Abstract
Cancer metabolic reprogramming has been considered an emerging hallmark in tumorigenesis and the antitumor immune response. Like cancer cells, immune cells within the tumor microenvironment or premetastatic niche also undergo extensive metabolic reprogramming, which profoundly impacts anti-tumor immune responses. Numerous evidence has illuminated that immunosuppressive TME and the metabolites released by tumor cells, including lactic acid, Prostaglandin E2 (PGE2), fatty acids (FAs), cholesterol, D-2-Hydroxyglutaric acid (2-HG), adenosine (ADO), and kynurenine (KYN) can contribute to CD8+ T cell dysfunction. Dynamic alterations of these metabolites between tumor cells and immune cells can similarly initiate metabolic competition in the TME, leading to nutrient deprivation and subsequent microenvironmental acidosis, which impedes immune response. This review summarizes the new landscape beyond the classical metabolic pathways in tumor cells, highlighting the pivotal role of metabolic disturbance in the immunosuppressive microenvironment, especially how nutrient deprivation in TME leads to metabolic reprogramming of CD8+ T cells. Likewise, it emphasizes the current therapeutic targets or strategies related to tumor metabolism and immune response, providing therapeutic benefits for tumor immunotherapy and drug development in the future.

Cancer metabolic reprogramming has been considered an emerging hallmark in tumorigenesis and the antitumor immune response. Dynamic alterations of metabolites between tumor cells and immune cells initiate metabolic competition in the TME, leading to nutrient deprivation and subsequent microenvironmental acidosis, which impedes immune response.
Similar content being viewed by others
Introduction
The homeostasis of the tumor microenvironment (TME) is controlled by intimate crosstalk between tumor cells, endothelial cells, stromal cells, and immune cells [1]. Such complex interactions commonly involve metabolic activity and extracellular metabolites, resulting in metabolic crosstalk, which is not only a source of energy supply but also the communication signal between different cellular compartments [2]. Tumor cells achieve rapid proliferation and escape lethal signals by increasing the capacity of glycolysis, lipid metabolism, or amino acid (e.g., glutamine) uptake [3]. Such changes in the metabolic process, in turn, also affect the metabolism pattern of adjacent cells in the TME, ultimately generating an immunosuppressive microenvironment [2]. Studies have shown that the Warburg effect reduces glucose consumption and increases lactate production of tumor cells in TME, reducing the activity of CD8+ T cells, natural killer cells (NKs), dendritic cells (DCs), and polarizing tumor-associated macrophages toward the toleratenic M2-like phenotype, which facilitate tumor immune escape [4,5,6]. Inhibiting glutamine metabolism in tumors and increasing the amino acid content in TME can enhance the cytotoxicity of immune cells. In addition, glutamine promotes T cell proliferation and cytokine production in lymphocytes, macrophages, and neutrophils [7, 8]. PGE2 promotes the polarization of M2-type macrophages and the generation and function of MDSCS and Tregs while inhibiting the function of T cells [9,10,11]. Tumor metabolites such as 2-HG, ADO, and KYN all exert immunosuppressive effects within the TME [12,13,14]. Notably, high cholesterol esterification rates in tumors impair T cell responses, and lowering cholesterol is expected to promote the proliferation and functions of CD8+ T cells, which are essential for long-term protective immunity [15].
Therefore, metabolic interventions hold promise for improving the effectiveness of immunotherapies. The comprehensive understanding and accurate evaluation of immune cell metabolism is essential for tumor immunotherapy. Herein, we focus on significant advances in tumor metabolic reprogramming, tumor immune microenvironment (TIME) remodeling, immunotherapy, targeting strategies, and drug development to pave the way for future anticancer therapy.
Metabolic reprogramming in cancer cells: beyond the classical metabolic pathways
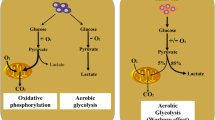
As the central elements of the TME, cancer cells are characterized by the extensive use of aerobic glycolysis (known as the Warburg effect) and increased uptake of amino acids (e.g., glutamine, lipids, etc.) to support their survival and growth (Fig. 1) [16]. Glucose mainly provides the energy material and carbon source for biosynthesis to meet the needs of cell growth and proliferation. Tumor cells can not only directly regulate the transcription level of critical metabolic enzymes in the glucose metabolism pathway but also regulate the activity, composition, subcellular localization, and other biological characteristics of critical metabolic enzymes through various post-translational modifications to enhance glucose uptake capacity, thus realizing metabolic reprogramming [17]. Glutamine, a nonessential hydrophilic amino acid, is the second primary energy substrate and an essential component in cancer cell culture. Compared with other cells in TME, tumor cells have the highest glutamine intake. The transmembrane transporter is critical for glutamine to enter cells and perform its physiological functions [18]. Alanine serine cysteine transporter 2 (ASCT2) (Fig. 1), encoded by the SLC1A5, is a sodium-dependent transporter that transports glutamine and other neutral amino acids across the plasma membrane [19], which is overexpressed in many cancers, such as triple-negative breast cancer (TNBC), hepatocellular carcinoma (HCC), colon cancer, cervical cancer, etc. [20]. To fulfill the biosynthetic demands associated with the structural components of the membrane matrix, proliferation, invasion, metastasis, etc., tumor cells also harness lipid metabolism, including lipid uptake, lipid catabolism, and lipid biosynthesis in TME.
Nonetheless, the inefficiency of ATP production in glycolysis gives a longstanding puzzle in cancer biology regarding how aerobic glycolysis provides a growth advantage to cancer cells in the long term. Studies have shown that mitochondria are essential for tumor cell growth or metastasis [21, 22]. Two mitochondrial functions exist in tumor cells, one capable of canonical tricarboxylic acid (TCA) cycle and the other whose enzymatic endowment is restricted to a downsized set of TCA enzymes (Fig. 2). These mitochondrial modifications are produced by highly stimulated glycolysis to inactivate OXPHOS pathway activity [23], thereby facilitating the formation of reduced mitochondrial activities [24]. However, it is worth noting that the non-canonical TCA cycle may sustain a faster protein synthesis and growth rate than the classical TCA cycle. In addition, studies have shown that primary solid tumors can slow down their TCA cycle, whereas metastatic cells exhibit higher TCA fluxes [25]. In the TME, T cells also utilize alternatives to glucose, such as inosine, which can be converted to phosphorylated ribose and fed into the TCA cycle, reducing tumor burden and improving patient survival rates [26]. As an alternative to glucose anaplerosis, acetate enables CD8+ T cells to increase global histone acetylation and chromatin accessibility, promoting tumor-infiltrating lymphocytes (TILs) cytokine production in vivo [27]. Furthermore, arginine is the primary source of ornithine and putrescine in normal cells [28]. By contrast, glutamine has been proven to be the primary source of ornithine in tumor cells [28]. Pancreatic ductal adenocarcinoma (PDA) prefers importing glutamate to the organic anion transporter (OAT) for de novo ornithine synthesis (DNS) rather than using arginine-derived ornithine for in vivo polyamine synthesis [28]. Another study identified that uridine-derived ribose released by uridine phosphorylase 1 (UPP1) could fuel central carbon metabolism, maintaining the redox balance and thus promoting the survival and proliferation of glucose-restricted PDA cells [29]. The cunning tumor cells oust the normally cytoplasmic gluconeogenic enzyme PCK1 from its usual role and activate AKT to induce cytosolic PCK1 phosphorylation at Ser90, followed by the translocation to the endoplasmic reticulum, where PCK1 uses GTP as a phosphate donor to phosphorylate INSIG1 at Ser207 and INSIG2 at Ser151 [30]. The phosphorylation of INSIG1 and INSIG2 reduces their binding to sterols and disrupts the interaction between INSIG and SCAP, leading to the translocation of the SCAP–SREBP complex to the Golgi apparatus, the activation of SREBP proteins (SREBP1 or SREBP2), and the transcription of downstream lipogenesis-related genes, ultimately tumorigenesis in mice [30]. Finally, one has to recall that inhibition of metabolic targets is often followed by metabolic rewiring, which may recover the growth capacity of cancer cells [30, 31].
