Abstract
Steroid remains the keystone therapy for Idiopathic Nephrotic Syndrome (NS). Besides genetic factors and histological changes, pharmacogenomic factors also affect the steroid response. The upregulation of P-glycoprotein (P-gp) and Multidrug resistance-associated protein 1 (MRP-1) modulate the pharmacokinetics of steroids and may contribute to steroid resistance. Flow-cytometric analysis of P-gp, MRP-1 expression and functional activity on peripheral blood mononuclear cells (PBMCs) was carried out in steroid-sensitive nephrotic syndrome (SSNS) (n = 171, male 103, mean age = 8.54 ± 4.3); and steroid-resistant nephrotic syndrome (SRNS) (n = 83, male 43, mean age = 7.43 ± 4.6) patients. The genotypings of MDR-1 gene were carried out using PCR-RFLP. We observed that the percentage expression of P-gp (10.01 ± 2.09 and 3.79 ± 1.13, p < 0.001); and MRP-1 (15.91 ± 3.99 and 7.40 ± 2.33, p < 0.001) on lymphocyte gated population were significantly higher in SRNS than that of SSNS. The functional activity of P-gp and MRP-1 was also significantly escalated in SRNS as compared to SSNS (68.10 ± 13.35 and 28.93 ± 7.57, p < 0.001); (72.13 ± 8.34 and 31.56 ± 8.65, p < 0.001) respectively. AUC-ROC curve analysis revealed that P-gp and MRP-1 expression with a cut-off value of 7.13% and 9.62% predicted SRNS with the sensitivity of 90% and 80.7%; and specificity 90% and 80%, respectively. Moreover, MDR-1 homozygous mutant TT+AA for G2677T/A (rs2032582) was significantly associated with SRNS (p = 0.025, OR = 2.86 CI = 1.14–7.14). The expression of P-gp (9.68 ± 4.99 v/s 5.88 ± 3.38, p = 0.002) was significantly higher in the patients of homozygous mutant alleles compared to wildtype GG. The increased expression and functionality of P-gp and MRP-1 contribute to steroid resistance, and MDR-1 homozygous mutant G2677T/A promotes steroid resistance by inducing P-gp expression in NS.
Similar content being viewed by others
Introduction
Idiopathic Nephrotic Syndrome (NS) is one of the most common chronic glomerular disorders characterized by alteration in permeability of the capillary of glomeruli, resulting in the excessive loss of proteins in urine. It manifests clinically by generalized edema, hypoalbuminemia (<2.5 g/dL), and proteinuria >40 mg /m2/h [1].
The exact pathogenesis of NS is not clear; however, alterations in T cell phenotypes have been reported in NS patients [2,3,4,5]. It has been postulated that in NS, there is an imbalance in Th1 and Th2 cytokines with an increase in cytokines secreted by activated Th2 that alter the glomerular permeability [6].
Steroid is the mainstay of therapy for NS patients. The International Study of Kidney Disease in Children established prednisolone as the standard initial treatment of idiopathic NS [7]. In addition to immunosuppressive effects and countering inflammatory cytokines signaling, steroids act directly through glucocorticoid receptors (GR) in podocytes [8]. Steroid exerts its effects either by up-regulating the expression of genes of anti-inflammatory cytokines [9] or by down-regulation of pro-inflammatory transcription factors, such as nuclear factor-κB complex [10]. The adequate bioavailability of glucocorticoids inside the cell is necessary to suppress the pro-inflammatory gene expression. Depending on the response to steroid, NS patients can be broadly categorized into two groups: steroid -sensitive nephrotic syndrome (SSNS) and steroid-resistant nephrotic syndrome (SRNS). Identification of SRNS patients, especially if they don’t have secondary or genetic causes of NS, is challenging. Many SRNS patients respond to the addition of calcineurin inhibitors (CNIs) to the treatment regimen [11].
