Cy regimens were similar at 11 and 10 days and 3672.5 and 3512.5 days*neutrophil/µL, respectively.
Our median D-index was lower than in previous studies with similar patients [11, 12]. A plausible explanation for this discrepancy is that days of neutropenia, and consequently the D-index that began prior to day 0, were not included in our calculation. Some patients who were neutropenic prior to day 0 may have had a falsely low D-index not reflective of their total neutropenia burden. When we examined the patients who were already neutropenic on day 0, indicative of prior neutropenia, we found that they had a higher incidence of IFI compared to those patients who were non-neutropenic at day 0 (10/123 [8%] vs 6/591 [1%]; P < 0.001). In addition, when we included data on the d-index prior to D0 for all patients who were neutropenic prior to day 0, the group difference approached significance (P = 0.054, analysis not shown). These results suggest that cumulative neutropenia prior to transplantation increases the risk for IFI.
However, among HSCT recipients neutropenic at day 0, the D-index was similar between those who developed IFI and those who did not (median, 4908 and 5500 days neutrophil/µl, respectively), again highlighting the poor performance of the D-index as a predictor of IFI. Similarly, the D-indices of those allo-HSCT recipients who were non-neutropenic at day 0 were comparable between those that did and did not develop IFI (median, 3235 and 3425) underscoring their relatively short span of neutropenia. A subgroup analysis of myeloid-only allo-HSCT recipients also showed results that are similar to the entire cohort (analysis not shown).
By employing the ROC curve, a cutoff D-index of 3420 provides a sensitivity of 81%, and specificity of 43%. Using an apparent optimal threshold of 4105 days neutrophil/µl results in a reduced sensitivity of 63% but an improved specificity of 63%. Although values below these cutoffs may be useful (e.g. negative predictive value), the D-index performed poorly, and we could not establish an association between D-index and IFI.
The concept of a cumulative D-index (c-D-index) was also introduced by Portugal et al. [6] who defined it as the index from the start of neutropenia until the date of first clinical manifestation of IFI. In our study, the c-D-index was calculable only in 7 of 16 patients, with a median c-D-index of 4105 days neutrophil/µl; for the rest, the IFI developed much later, after resolution of neutropenia. As such, in our cohort, the c-D-index was not as useful a parameter.
Although our study failed to validate the use of the D-index as a tool to predict IFI in the early post-engraftment period, we believe the D-index may still prove useful if applied in a different setting. Based on the results of earlier studies [6, 13], it seems to perform best during induction chemotherapy for AML, where duration of profound neutropenia is considerably protracted and is the dominant risk factor for IFI. In this setting, a tool that automatically calculates the D-index can be used to help determine the risk of IFI; above a pre-determined threshold value, mold-active prophylaxis should be started. Other risk factors for IFI in the early post-transplant period, such as transplant characteristics, conditioning regimen, and GVH treatment, may render the D-index a less than ideal tool in this setting. In the future, it may prove useful in calculating cumulative duration of neutropenia from time of induction through consolidation chemotherapy, and then used to guide both choice of anti-fungal prophylaxis and pre-emptive versus empiric anti-fungal therapy thereafter. There are plans to proceed with a clinical trial using the index to guide pre-emptive therapy among high-risk hematologic patients [13].
We included a large number of patients who underwent allogeneic transplantation but there were only 2% who developed probable or proven IFIs within the first 100 days after transplantation. This low rate of infection is similar to recent epidemiologic data [14], and likely reflective of the almost universal use of mold active antifungal prophylaxis in recent years in this patient population. Notably, 7 patients developed IFI within 2 weeks, which may imply the presence of an underlying IFI prior to transplant, despite the lack of symptoms and the use of appropriate infection control measures. Although uncommon, it can occur, especially with prior episodes of profound neutropenia and exposure. In addition 10 of 16 IFIs developed despite intake of a mold-active agent. This underscores the need for other serologic markers that can help guide pre-emptive versus empiric therapy. In our study, factors which were associated with increased risk for IFI included higher number of packed red blood cell transfusion and CMV seropositive recipient status, which have been previously reported in the literature [1, 4, 8]. The presence of COPD almost reached statistical significance as a risk factor, perhaps implicating the greater use of corticosteroid use or greater presence of mold airway colonization in this patient population.