Metabolic disorder of immune cells
Metabolic disorder of CD8+ T cells
T cells exhibit different metabolic patterns under different activation states (Fig. 3). Cytotoxic CD8+ T cells are vital in eliminating malignant cells and can provide long-term protective immunity. Nonetheless, tumor-infiltrating CD8+ T cells face fierce competition with cancer cells for limited nutrients. A clear example of nutrient competition is the depletion of glucose, which is associated with reduced infiltration and antitumor function of CD8+ T cells [32,33,34]. Exhausted T cells diminished glucose uptake [32, 34,35,36,37] but bypassed aerobic glycolysis enzymes and supplied cells with pyruvate, directly improving the cytokine function of TILs in vitro (Fig. 2) [38]. In addition, excessive inhibitory signals in the TME, such as the suppression of glucose uptake and mitochondria, contribute to metabolic inelasticity and hypoxia’s negative effect. Hypoxia can create a barrier to TILs in transcriptionally mediated responses to hypoxia via HIF-1α and mitochondrial respiration [39,40,41]. Under hypoxia, electrons in the mitochondria move from complex V to complex I, thereby generating ROS superoxide, a phenomenon that is a driver of TILs exhaustion [42,43,44,45]. Thus, low metabolites in the TME can negatively affect TILs function through multiple metabolic pathways. Glutaminase inhibition exerts distinct effects on tumor cells and CD8+ T cells, with a reduction in intermediates of the TCA cycle in tumor cells. In contrast, CD8+ T cells exhibited metabolic plasticity and upregulated glucose anaplerosis [46]. Lipid accumulation leads to immune suppression in the TME of solid tumors [47, 48]. Specific CD8+ T cells exhibit metabolic flexibility in response to the lipid-rich TME by upregulating fatty acid catabolism, thereby preventing lipid accumulation, which is particularly prominent in low-glucose environments, where lipids serve as a crucial biomass source for ATP synthesis [49]. CD8+ T cells accumulate specific long-chain fatty acids, resulting in reduced mitochondrial activity and triggering transcriptional changes, thereby diminishing the ability of TILs to extract energy from lipids through fatty acid oxidation, consequently suppressing their antitumor functions [49]. Studies have shown that increasing fatty acid catabolism with pharmacological agonists of PPAR further enhances the antitumor efficacy of TILs [50] (Fig. 3). Tumor cells also increase fat uptake through a high-fat diet (HFD), whereas tumor-infiltrating CD8+ T cells do not, impairing function and accelerating tumor growth. Blocking metabolic reprogramming of tumor cells in obese mice and promoting beneficial fatty acids competition between tumor cells and CD8+ T cells may improve anti-tumor immunity [51]. The cytotoxic T cells rely on pyruvate carboxylase (PC) to replenish TCA cycle intermediates. By contrast, lactate reduces PC-mediated anaplerosis, so the inhibition of pyruvate dehydrogenase (PDH) is sufficient to restore PC activity [51]. When type IVA phospholipase A was inhibited in T cells of breast cancer and melanoma, the lipid metabolism of T cells was reprogrammed, and the anti-tumor ability of T cells was enhanced [51].
T cells exhibit different metabolic patterns under different activation states. Sufficient glucose facilitates NK cell proliferation and direct cytotoxicity. Resting-state DCs display OXPHOS ability, while activated DCs have vigorous glycolysis capacity. Tumor-derived MDSCs exhibit increased central carbon metabolism, including glycolysis, PPP, and the TCA cycle. The cytocidal functions of M1-like macrophages are based on the high glycolytic metabolism and increased ROS production. M2-like macrophages mainly rely on FAO-derived OXPHOS and glutamine to promote tumor progression.
Exhausted CD8+ T cells exhibit diminished oxygen consumption, depolarized mitochondrial membrane potential, punctate mitochondrial morphology, loss of cristae ultrastructure, and decreased mitochondrial mass that negatively affect TILs function [37, 39, 43, 52,53,54]. Notably, the remaining mitochondria of exhausted T cells generate excessive ROS, regulating disease progression [43, 44, 54,55,56,57]. PGC1α is the master regulator of mitochondrial programming [53, 56]. The activated AKT and BLIMP1 downregulate the expression of PGC1α, disrupting mitochondrial function in tumor cells [53, 56]. Moreover, overexpressing PGC1α or glutathione peroxidase 1 (GPX1) or decreasing the degree of tumor hypoxia by NDUFS4-knockout or pharmacological intervention can alleviate the negative impact of ROS on TILs [40, 56]. The use of AKT inhibitors restored PGC1α expression and augmented mitochondrial mass in solid tumors and chronic viral infections, indicating that the suppression of PGC1α in CD8+ T cells is partially mediated by AKT and mTOR [53, 58].
NK cells
NK cells depend on highly increased glucose content to survive after full activation. Studies have shown sufficient glucose facilitates NK cells proliferation and direct cytotoxicity. GLUT1 helps NK cells utilize glucose to generate ATP and pyruvate, promoting glycolysis and OXPHOS, leading to elevated IFN-γ and Fas ligands [59]. Transforming growth factor-β (TGF-β) accumulated in TME induces NK cells to up-regulate fructose-1,6-bisphosphatase (FBP1), which inhibits glycolysis metabolism and disrupts the cell viability, ultimately resulting in the dysfunction of NK cells [60]. Therefore, FBP1 inhibition has been used as a new strategy to restore the function and viability of NK cells in vivo. Recent studies have also pointed out that the cholesterol accumulation in NK cells increases their anti-tumor ability by facilitating the formation of lipid rafts [61]. In addition, the hypoxic microenvironment in tumors harms the function of NK cells by downregulating activating signals, such as NKG2D, NKp30, CD16, and granzyme B, thereby limiting cytokine production and cytotoxicity and resulting in tumor metastasis [62]. When NK cells make direct contact with tumor cells to form an immune synapse in response to local energy consumption, the mitochondria of NK cells are depolarized, indicating a rapid consumption of their metabolic energy [63]. The STAT3-activated proliferating NK (STAT3-exNK) cells resist oxidative stress by up-regulating the glycolysis, down-regulating OXPHOS and the expression of the related proteins to oxidative damage, and up-regulating proteins expression associated with DNA repair [64]. Furthermore, STAT3-exNK cells flexitively utilize metabolic substrates through expressing enzymes related to one-carbon metabolism, folate metabolism, and serine synthesis pathways, whose metabolic flexibility and adaptability can better adapt to the TME and exhibit enhanced anti-tumor ability.