P-glycoprotein (P-gp) and Multi-Drug Resistant Associated protein -1 (MRP-1) are two energy-dependent efflux pumps, which are associated with steroid non-responsiveness in autoimmune diseases like systemic lupus erythematosus [12] as well as NS patients [13, 14]. It has been reported that overexpression of both P-gp and MRP-1 is associated with drug resistance in renal cell carcinoma and other cancer treatment [15]. As steroids are substrate for P-gp and MRP-1, the overexpression or overactivity of these pumps may reduce the bioavailability of steroids at the site of actions in the immune cells leading to SRNS state and the addition of CNIs may enhance the response of the steroid [11]. In addition to the immunosuppressive effect, CNIs act directly through calcineurin receptors on podocyte to induce remission in NS [16]. However, there is another pharmacogenomic aspect of CNI action that it acts as an inhibitor of P-gp and increases the bioavailability of glucocorticoids inside the immune cells, including T-cells, thereby enhancing the response to glucocorticoids [17]. This could be the reason why many SRNS patients become steroid-responsive after the addition of CNI.
It has been observed that there are genetic variations linked with steroid resistance [18]. In our previous study, we have observed increased expression of MDR-1 gene polymorphism (2677A/T) in SRNS patients [19]. Herein, we investigated the expression and functional activity of P-gp and MRP-1 on peripheral blood mononuclear cells along with single nucleotide polymorphisms (SNPs) of the MDR-1 gene; C3435T, C1236T, and G2677A/T, with an attempt to identify SRNS patients.
Materials and methods
Patients recruitment criteria
In the present study, SSNS (n = 171) and SRNS (n = 83) subjects were included. Children of <2 years and >16 years, and those with a family history of NS, secondary and genetic causes of NS were excluded. Patients enrolled in this study did not have any (i) underlying secondary causes; they were negative for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus infection. All had normal serum complement (C3 and C4) levels.
Sample size was calculated using OSSE online sample size calculator accessed an 20th Sep 2010 (http://osse.bii.a-star.edu.sg/calculation1.php) with an assumption; desired power 80%, significance level 5%, minor allele frequency in steroid resistance cases 17.70% (data from preliminary study), minor allele frequency in steroid sensitive cases 5.80% (data from preliminary study) and ratio of steroid resistance to steroid sensitive cases as 1:2. With these assumptions a total of 83 cases of steroid resistance and 171 cases of steroid sensitive nephrotic syndromes was required for the study. A total of 256 patients with NS was required for the study.
NS in children was defined as proteinuria of 40 mg/m2/h or a ratio of 2 for spot urine protein (milligram)/creatinine (milligram) in the first morning urine sample with hypoalbuminemia (serum albumin <2.5 g/dL) and presence of edema. All children were started with the prednisolone dose of 60 mg/m2daily. Children who did not achieve complete or partial remission after four weeks of steroid therapy were defined as SRNS. Remission of NS was defined by urinary protein excretion <4 mg/m2/h or urine dipstick nil/trace for 3 consecutive days. All SRNS patients were biopsied and subjected to light microscopy, immunofluorescence, and electron microscopy to confirm minimal change disease. At the end of 4 weeks of therapy, blood samples from all children were collected for peripheral blood mononuclear cell isolation, flow-cytometric analysis, the functional assay for -P-gp and MRP-1, and genetic analysis.
Informed written consent was obtained from a parent or guardian of patients when participants were <15 years and from the participant when age was >15 years as per institute guidelines. This study was approved by the Institute Ethics Committee (Research Protocol No.:A09:PGI/EMP/IEC/52/19/11/2010), Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, India.
Human blood collection and peripheral blood mononuclear cells isolation
Total 10 ml blood (8 ml in heparinized tube and 2 ml in EDTA tube) were collected from all screened patients from the out-patient department of Nephrology of our institute. Peripheral Blood Mononuclear Cells (PBMCs) were isolated by density gradient centrifugation method from human venous blood as described previously [4].