Our study has both strengths and limitations. We used a much larger sample size and a homogenous high-risk population (e.g. allogeneic HSCT), focusing on the early period post-transplantation where the risk of IFI driven by neutropenia is highest. We also followed established definitions to determine probable and proven IFI, as compared to prior studies where non-standard definitions were used [11, 12]. We also excluded transplant recipients with prior probable and proven IFI’s. However, this was a retrospective cohort, and the D-index was only calculated from day 0 until engraftment, and we were unable to account for episodes of neutropenia that occurred prior to transplantation (e.g., at the start of the conditioning regimen) which may have impacted the overall D-index and risk for IFI. Also, despite the large sample size of transplant patients, the statistical power for analyzing the association with IFI is largely dependent on the frequency of the outcome, and the availability of only 16 events was a limiting factor.
In conclusion, the pre-engraftment D-index did not predict the development of IFI during the early-post engraftment phase following allogeneic transplantation in this large cohort study using carefully defined outcomes. The D-index may be more useful during the setting of induction chemotherapy, although well-powered, and controlled studies are necessary to determine its clinical use.
References
Engels EA, Ellis CA, Supran SE, Schmid CH, Barza M, Schenkein DP, et al. Early infection in bone marrow transplantation: quantitative study of clinical factors that affect risk. Clin Infect Dis. 1999;28(2):256–66.
Ketterer N, Espinouse D, Chomarat M, Dumontet C, Moullet I, Rieux C, et al. Infections following peripheral blood progenitor cell transplantation for lymphoproliferative malignancies: etiology and potential risk factors. Am J Med. 1999;106(2):191–7.
Kolbe K, Domkin D, Derigs HG, Bhakdi S, Huber C, Aulitzky WE. Infectious complications during neutropenia subsequent to peripheral blood stem cell transplantation. Bone Marrow Transplant. 1997;19(2):143–7.
Offidani M, Corvatta L, Olivieri A, Rupoli S, Frayfer J, Mele A, et al. Infectious complications after autologous peripheral blood progenitor cell transplantation followed by G-CSF. Bone Marrow Transplant. 1999;24(10):1079–87.
Saavedra S, Jarque I, Sanz GF, Moscardo F, Jimenez C, Martin G, et al. Infectious complications in patients undergoing unrelated donor bone marrow transplantation: experience from a single institution. Clin Microbiol Infect. 2002;8(11):725–33.
Portugal RD, Garnica M, Nucci M. Index to predict invasive mold infection in high-risk neutropenic patients based on the area over the neutrophil curve. J Clin Oncol. 2009;27(23):3849–54.
Cornillet A, Camus C, Nimubona S, Gandemer V, Tattevin P, Belleguic C, et al. Comparison of epidemiological, clinical, and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients: a 6-year survey. Clin Infect Dis. 2006;43(5):577–84.
Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100(13):4358–66.
Nucci M, Anaissie EJ, Queiroz-Telles F, Martins CA, Trabasso P, Solza C, et al. Outcome predictors of 84 patients with hematologic malignancies and Fusarium infection. Cancer. 2003;98(2):315–9.
De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–21.
Kimura S, Oshima K, Sato K, Sato M, Terasako K, Nakasone H, et al. Retrospective evaluation of the area over the neutrophil curve index to predict early infection in hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2010;16(10):1355–61.
Aoki J, Tsubokura M, Kakihana K, Oshikawa G, Kobayashi T, Doki N, et al. The predictive value for pulmonary infection by area over the neutrophil curve (D-index) in patients who underwent reduced intensity hematopoietic stem cell transplantation. Pathol Oncol Res. 2014;20(4):879–83.
Kimura S, Wada H, Ishihara Y, Kawamura K, Sakamoto K, Yamasaki R, et al. D-index dose not predict the development of pulmonary infection in acute myeloid leukemia patients undergoing consolidation chemotherapy with high-dose cytarabine. Hematology. 2014;19(2):107–12.
Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50(8):1091–100.
Funding
This work was supported by the Mayo Clinic Small Grants Award [FP85701].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Abad, C.L.R., Lahr, B., O’Horo, J.C. et al. The D-index is not correlated with invasive fungal infection during the early-post engraftment phase among allogeneic hematopoietic stem cell transplant recipients. Int J Hematol 111, 293–302 (2020). https://doi.org/10.1007/s12185-019-02776-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-019-02776-x