Dendritic cells
DCs are the quintessential antigen-presenting cells (APC) in the immune system, which can efficiently ingest, process, and present antigens. The metabolism pattern of DCs in a resting state is mainly through oxidative phosphorylation, while activated DCs have vigorous glycolysis [65]. Changes in the lipid metabolism of DCs lead to a shift in its overall function. In both tumor-bearing mice and cancer patients, a notable proportion of DCs exhibit elevated triglyceride levels and reduced capacity for antigen processing. The effectiveness of cancer vaccines was greatly improved by normalizing lipid levels of DCs using an acetyl-CoA carboxylase inhibitor [48], suggesting that the enhanced cancer immune response is expected by regulating lipid levels of DCs.
Tumor-associated macrophages
There are two separate categories of tumor-associated macrophages (TAMs), which are stimulated by varying polarizing cytokines, including the pro-inflammatory (M1) state (activated by lipopolysaccharide alone or with Th1 cytokines), which usually has the anti-tumor function, and the anti-inflammatory (M2) state (activated by Th2 cytokines), which generally has a pro-tumor function. The metabolic profile of TAMs is indeed very dynamic. M1-like macrophages are generally associated with highly glycolytic metabolism and a robust ability to generate ROS, underlying their cytocidal functions. Conversely, M2-like macrophages mainly rely on FAO-derived OXPHOS and glutamine for energy supply. Inhibition of FAO could polarize TAMs from M2 to M1 in mouse models of lung and colon cancer [66]. When glutamine-synthetase (GS) is inhibited in macrophages, the intracellular glutamine is reduced, glycolysis is increased, and the phenotype switches from M2 to M1. In vitro experiments demonstrate that glutamine ligase (GLUL) promotes the polarization of TAMs towards the M2 type by catalyzing the conversion of glutamate to glutamine [66]. Therefore, inhibiting glutamine uptake can promote the polarization of TAMs to M1. In contrast, colony-stimulating factor 1 (CSF1) released from tumor cells can induce high expression of FASN in TAMs, producing fatty acids to activate peroxisome proliferator-activated receptor δ (PPARδ). PPARδ releases immunosuppressive cytokine IL-10 to downstream signals, thus inducing TAMs to polarize into type M2 [67]. In the early stage of tumor development, FABP5 is overexpressed by TAMs, which leads to more type I interferon (IFN-1) secretion and promotes an anti-tumor immune response. When the tumor is advanced, the high expression of FABP4 in TAMs can promote the signal transduction of IL-6/STAT3, thus promoting tumor development [68]. However, it remains to be seen whether TAMs can promote tumor development by supplying lipids directly, such as adipocytes. These above studies suggest the potential metabolic crosstalk between tumor cells and TAMs.
Myeloid-derived suppressor cells
Myeloid-derived suppressor cells (MDSC) accumulate in almost all malignancy patients. During maturation and activation, tumor-derived MDSCs exhibit increased central carbon metabolism, including glycolysis, PPP, and the TCA cycle. Through dynamic metabolic flux analysis, it has been found that MDSCs exhibited the Warburg effect during maturation, which produced about 95% of ATP through the glycolytic pathway and significantly increased glucose and glutamine uptake rates, accompanied by a decrease in oxygen consumption rate (OCR) [69]. Notably, the phosphoenolpyruvate, a metabolite of tumor-derived MDSCs during glycolysis, protects MDSCs from apoptosis and facilitates their survival [70]. MDSC consists of two major cell subsets, monocyte MDSCs (M-MDSCs) and granulocyte MDSCs (G-MDSCs), promoting tumor growth through non-immune or immunosuppressive mechanisms. G-MDSCs have also been reported to utilize glycolysis and OXPHOS in the tumor-bearing mouse model of nasopharyngeal carcinoma. Owing to the high glucose uptake rate of both tumor cells and MDSCs, immune cells do not have any metabolic elasticity to acclimate to low oxygen tension and limited glucose availability, resulting in dysfunction and death, indirectly facilitating tumor escape and progression [71].
Tumor-derived metabolites mediate TIME remodeling
Lactic acid
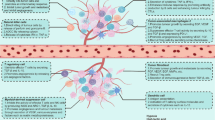
Regardless of normal or hypoxic conditions, tumor cells consume glucose and produce large amounts of lactic acid, which can be accumulated on the cell membrane through MCTs (especially MCT4), forming an acidic TIME and suppressing anti-tumor immune responses. In addition, lactic acid induces PD-1 expression on Tregs to suppress human T cell proliferation in vitro, indicating that lactic acid is an essential substrate for Treg cells [40, 72]. Tumor-derived lactic acid (TDLA) also reduces the number and activity of CD8+ T cells and NK cells and increases the number of MDSC cells, resulting in accelerated tumor growth of B16 melanoma [4]. In addition, TDLA has a critical signaling function in the TME to induce M2 polarization. Specifically, expressions of pro-inflammatory M1 markers (e.g., iNOS, MCP1, IL-6) are lower at an acidic pH, while expressions of M2 markers (e.g., MRC1, arginase 1 (Arg1), chitinase-3-like protein) are higher. For example, El-Kenawi et al. discovered that the acidic TME contributes to the M2-polarization of macrophages in prostate cancer [6]. Moreover, adequate DC functions are required for sufficient T-cell activation, while the function of cancer-associated DCs is suppressed in the acidic TME (Fig. 4) [5].
Tumor-derived lactic acid (TDLA) increases the number of MDSC cells and induces M2 polarization. Fatty acids can cause the conversion of M1 macrophages to M2 macrophages and inhibit the function of T cells, NK cells, and DC cells. High cholesterol esterification rates in tumors impair T-cell responses. Accumulation of 2-HG reduces the production of chemokines that typically attract CD8+ T cells to tumors. Furthermore, 2-HG-mediated infiltration of MDMs with an immunosuppressive phenotype further suppresses the activity and proliferation of T cells. ADO inhibits the density and activity of CD8+ T cells and NK cells. The immunosuppressive effect of KYN in the TME is predominantly mediated by the AhR. After entering the nucleus, AhR regulates the transcription of CYP1A1 and AhRR and regulates T cell and DC cell function.
PGE2
PGE2, an essential regulator for cell growth and inflammatory mediators, participates in the immune response as an immunosuppressive factor through autocrine and paracrine. Studies have shown that the PGE2 secreted by CAFs can stimulate angiogenesis, thereby inducing tumor cell invasion and metastasis. In addition, tumor cell-derived PGE2 converts M1-type macrophages into cancer-promoting M2-type macrophages [9, 73] and promotes the immunosuppressive functions of MDSCs [10]. Moreover, PGE2 promotes the differentiation of Treg cells, inhibiting IL-2 and IFNγ of T cells and activating the cAMP-PKA pathway, resulting in CD8+ T cell growth arrest [74]. PGE2 can further impede T cell infiltration by downregulating conventional type 1 dendritic cells (cDC1) mediated by NK cells, contributing to cancer immune evasion (Fig. 4) [11].
Fatty acids
Emerging evidence supports that fatty acids can regulate immune cells through intracellular signaling. Treg cells predominantly utilize fatty acid oxidation for their metabolism, whereas the activity of conventional CD4+ T cells and CD8+ T cells is impaired [75]. Elevated de novo fatty acid synthesis in tumor cells supports the proliferation, differentiation, and function of MDSCs. Abnormal accumulation of short-chain fatty acids, long-chain fatty acids (LCFAs), and cholesterol can be observed in immunosuppressive cells, such as MDSCs. Polymorphonuclear MDSCs (PMN-MDSC) pathologically activated neutrophils and overexpress FATP2 by activating the transcription factor of STAT5, thus promoting immune suppressive activity [76]. Studies have found that the accumulation of specific LCFAs in CD8+ T cells in pancreatic cancer impairs the function of mitochondria and reduces the fatty acid catabolism, thereby exacerbating the accumulation of LCFAs and very-long-chain fatty acids (VLCFAs) that mediate lipotoxicity. In addition, TAMs express high levels of the scavenger receptor CD36 to accumulate lipids and use FAO instead of glycolysis to provide energy, which is critical for the differentiation and pro-tumor function of TAMs in the TME [77].