Flow-cytometric analysis
P-gp expression on PBMCs
From the whole blood, P-gp expression was measured by the percentage of P-gp positive cells. 50 µl heparinized blood was incubated in flow tubes with 20 µl PE-conjugated human anti-P-gp mAb (BD Pharmingen CA, USA), appropriate 20 µl PE-conjugated matched isotype control samples were incubated in separate tubes. Both the tubes were incubated in dark for 30 min. Then, RBCs were lysed by incubation in 1 ml 1X BD FACS lysing solution for 10 min and washed twice in phosphate-buffered saline (PBS). Later, a minimum of 10,000 events was acquired and analyzed on FACS Canto flow-cytometer (Becton Dickinson, Mount View, CA, USA). Lymphocytes were gated by using forward scatter and side scatter, the P-gp positivity was evaluated by gating the P-gp positive cells on lymphocytes. The gating strategy for P-gp is shown in Supplementary Fig. 1.
MRP-1 expression on PBMCs
MRP-1 expression was quantified by the percentage of MRP-1 positive cells. RBCs were lysed by incubation in 1 ml 1X BD FACS lysing solution for 10 min of 50 µl heparinized blood. Cells were then washed, fixed, and permeabilized with Cytofix/Cytoperm kit (BD Biosciences, San Diego, CA, USA) according to the manufacturer’s instruction. After that, 20 µl PE-conjugated human anti-MRP-1mAb (R&D System, Minneapolis, Minnesota, USA) was incubated in the dark for 30 min at room temperature. Appropriate 20 µl PE-conjugated matched isotype control samples were incubated in separate tubes. After incubation, both the tubes were washed twice in PBS. Then cells were acquired and analyzed on the FACS Canto flow-cytometer (Becton Dickinson, Mount View, CA, USA). Lymphocytes were gated by using forward scatter and side scatter, and the MRP-1 positivity was evaluated by gating the MRP-1 positive cells on lymphocytes. The gating strategy for MRP-1 is shown in Supplementary Fig. 2. The details of the gating strategies have also been shown in our previous study [4].
Functional assay of P-gp and MRP-1
PBMCs were isolated from the heparinized blood samples by the Ficoll-hypaque density gradient method. Cell viability was determined by trypan blue staining (Gibco, Auckland, New Zealand), and cells were counted on hemocytometer as per the standard procedure.
The functional activity of P-gp and MRP-1 was measured by EFLUXX-ID® Green multidrug resistance assay kit (Enzo Life Sciences, Farmingdale, New York, USA). Then the cell suspension was subjected to flow-cytometric assay for the evaluation of the functional activity of P-gp and MRP-1. Multi Resistance Activity Factor (MAF) for each transporter were calculated using the formula (MAF Pgp = (100*F Pgp-F0)/Fpgp). Functionality of P-gp and MRP-1 was analyzed in duplicate for each patient. The lymphocytes were gated in forward and side scatter plot, whereas the functionality was assessed in FITC tagged dye. The gating strategy for functionality of P-gp and MRP-1 is shown in Supplementary Fig. 3.
Genotyping
DNA was extracted by a commercially available kit (QIAGEN, QIAamp DNA mini kit, USA). The genetic variants of the MDR-1 gene were genotyped by using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP), as described earlier [20]. All MDR-1 gene SNPs (C3435T, C1236T, and G2677A/T) were genotyped in triplicate.
Reagents
PE Mouse Anti-Human P-gp Antibody (Cat. No. 340555; BD Biosciences, San Jose, CA 95131 USA); PE Mouse IgG1 Isotype (Cat. No. 349043; BD PharmingenTM, BD Biosciences, San Jose, CA 95131 USA); Human MRP1 PE-Conjugated Antibody (Cat. No. IC1929P; R&D Systems, MN 55413, USA); Mouse IgG1 PE-Conjugated Antibody (Cat. No. IC002P; R&D Systems, MN 55413, USA); EFLUXX-ID® Green multidrug resistance assay kit (Cat No. ENZ-51029-K100; Enzo Life Sciences, Farmingdale, New York, USA); Histopaque®-1077 (Cat No. 107710; United Kingdome); BD Fixation/Permeabilization Solution Kit with GolgiStop™ (Cat. No. 554715; BD Biosciences, San Diego, USA); Lysing Solution 10X™ (Cat. No. 349202; BD Biosciences, CA 95131, USA); GibcoTM Trypan Blue Solution 0.4% (Cat. No. 15-250-061; Gibco, Aucckland, New Zealand); QIAamp DNA Blood Mini Kit [50] (Cat.No.51104; Germany); HaeIII-15000, units (Cat. No. R0108L; New England Biolabs, USA); Sau3AI-1000 units (Cat. No. R0169L; New England Biolabs, USA); Rsal-5,000 units (Cat. No. R0167L; New England Biolabs, USA); Banl-5000 units (Cat. No. R0118S; New England Biolabs, USA); Deoxynucleotide (dNTP) Solution Mix (Cat. No. N04475; New England Biolabs, USA); Taq DNA Polymerase with Standard Taq Buffer-2,000 unit (Cat. No. M0273L; New England Biolabs, USA).