Cholesterol
Cholesterol is one of the essential components of the cell membrane, and its high expression helps tumor cells evade immune surveillance. Studies have shown that high cholesterol expression predisposes immune cells to apoptosis, along with higher expression of immune checkpoint molecules such as PD-1, LAG-3, and TIM-3. Sustained expression of immune checkpoints on T cells considerably dampens their function and induces cell exhaustion (Fig. 4) [15].
2-HG
2-HG suppresses antitumor T-cell responses through direct and indirect mechanisms. Within the isocitrate dehydrogenase (IDH)-mutant glioma microenvironment, impaired antitumor T-cell immunity is partly due to the intracellular accumulation of 2-HG generated by the mutant IDH, which reduces the production of chemokines that typically attract CD8+ T cells to tumors [12]. Additionally, 2-HG can be exported from tumor cells, directly inhibiting effector T-cell function [12]. Furthermore, 2-HG-mediated infiltration of monocyte-derived macrophages (MDMs) with an immunosuppressive phenotype further suppresses T cell activity and proliferation [78]. In preclinical models, inhibition of IDH reactivates T-cell activity and reprograms the immunosuppressive myeloid phenotype, thereby reversing 2-HG-mediated immune suppression [78]. Therefore, using IDH inhibitors can potentially enhance the sensitivity of IDH-mutant glioma to immunotherapy.
Adenosine (ADO)
ADO is a joint chemotherapy-associated immune checkpoint that hinders the anti-tumor immunity-mediated efficacy of chemotherapy. Inhibiting ADO generation improved the density and activity of CD8+ T cells and NK cells, relieving the immunosuppressive microenvironment and leading to a substantial proliferation inhibition of breast cancer cells (Fig. 4) [13, 79]. In addition, facilitating the release of immunostimulatory ATP and reducing the levels of immunosuppressive extracellular ADO can effectively eliminate melanoma and colorectal adenocarcinoma by recruiting ectonucleotidases CD39-expressing immune cells [80].
Kynurenine (KYN)
The metabolism of KYN by indoleamine-2,3-dioxygenase (IDO1) or tryptophan-2,3-dioxygenase (TDO2) is a critical link in constitutive and adaptive tumor immunity [14]. The immunosuppressive effect of KYN in the TME is predominantly mediated by the aryl hydrocarbon receptor (AhR), a cytosolic transcription factor that widely suppresses immune cell function [14]. AhR binds to different ligands such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), benzo[a] pyrene (BaP), Trp catabolic products, and kynurenic acid (KA), and then translocates to the nucleus, where it regulates the transcription of CYP1A1 and AhR repressor (AhRR) by binding to the dioxin response elements (DREs) in their promoter region [81]. In addition, AhR modulates the maturation and function of DCs, regulates the generation and function of Tregs, and inhibits tumor-specific CD8+ T cells [82,83,84] (Fig. 4). Therefore, inhibition of AhR offers an opportunity for antitumor therapy via restoring immune system functions. Recent studies have linked the presence of KYN and IDO activity to the resistance to anti-PD-1 therapy [85], suggesting that inhibition of AhR may also provide new insights into immunosuppressive treatment.
Targeting metabolism for cancer therapy
Cancer cells use metabolic reprogramming to produce ATP and maintain redox homeostasis and biosynthesizing substances required for tumor cell survival. This contributes to immune escape and metastatic invasion of tumor cells. Therefore, targeting tumor metabolism by exploiting the metabolic differences between tumor cells and normal cells becomes a promising anti-cancer strategy.
Metabolic intervention in tumor cells
Increased glucose uptake is a characteristic hallmark of various tumors, inhibition of which is considered a promising therapeutic direction for cancer therapy (Table 1). The most successful example approved for targeting glucose metabolism is metformin, which induces energy stress and the activation of the AMPK signaling pathway, further inhibiting hepatic gluconeogenesis and activating glycolysis. In addition, metformin induces the dissociation of HK2 in mitochondria by specifically binding to the site of G-6-P, thus altering the subcellular localization of HK2 [86]. Other markers associated with glucose metabolism that can be targeted include GLUT1/2/3/4, PHGDH, MCT1, and LDHA, and are currently being investigated in preclinical studies (Fig. 5). However, targeting aerobic glycolysis has not been successfully exploited clinically. For example, the inhibitor of glycolysis, 2-deoxyglucose, has been proven to have undesirable side effects and limited efficacy in humans [87]. Similarly, markers associated with glutamine metabolism that can be targeted mainly include GLS, ASCT2, SLC6A14, and GAC (Fig. 6). For example, the clinically tested CB-839, co-crystallized with GLS1 [88], is a particular metabolic inhibitor of GLS. In contrast to GLS1-specific inhibition, 6-diazo-5-oxo-l-norleucine (DON) covalently binds to multiple glutamine enzymes. Although DON has remarkable anti-tumor activity, it poses challenges for precision therapies due to drug resistance development [89].
In addition to the above-mentioned metabolism patterns, tumor cells rely on lipid uptake, lipid catabolism, and lipid biosynthesis. Markers associated with fatty acid metabolism mainly include FASN and ACLY (Fig. 7). Metformin can inhibit the lipogenesis, adipocyte-mediated proliferation, and metastasis of ovarian tumors, a therapeutic option in the early stages of ovarian cancer [90]. The combination of metformin with fatty acid synthase inhibitors can affect the survival of diffuse large B-cell lymphoma (DLBCL) cells by regulating de novo fatty acid synthesis, thus exerting anti-tumor effects. Although many tumors depend on fatty acid oxidation [91], particular inhibitors for fatty acid oxidation are still lacking.
Clinical application of metabolic regulatory drugs combined with immunotherapy
Currently, checkpoint blockade and adoptive T-cell therapy (ACT) in the form of chimeric antigen receptor (CAR)-T cells represent the two clinically approved cancer immunotherapies. In addition to targeting tumor metabolism and regulating immune metabolism, metabolic therapy can also potentially enhance antitumor immune responses. For instance, blocking lactate released from tumor cells or inhibiting carnitine palmitoyltransferase can activate antitumor immunity and improve immunotherapy [92,93,94,95]. Additionally, targeting specific amino acid pathways and nucleotide metabolism can enhance tumor sensitivity to immunotherapy [14, 96, 97]. The first oral small-molecule inhibitor, ivosidenib, approved by the FDA for the treatment of IDH1-mutated acute myeloid leukemia (AML), was also used to treat the chemotherapy-refractory cholangiocarcinoma with IDH1 mutations [98, 99]. Another notable example involves the progress of IDO1 inhibitors. Numerous preclinical investigations have highlighted the potent effect of IDO1 inhibitors in activating antitumor immunity and in synergy with anti-PD-(L)1 therapy [100]. Subsequent clinical phase I/II trials evaluated epacadostat in combination with pembrolizumab (ECHO-202; NCT02178722) and epacadostat plus nivolumab (ECHO-204; NCT02327078) demonstrate promising antitumor activity [98]. In addition, direct targeting of metabolic reprogramming can also sensitize tumor cells to radiotherapy and chemotherapy. For instance, ascorbate sensitized non-small-cell lung cancer and glioblastoma multiforme (GBM) cells to radiotherapy and chemotherapy by disrupting intracellular iron metabolism [101] and targeting estrogen-related receptor α-sensitized tumors to immunotherapy by downregulating energy metabolism in tumor cells [102]. CAR-T cells are generated through ex vivo activation and expansion, offering opportunities for metabolic modulation. Expanded T cells in the presence of glycolysis or AKT inhibitors can promote the generation of memory cells, thereby improving their persistence and functionality upon adoptive transfer to tumor-bearing mice [103, 104]. Furthermore, overexpression of phosphoenolpyruvate carboxykinase 1 (PCK1), which converts oxaloacetate (OAA) into phosphoenolpyruvate (PEP) to genetically engineer T cells, has been demonstrated to improve the effectiveness of T cells transferred adoptively [34]. Alternatively, pharmacologically promoting mitochondrial fusion and inhibiting mitochondrial fission can lead to superior control of adoptively transferred T cells by enhancing memory generation with increased mitochondrial mass, OXPHOS, and spare respiratory capacity [105]. Even after adoptive cellular therapy, continuous administration of glutamine metabolism inhibitors is expected to control tumor growth and foster enduring memory in metastasized cells [106].
Conclusion
The metabolic alterations in cancer cells significantly impact the immune system in recognizing and presenting antigens. Conversely, metabolic reprogramming of immune cells affects the function of tumor cells, leading to variations in local immune activity [107]. In addition, certain specific metabolites in TME, such as adenosine, Ca2+, K+, and Cl−, suppress the antitumor immune response. Although ATP has an immunostimulatory effect, adenosine suppresses the effector functions of immune cells [108]. The antagonism of the adenosine A2A receptor can improve the effector function of CD8+ T cells. Moreover, the adenosine A2A receptor targeted therapy combined with CAR T cell therapy enhances the treatment efficacy in breast cancer [109]. The inhibition of extracellular adenosine (eADO)-generating enzymes and eADO receptors can promote the function of T cells and NK cells function, inhibiting the pro-tumor effect of myeloid cells and other immunomodulatory cells by promoting the antigen presentation [108]. Furthermore, T cell function can be disrupted by the low pH environment resulting from the imbalance of Ca2+, K+, and Cl− in the TME [110]. So, the comprehensive knowledge and skillful manipulation of the interaction between malignant cells and the body’s defense mechanism within the TME could enhance the efficacy of immunotherapy.
Compared with malignant tumor cells, immune cells exhibit unique metabolic characteristics. The failure of GLS and IDO inhibitors (epacadostat) in clinical trials has inspired a greater understanding of the interdependent metabolic functions of cancer cells and host cells and the overlapping pathways contributing to metabolic adaptation and evasion of therapeutic intervention. Hence, insights into the unique metabolic patterns of immune cells enable us to improve the surveillance ability and inhibition effect on tumor progression of the immune system. For example, while aerobic glycolysis may be an obvious therapeutic target due to its vital role in supporting the growth of cancer cells, the same metabolic processes are also critical for optimal immune cell effector function in anti-tumor immune responses. It is crucial to have a comprehensive understanding of the intricate interplay between cancer cells and host cell metabolism and the redundant mechanisms that regulate their co-dependence within TME. Therefore, in future research, it would be prudent to focus on developing a strategy that inhibits cancer cell metabolism and maintains the effectiveness of anti-tumor immune cells. In addition, methods for assessing and measuring the metabolic characteristics of cancers need to be further improved, for example, the use of metabolomics, isotope tracking, and metabolic imaging techniques, which may eventually allow clinical oncologists to tailor treatment strategies by matching treatments to the metabolism of patient-specific tumors.
References
Dey P, Kimmelman AC, DePinho RA. Metabolic codependencies in the tumor microenvironment. Cancer Discov. 2021;11:1067–81.
Bader JE, Voss K, Rathmell JC. Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol Cell. 2020;78:1019–33.
Stine ZE, Schug ZT, Salvino JM, Dang CV. Targeting cancer metabolism in the era of precision oncology. Nat Rev Drug Discov. 2022;21:141–62.
Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016;24:657–71.
Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–63.
El-Kenawi A, Gatenbee C, Robertson-Tessi M, Bravo R, Dhillon J, Balagurunathan Y, et al. Acidity promotes tumour progression by altering macrophage phenotype in prostate cancer. Br J Cancer. 2019;121:556–66.
Johnson MO, Wolf MM, Madden MZ, Andrejeva G, Sugiura A, Contreras DC, et al. Distinct regulation of Th17 and Th1 cell differentiation by glutaminase-dependent metabolism. Cell. 2018;175:1780–95.e19.
Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. 2018;10:1564.
Larsson K, Kock A, Idborg H, Arsenian Henriksson M, Martinsson T, Johnsen JI, et al. COX/mPGES-1/PGE2 pathway depicts an inflammatory-dependent high-risk neuroblastoma subset. Proc Natl Acad Sci USA. 2015;112:8070–5.
Obermajer N, Wong JL, Edwards RP, Odunsi K, Moysich K, Kalinski P. PGE (2)-driven induction and maintenance of cancer-associated myeloid-derived suppressor cells. Immunol Invest. 2012;41:635–57.
Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172:1022–1037.e14.
Bunse L, Pusch S, Bunse T, Sahm F, Sanghvi K, Friedrich M, et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med. 2018;24:1192–203.
Gong X, Zheng C, Cai Y, Zhang W, Zhu B, Rong R, et al. Adenosine-modulating synthetic high-density lipoprotein for chemoimmunotherapy of triple-negative breast cancer. J Control Release. 2024;367:637–48.
Platten M, Nollen EAA, Röhrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18:379–401.
Ma X, Bi E, Lu Y, Su P, Huang C, Liu L, et al. Cholesterol induces CD8+ T cell exhaustion in the tumor microenvironment. Cell Metab. 2019;30:143–156.e5.
Thompson CB, Vousden KH, Johnson RS, Koppenol WH, Sies H, Lu Z, et al. A century of the Warburg effect. Nat Metab. 2023;5:1840–3.
Xu D, Shao F, Bian X, Meng Y, Liang T, Lu Z. The evolving landscape of noncanonical functions of metabolic enzymes in cancer and other pathologies. Cell Metab. 2021;33:33–50.
Reinfeld BI, Madden MZ, Wolf MM, Chytil A, Bader JE, Patterson AR, et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature. 2021;593:282–8.
Yoo HC, Park SJ, Nam M, Kang J, Kim K, Yeo JH, et al. A variant of SLC1A5 is a mitochondrial glutamine transporter for metabolic reprogramming in cancer cells. Cell Metab. 2020;31:267–283.e12.
Liu Y, Zhao T, Li Z, Wang L, Yuan S, Sun L. The role of ASCT2 in cancer: A Review. Eur J Pharmacol. 2018;837:81–7.
Morais R, Zinkewich-Péotti K, Parent M, Wang H, Babai F, Zollinger M. Tumor-forming ability in athymic nude mice of human cell lines devoid of mitochondrial DNA. Cancer Res. 1994;54:3889–96.