Statistical analysis
The values of continuous data were presented in mean ± standard deviation, analyzed by t-test. Categorical variable data were analyzed using the chi-square test. The continuous data were considered to be normally distributed if standard deviation ≤ ½ of the mean value. The SNPs were analyzed for their association with SRNS, by using SPSS 20.0 statistical package (SPSS Inc., Chicago, IL, USA). The association was determined by binary logistic regression analysis. The risks conferred by alleles were evaluated by calculating the odds ratio (OR) and 95% confidence interval. The Area Under the Receiver Operating Characteristics (AUC-ROC) curve was constructed to determine the cut-off values of P-gp and MRP-1 expression to predict the sensitivity and specificity of SRNS patients curve. The statistical significance was considered p < 0.05.
Results
Two hundred fifty-four patients fulfilled the inclusion criteria for the study. One hundred seventy-one patients who responded to steroid therapy were categorized as SSNS and 83 patients as SRNS. The demographic and clinical details of the patients are mentioned in Table 1.
Expression of P-gp and MRP-1 on lymphocytes from NS patients
Expression of P-gp (3.79 ± 1.13 and 10.01 ± 2.09, p < 0.001) and MRP-1 (7.40 ± 2.33 and 15.91 ± 3.99, p < 0.001) were significantly lower in SSNS patients as compared to that of SRNS patients. (Fig. 1A).
Functional activity of P-gp and MRP-1 efflux pumps in lymphocytes of NS patients
There was a significant decrease in the functional activity of P-gp (28.93 ± 7.57 and 68.10 ± 13.35, p < 0.001) and MRP-1 (31.56 ± 8.65 and 72.13 ± 8.34, p < 0.001) in SSNS patients as compared to SRNS patients. (Fig. 1B).
Area under -receiver operating characteristic (AUC-ROC) curve analysis
AUC-ROC curve showed P-gp expression on PBMC with a cut-off value of 7.13% predicted steroid resistance with a sensitivity of 86.4% and specificity 90% (Fig. 2A). Similarly, the MRP-1 percentage of 9.62% predicted steroid resistance with a sensitivity of 80.7% and specificity of 80% (Fig. 2B).
MDR-1 SNPs genotypes and alleles in patients with SSNS v/s patients with SSNS
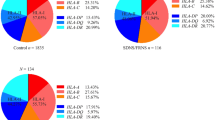
Homozygous mutant allele 2677 TT + AA was significantly associated with SRNS (p = 0.025, OR = 2.86 CI = 1.14–7.14). However, heterozygous (GT + GA) and mutant alleles (T + A) were not significantly associated with SRNS patients as compared to SSNS patients. In terms of the C3435T and C1236T SNPs, there were no differences in the frequency distribution of genotypes and alleles between patients with SSNS and patients with SRNS. (Table 2).
Genetic association of G2677T/A with P-gp expression
In our data set, the expression of P-gp (9.68 ± 4.99 v/s 5.88 ± 3.38, p = 0.002) was significantly higher in the patients of homozygous mutant alleles compared to wildtype GG. (Fig. 3).
Discussion
The data of the present study revealed that there is increased expression and function of two efflux pumps P-gp and MRP-1 and the presence of homozygous mutant genotype G2677T/A (TT + AA) in the MDR-1 gene identifies NS patients with steroid resistance. Expression of P-gp (7.13%) and MRP-1 (9.62%) predicted steroid resistance with a sensitivity of 86.4% and 80.7% and specificity of 90% and 80%, respectively. In addition, homozygous mutant genotype G2677T/A (TT+AA) predicted the steroid resistance with high sensitivity and specificity. This may help in early identification of SRNS patients.