Cavalli LR, Varella-Garcia M, Liang BC. Diminished tumorigenic phenotype after depletion of mitochondrial DNA. Cell Growth Differ. 1997;8:1189–98.
Díaz-Ruiz R, Avéret N, Araiza D, Pinson B, Uribe-Carvajal S, Devin A, et al. Mitochondrial oxidative phosphorylation is regulated by fructose 1,6-bisphosphate. A possible role in Crabtree effect induction? J Biol Chem. 2008;283:26948–55.
Alberghina L. The Warburg Effect Explained: integration of enhanced glycolysis with heterogeneous mitochondria to promote cancer cell proliferation. Int J Mol Sci. 2023;24:15787.
Bartman CR, Weilandt DR, Shen Y, Lee WD, Han Y, TeSlaa T, et al. Slow TCA flux and ATP production in primary solid tumours but not metastases. Nature. 2023;614:349–57.
Wang T, Gnanaprakasam JNR, Chen X, Kang S, Xu X, Sun H, et al. Inosine is an alternative carbon source for CD8+-T-cell function under glucose restriction. Nat Metab. 2020;2:635–47.
Qiu J, Villa M, Sanin DE, Buck MD, O’Sullivan D, Ching R, et al. Acetate promotes T cell effector function during glucose restriction. Cell Rep. 2019;27:2063–2074.e5.
Lee MS, Dennis C, Naqvi I, Dailey L, Lorzadeh A, Ye G, et al. Ornithine aminotransferase supports polyamine synthesis in pancreatic cancer. Nature. 2023;616:339–47.
Nwosu ZC, Ward MH, Sajjakulnukit P, Poudel P, Ragulan C, Kasperek S, et al. Uridine-derived ribose fuels glucose-restricted pancreatic cancer. Nature. 2023;618:151–8.
Xu D, Wang Z, Xia Y, Shao F, Xia W, Wei Y, et al. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. Nature. 2020;580:530–5.
Bonanomi M, Salmistraro N, Porro D, Pinsino A, Colangelo AM, Gaglio D. Polystyrene micro and nano-particles induce metabolic rewiring in normal human colon cells: a risk factor for human health. Chemosphere. 2022;303:134947.
Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–41.
Ottensmeier CH, Perry KL, Harden EL, Stasakova J, Jenei V, Fleming J, et al. Upregulated glucose metabolism correlates inversely with CD8+ T-cell infiltration and survival in squamous cell carcinoma. Cancer Res. 2016;76:4136–48.
Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell. 2015;162:1217–28.
Van Bruggen JAC, Martens AWJ, Fraietta JA, Hofland T, Tonino SH, Eldering E, et al. Chronic lymphocytic leukemia cells impair mitochondrial fitness in CD8+ T cells and impede CAR T-cell efficacy. Blood. 2019;134:44–58.
Gemta LF, Siska PJ, Nelson ME, Gao X, Liu X, Locasale JW, et al. Impaired enolase 1 glycolytic activity restrains effector functions of tumor-infiltrating CD8+ T cells. Sci Immunol. 2019;4:eaap9520.
Siska PJ, van der Windt GJ, Kishton RJ, Cohen S, Eisner W, MacIver NJ, et al. Suppression of Glut1 and glucose metabolism by decreased Akt/mTORC1 signaling drives T cell impairment in B cell leukemia. J Immunol. 2016;197:2532–40.
Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–82.
Najjar YG, Menk AV, Sander C, Rao U, Karunamurthy A, Bhatia R, et al. Tumor cell oxidative metabolism as a barrier to PD-1 blockade immunotherapy in melanoma. JCI Insight. 2019;4:e124989.
Scharping NE, Menk AV, Whetstone RD, Zeng X, Delgoffe GM. Efficacy of PD-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer Immunol Res. 2017;5:9–16.
Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465–75.
Chinopoulos C. Which way does the citric acid cycle turn during hypoxia? The critical role of α-ketoglutarate dehydrogenase complex. J Neurosci Res. 2013;91:1030–43.
Sukumar M, Liu J, Mehta GU, Patel SJ, Roychoudhuri R, Crompton JG, et al. Mitochondrial membrane potential identifies cells with enhanced stemness for cellular therapy. Cell Metab. 2016;23:63–76.
Siska PJ, Beckermann KE, Mason FM, Andrejeva G, Greenplate AR, Sendor AB, et al. Mitochondrial dysregulation and glycolytic insufficiency functionally impair CD8+ T cells infiltrating human renal cell carcinoma. JCI Insight. 2017;2:e93411.
Hurst KE, Lawrence KA, Essman MT, Walton ZJ, Leddy LR, Thaxton JE. Endoplasmic reticulum stress contributes to mitochondrial exhaustion of CD8+ T cells. Cancer Immunol Res. 2019;7:476–86.
Leone RD, Zhao L, Englert JM, Sun IM, Oh MH, Sun IH, et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366:1013–21.
Zhang Y, Kurupati R, Liu L, Zhou XY, Zhang G, Hudaihed A, et al. Enhancing CD8+ T cell fatty acid catabolism within a metabolically challenging tumor microenvironment increases the efficacy of melanoma immunotherapy. Cancer Cell. 2017;32:377–391.e9.
Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16:880–6.
Manzo T, Prentice BM, Anderson KG, Raman A, Schalck A, Codreanu GS, et al. Accumulation of long-chain fatty acids in the tumor microenvironment drives dysfunction in intrapancreatic CD8+ T cells. J Exp Med. 2020;217:e20191920.
Chekaoui A, Ertl HCJ. PPARα agonist fenofibrate enhances cancer vaccine efficacy. Cancer Res. 2021;81:4431–40.
Reina-Campos M, Scharping NE, Goldrath AW. CD8+ T cell metabolism in infection and cancer. Nat Rev Immunol. 2021;21:718–38.
Simula L, Pacella I, Colamatteo A, Procaccini C, Cancila V, Bordi M, et al. Drp1 controls effective T cell immune-surveillance by regulating T cell migration, proliferation, and cmyc-dependent metabolic reprogramming. Cell Rep. 2018;25:3059–3073.e10.
Scharping NE, Menk AV, Moreci RS, Whetstone RD, Dadey RE, Watkins SC, et al. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity. 2016;45:701–3.
Yu YR, Imrichova H, Wang H, Chao T, Xiao Z, Gao M, et al. Disturbed mitochondrial dynamics in CD8+ TILs reinforce T cell exhaustion. Nat Immunol. 2020;21:1540–51.
Russell SL, Lamprecht DA, Mandizvo T, Jones TT, Naidoo V, Addicott KW, et al. Compromised metabolic reprogramming is an early indicator of CD8+ T cell dysfunction during chronic mycobacterium tuberculosis infection. Cell Rep. 2019;29:3564–3579.e5.
Scharping NE, Rivadeneira DB, Menk AV, Vignali PDA, Ford BR, Rittenhouse NL, et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat Immunol. 2021;22:205–15.
Vardhana SA, Hwee MA, Berisa M, Wells DK, Yost KE, King B, et al. Impaired mitochondrial oxidative phosphorylation limits the self-renewal of T cells exposed to persistent antigen. Nat Immunol. 2020;21:1022–33.
Bengsch B, Johnson AL, Kurachi M, Odorizzi PM, Pauken KE, Attanasio J, et al. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8+ T cell exhaustion. Immunity. 2016;45:358–73.
Wang Z, Guan D, Wang S, Chai LYA, Xu S, Lam KP. Glycolysis and oxidative phosphorylation play critical roles in natural killer cell receptor-mediated natural killer cell functions. Front Immunol. 2020;11:202.
Cong J, Wang X, Zheng X, Wang D, Fu B, Sun R, et al. Dysfunction of natural killer cells by FBP1-induced inhibition of glycolysis during lung cancer progression. Cell Metab. 2018;28:243–255.e5.
Qin WH, Yang ZS, Li M, Chen Y, Zhao XF, Qin YY, et al. High Serum Levels of cholesterol increase antitumor functions of nature killer cells and reduce growth of liver tumors in mice. Gastroenterology. 2020;158:1713–27.
Balsamo M, Manzini C, Pietra G, Raggi F, Blengio F, Mingari MC, et al. Hypoxia downregulates the expression of activating receptors involved in NK-cell-mediated target cell killing without affecting ADCC. Eur J Immunol. 2013;43:2756–64.
Abarca-Rojano E, Muñiz-Hernández S, Moreno-Altamirano MM, Mondragón-Flores R, Enriquez-Rincón F, Sánchez-García FJ. Re-organization of mitochondria at the NK cell immune synapse. Immunol Lett. 2009;122:18–25.
Poznanski SM, Singh K, Ritchie TM, Aguiar JA, Fan IY, Portillo AL, et al. Metabolic flexibility determines human NK cell functional fate in the tumor microenvironment. Cell Metab. 2021;33:1205–1220.e5.
Hu Z, Yu X, Ding R, Liu B, Gu C, Pan XW, et al. Glycolysis drives STING signaling to facilitate dendritic cell antitumor function. J Clin Invest. 2023;133:e166031.
Liu PS, Wang H, Li X, Chao T, Teav T, Christen S, et al. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol. 2017;18:985–94.
Li Z, Li H, Zhao ZB, Zhu W, Feng PP, Zhu XW, et al. SIRT4 silencing in tumor-associated macrophages promotes HCC development via PPARδ signalling-mediated alternative activation of macrophages. J Exp Clin Cancer Res. 2019;38:469.
Hao J, Yan F, Zhang Y, Triplett A, Zhang Y, Schultz DA, et al. Expression of adipocyte/macrophage fatty acid-binding protein in tumor-associated macrophages promotes breast cancer progression. Cancer Res. 2018;78:2343–55.
Goffaux G, Hammami I, Jolicoeur M. A dynamic metabolic flux analysis of myeloid-derived suppressor cells confirms immunosuppression-related metabolic plasticity. Sci Rep. 2017;7:9850.
Jian SL, Chen WW, Su YC, Su YW, Chuang TH, Hsu SC, et al. Glycolysis regulates the expansion of myeloid-derived suppressor cells in tumor-bearing hosts through prevention of ROS-mediated apoptosis. Cell Death Dis. 2017;8:e2779.
Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol. 2021;21:485–98.
Watson MJ, Vignali PDA, Mullett SJ, Overacre-Delgoffe AE, Peralta RM, Grebinoski S, et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature. 2021;591:645–51.
Luan B, Yoon YS, Le Lay J, Kaestner KH, Hedrick S, Montminy M. CREB pathway links PGE2 signaling with macrophage polarization. Proc Natl Acad Sci USA. 2015;112:15642–7.
Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–20.
Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–303.
Veglia F, Tyurin VA, Blasi M, De Leo A, Kossenkov AV, Donthireddy L, et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature. 2019;569:73–8.
Su P, Wang Q, Bi E, Ma X, Liu L, Yang M, et al. Enhanced lipid accumulation and metabolism are required for the differentiation and activation of tumor-associated macrophages. Cancer Res. 2020;80:1438–50.
Friedrich M, Sankowski R, Bunse L, Kilian M, Green E, Ramallo Guevara C, et al. Tryptophan metabolism drives dynamic immunosuppressive myeloid states in IDH-mutant gliomas. Nat Cancer. 2021;2:723–40.
Wu Y, Lin JY, Zhou YD, Liu HJ, Lu SX, Zhang XK, et al. Oncolytic peptide-nanoplatform drives oncoimmune response and reverses adenosine-induced immunosuppressive tumor microenvironment. Adv Healthc Mater. 2024:e2303445.
Shi C, Chen M, Li X, Fu Y, Yang D, Wen T, et al. ATP-adenosine axis regulation combined with microneedle assisted photoimmunotherapy to boost the immunotherapy efficiency. J Control Release. 2024;367:1–12.
Gutiérrez-Vázquez C, Quintana FJ. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity. 2018;48:19–33.
Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203.
Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–8.
Wang C, Ye Z, Kijlstra A, Zhou Y, Yang P. Activation of the aryl hydrocarbon receptor affects activation and function of human monocyte-derived dendritic cells. Clin Exp Immunol. 2014;177:521–30.
Botticelli A, Mezi S, Pomati G, Cerbelli B, Cerbelli E, Roberto M, et al. Tryptophan catabolism as immune mechanism of primary resistance to Anti-PD-1. Front Immunol. 2020;11:1243.
Salani B, Marini C, Rio AD, Ravera S, Massollo M, Orengo AM, et al. Metformin impairs glucose consumption and survival in Calu-1 cells by direct inhibition of hexokinase-II. Sci Rep. 2013;3:2070.
Landau BR, Laszlo J, Stengle J, Burk D. Certain metabolic and pharmacologic effects in cancer patients given infusions of 2-deoxy-D-glucose. J Natl Cancer Inst. 1958;21:485–94.
Ramachandran S, Pan CQ, Zimmermann SC, Duvall B, Tsukamoto T, Low BC, et al. Structural basis for exploring the allosteric inhibition of human kidney type glutaminase. Oncotarget. 2016;7:57943–54.
Lemberg KM, Vornov JJ, Rais R, Slusher BS. We’re not “DON” yet: optimal dosing and prodrug delivery of 6-Diazo-5-oxo-L-norleucine. Mol Cancer Ther. 2018;17:1824–32.
Tebbe C, Chhina J, Dar SA, Sarigiannis K, Giri S, Munkarah AR, et al. Metformin limits the adipocyte tumor-promoting effect on ovarian cancer. Oncotarget. 2014;5:4746–64.
Smyth L, Blunt DN, Gatov E, Nagamuthu C, Croxford R, Mozessohn L, et al. Statin and cyclooxygenase-2 inhibitors improve survival in newly diagnosed diffuse large B-cell lymphoma: a large population-based study of 4913 subjects. Br J Haematol. 2020;191:396–404.
Chen F, Chen J, Yang L, Liu J, Zhang X, Zhang Y, et al. Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat Cell Biol. 2019;21:498–510.
Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C, et al. JAK/STAT3-regulated fatty acid β-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab. 2018;27:1357.
Han S, Wei R, Zhang X, Jiang N, Fan M, Huang JH, et al. CPT1A/2-mediated FAO enhancement-a metabolic target in radioresistant breast cancer. Front Oncol. 2019;9:1201.
Zadra G, Ribeiro CF, Chetta P, Ho Y, Cacciatore S, Gao X, et al. Inhibition of de novo lipogenesis targets androgen receptor signaling in castration-resistant prostate cancer. Proc Natl Acad Sci USA. 2019;116:631–40.
Kidd JG. Regression of transplanted lymphomas induced in vivo by means of normal guinea pig serum. I. Course of transplanted cancers of various kinds in mice and rats given guinea pig serum, horse serum, or rabbit serum. J Exp Med. 1953;98:565–82.