Management of SRNS patients is a major challenge faced by the clinicians, and it remains an area of concern because of futile use of steroid in case of resistance, and unnecessary steroid toxicities produced in children. Steroids are one of the major substrates for P-gp and MRP-1, and overexpression of these may reduce the bioavailability of steroids to the cells, and may be associated with the low response of steroid in NS [14]. It has been reported that P-gp overexpression on CD3 lymphocytes was associated with poor response to steroids [13]; similarly, Youssef et al. in a small study reported a significant increase in MDR1 gene expression in peripheral blood lymphocytes and serum IL2r levels in the patients with SRNS than that of SSNS patients, which is in agreement with our study [21]. Another study reported that an increase in MDR-1 gene expression in PBMCs of patients who did not respond to steroids or relapsed during treatment with steroids [22]. However, the present study carries edge over the previous studies as besides the expression of P-gp and MRP-1, the functionality of the efflux pumps were also studied and found to be significantly increased in SRNS patients as compared to SSNS patients.
MRP-1 is expressed abundantly throughout the body with relatively high levels found in the lung, testis, kidneys, skeletal muscle, and PBMCs [23,24,25]. Human kidney glomeruli also express MRP-1, where it serves to protect these cells from the toxic effects of exogenous and endogenous compounds [26]. MRP-1 overexpression has been reported in many cancer cell lines [27, 28]. In the present study, we observed that the expression and functionality of MRP-1 on PBMCs were increased in patients with SRNS. Further studies are needed to confirm its exact role in developing resistance to steroid in SRNS. To the best of our knowledge, this is the first report of MRP-1 overexpression and functionality in childhood NS patients.
In our previous work, we have identified a reciprocal relationship between P-gp and histone deacetylase-2 and its role in SRNS and SSNS patients in a small longitudinal study [29]. In addition to drug efflux pumps, histone deacetylation may affect pro-inflammatory cytokines genes expression by tightly wrapping the chromatin on the histones and thus closing access of the transcription factors to the promoter regions of the cytokine genes. The significance of the previous work lies in the fact that either P-gp or HDAC-2 modulators may be tried as an adjunct in the treatment of SRNS patients [30]. However, the present study is aimed to identify patients who might not respond to steroids right as there is no biomarker available for it, at present. ROC curve analysis of P-gp and MRP-1 in the present study for the first time identifies a cut-off of both P-gp and MRP-1 that delineates resistance with high sensitivity and specificity. In addition, identification of MDR-1 gene homozygous mutant SNP G2677T/A (TT+AA) also identifies NS patients with steroid resistance [31]. Similar to our previous observation, SNP G2677T/A (TT+AA) is associated with increased expression of P-gp in the present study as well.
Our study carries translational potential, drugs that target P-gp like CNIs (cyclosporine-A, and tacrolimus), metformin, clarithromycin, ketoconazole, proton pump inhibitors (omeprazole, lansoprazole, and others), and verapamil may have potential to modulate the P-gp expression and function. Thus, it may be useful adjunct in treatment of SRNS patients. Tacrolimus directly binds and blocks the function of P-gp and reduces the IL-2 mediated MDR-1 gene expression [32]. Other than immunosuppressive effect and action through calcineurin receptors on podocytes, the CNIs act through inhibition of P-gp and enhances steroid response in SRNS patients [33]. Moreover, P-gp requires a constant supply of ATP for its functioning whereas Metformin inhibits P-gp expression by depletion of ATP. Metformin also reduces NF κβ via AMP kinase and inhibits transcription of P-gp [34]. Similarly, MRP-1 inhibitors, too, may be exploited for potential usage in SRNS.
The strength of our study is that we have recruited a large sample size, and for the first time, we have tried to calculate a cut-off value of expression of P-gp and MRP-1 for predicting the steroid responsiveness. Lack of longitudinal follow-up to assess the association between expression and functions of multidrug resistance proteins and MDR-1 gene polymorphism remains a limitation of the study.