Broome JD. Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects. I. Properties of the L-asparaginase of guinea pig serum in relation to those of the antilymphoma substance. J Exp Med. 1963;118:99–120.
Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:796–807.
Zhu AX, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: the phase 3 randomized clinical claridhy trial. JAMA Oncol. 2021;7:1669–77.
Opitz CA, Somarribas Patterson LF, Mohapatra SR, Dewi DL, Sadik A, Platten M, et al. The therapeutic potential of targeting tryptophan catabolism in cancer. Br J Cancer. 2020;122:30–44.
Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Wagner BA, Cramer-Morales KL, Furqan M, et al. O2- and H2O2- mediated disruption of Fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell. 2017;31:487–500.e8.
Sahu A, Wang X, Munson P, Klomp JPG, Wang X, Gu SS, et al. Discovery of targets for immune-metabolic antitumor drugs identifies estrogen-related receptor alpha. Cancer Discov. 2023;13:672–701.
Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest. 2013;123:4479–88.
Crompton JG, Sukumar M, Roychoudhuri R, Clever D, Gros A, Eil RL, et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 2015;75:296–305.
Buck MD, O’Sullivan D, Klein Geltink RI, Curtis JD, Chang CH, Sanin DE, et al. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell. 2016;166:63–76.
Nabe S, Yamada T, Suzuki J, Toriyama K, Yasuoka T, Kuwahara M, et al. Reinforce the antitumor activity of CD8+ T cells via glutamine restriction. Cancer Sci. 2018;109:3737–50.
Leone RD, Powell JD. Metabolism of immune cells in cancer. Nat Rev Cancer. 2020;20:516–31.
Allard B, Allard D, Buisseret L, Stagg J. The adenosine pathway in immuno-oncology. Nat Rev Clin Oncol. 2020;17:611–29.
Beavis PA, Henderson MA, Giuffrida L, Mills JK, Sek K, Cross RS, et al. Targeting the adenosine 2A receptor enhances chimeric antigen receptor T cell efficacy. J Clin Invest. 2017;127:929–41.
Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T, et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013;13:89.
Ganapathy-Kanniappan S, Geschwind JF, Kunjithapatham R, Buijs M, Vossen JA, Tchernyshyov I, et al. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is pyruvylated during 3-bromopyruvate mediated cancer cell death. Anticancer Res. 2009;29:4909–18.
Chan DA, Sutphin PD, Nguyen P, Turcotte S, Lai EW, Banh A, et al. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med. 2011;3:94ra70.
Siebeneicher H, Cleve A, Rehwinkel H, Neuhaus R, Heisler I, Müller T, et al. Identification and optimization of the first highly selective GLUT1 inhibitor BAY-876. ChemMedChem. 2016;11:2261–71.
Reckzeh ES, Karageorgis G, Schwalfenberg M, Ceballos J, Nowacki J, Stroet MCM, et al. Inhibition of glucose transporters and glutaminase synergistically impairs tumor cell growth. Cell Chem Biol. 2019;26:1214–1228.e25.
Wei C, Bajpai R, Sharma H, Heitmeier M, Jain AD, Matulis SM, et al. Development of GLUT4-selective antagonists for multiple myeloma therapy. Eur J Med Chem. 2017;139:573–86.
Curtis NJ, Mooney L, Hopcroft L, Michopoulos F, Whalley N, Zhong H, et al. Pre-clinical pharmacology of AZD3965, a selective inhibitor of MCT1: DLBCL, NHL and Burkitt’s lymphoma anti-tumor activity. Oncotarget. 2017;8:69219–36.
Guan X, Morris ME. In vitro and in vivo efficacy of AZD3965 and alpha-cyano-4-hydroxycinnamic acid in the murine 4T1 breast tumor model. AAPS J. 2020;22:84.
Billiard J, Dennison JB, Briand J, Annan RS, Chai D, Colón M, et al. Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells. Cancer Metab. 2013;1:19.
Rais R, Lemberg KM, Tenora L, Arwood ML, Pal A, Alt J, et al. Discovery of DRP-104, a tumor-targeted metabolic inhibitor prodrug. Sci Adv. 2022;8:eabq5925.
Mullard A. FDA approves first-in-class cancer metabolism drug. Nat Rev Drug Discov. 2017;16:593.
Lee JS, Kang JH, Lee SH, Hong D, Son J, Hong KM, et al. Dual targeting of glutaminase 1 and thymidylate synthase elicits death synergistically in NSCLC. Cell Death Dis. 2016;7:e2511.
Schulte ML, Fu A, Zhao P, Li J, Geng L, Smith ST, et al. Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat Med. 2018;24:194–202.
Edwards DN, Ngwa VM, Raybuck AL, Wang S, Hwang Y, Kim LC, et al. Selective glutamine metabolism inhibition in tumor cells improves antitumor T lymphocyte activity in triple-negative breast cancer. J Clin Invest. 2021;131:e140100.
Jin H, Wang S, Zaal EA, Wang C, Wu H, Bosma A, et al. A powerful drug combination strategy targeting glutamine addiction for the treatment of human liver cancer. Elife. 2020;9:e56749.
Alli PM, Pinn ML, Jaffee EM, McFadden JM, Kuhajda FP. Fatty acid synthase inhibitors are chemopreventive for mammary cancer in neu-N transgenic mice. Oncogene. 2005;24:39–46.
Zaytseva YY, Rychahou PG, Le AT, Scott TL, Flight RM, Kim JT, et al. Preclinical evaluation of novel fatty acid synthase inhibitors in primary colorectal cancer cells and a patient-derived xenograft model of colorectal cancer. Oncotarget. 2018;9:24787–800.
Verrelli D, Dallera L, Stendardo M, Monzani S, Pasqualato S, Giorgio M, et al. Hydroxycitric acid inhibits chronic myelogenous leukemia growth through activation of AMPK and mTOR pathway. Nutrients. 2022;14:2669.
Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–21.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82322073, 82173846, 82304790), China Postdoctoral Innovative Talent Support Program (BX20220213), Oriental Scholars of Shanghai Universities (TP2022081), Jiangxi Province Thousand Talents Program (jxsq2023102168), Young Talent Lifting Project of China Association of Chinese Medicine [CACM-(2021-QNRC2-A08)], Shanghai Rising-Star Program (22QA1409100), 2021 Shanghai Science and Technology Innovation Action Plan (21S11902800), Three-year Action Plan for Shanghai TCM Development and Inheritance Program [ZY (2021-2023)-0401; ZY (2021-2023)-0208], Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-D-202004), CAMS Innovation Fund for Medical Sciences (CIFMS) (2023-I2M-3-009), Key Project at Central Government Level: The ability establishment of sustainable use for valuable Chinese medicine resources (2060302), High Level Key Discipline of National Administration of Traditional Chinese Medicine (71), Innovation team of high-level local universities in Shanghai: Strategic Innovation Team of TCM Chemical Biology, Shanghai Sailing Program (22YF1445000, 23YF1442600). We acknowledge the online drawing software for figure creation (BioRender, https://biorender.com).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, Xh., Chen, Xy., Yan, Y. et al. Targeting metabolism to enhance immunotherapy within tumor microenvironment. Acta Pharmacol Sin (2024). https://doi.org/10.1038/s41401-024-01304-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-024-01304-w
- Springer Nature Singapore Pte Ltd.