Thus, we conclude that the expression and function of MDR efflux pumps (P-gp and MRP-1) may contribute to steroid resistance in NS children, and the presence of MDR-1 SNP G2677T/A may confer SRNS phenotype by inducing expression of P-gp on PBMCs.
References
McBryde KD, Kershaw DB, Smoyer WE. Pediatric steroid-resistant nephrotic syndrome. Curr Probl Pediatr Adolesc Health Care. 2001;31:280–307.
Frank C, Herrmann M, Fernandez S, Dirnecker D, Boswald M, Kolowos W, et al. Dominant T cells in idiopathic nephrotic syndrome of childhood. Kidney Int. 2000;57:510–7.
Colucci M, Corpetti G, Emma F, Vivarelli M. Immunology of idiopathic nephrotic syndrome. Pediatr Nephrol. 2018;33:573–84.
Prasad N, Jaiswal AK, Agarwal V, Yadav B, Sharma RK, Rai M, et al. Differential alteration in peripheral T-regulatory and T-effector cells with change in P-glycoprotein expression in Childhood Nephrotic Syndrome: a longitudinal study. Cytokine. 2015;72:190–6.
Liu LL, Qin Y, Cai JF, Wang HY, Tao JL, Li H, et al. Th17/Treg imbalance in adult patients with minimal change nephrotic syndrome. Clin Immunol. 2011;139:314–20.
Singh VK, Mehrotra S, Agarwal SS. The paradigm of Th1 and Th2 cytokines: its relevance to autoimmunity and allergy. Immunol Res. 1999;20:147–61.
Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A Report of the International Study of Kidney Disease in Children. Kidney Int. 1981;20:765–71.
Goodwin JE. Role of the glucocorticoid receptor in glomerular disease. Am J Physiol Ren Physiol. 2019;317:F133–F6.
Hebbar PB, Archer TK. Chromatin remodeling by nuclear receptors. Chromosoma. 2003;111:495–504.
McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–59.
Tullus K, Webb H, Bagga A. Management of steroid-resistant nephrotic syndrome in children and adolescents. Lancet Child Adolesc Health. 2018;2:880–90.
Kansal A, Tripathi D, Rai MK, Agarwal V. Persistent expression and function of P-glycoprotein on peripheral blood lymphocytes identifies corticosteroid resistance in patients with systemic lupus erythematosus. Clin Rheumatol. 2016;35:341–9.
Badr HS, El-Hawy MA, Helwa MA. P-Glycoprotein Activity in Steroid-Responsive vs. Steroid-Resistant Nephrotic Syndrome. Indian J Pediatr. 2016;83:1222–6.
Wasilewska AM, Zoch-Zwierz WM, Pietruczuk M. Expression of P-glycoprotein in lymphocytes of children with nephrotic syndrome treated with glucocorticoids. Eur J Pediatr. 2006;165:839–44.
Walsh N, Larkin A, Kennedy S, Connolly L, Ballot J, Ooi W, et al. Expression of multidrug resistance markers ABCB1 (MDR-1/P-gp) and ABCC1 (MRP-1) in renal cell carcinoma. BMC Urol. 2009;9:6.
Muller-Deile J, Schiffer M. Podocyte directed therapy of nephrotic syndrome-can we bring the inside out? Pediatr Nephrol. 2016;31:393–405.
Parasrampuria DA, Lantz MV, Birnbaum JL, Vincenti FG, Benet LZ. Effect of calcineurin inhibitor therapy on P-gp expression and function in lymphocytes of renal transplant patients: a preliminary evaluation. J Clin Pharm. 2002;42:304–11.
Kara A, Gurgoze MK, Kara M, Aydin M. Evaluation of Genetic Polymorphisms for Determining Steroid Response in Nephrotic Children. Ann Clin Lab Sci. 2018;48:478–83.
Jafar T, Prasad N, Agarwal V, Mahdi A, Gupta A, Sharma RK, et al. MDR-1 gene polymorphisms in steroid-responsive versus steroid-resistant nephrotic syndrome in children. Nephrol Dial Transpl. 2011;26:3968–74.
Prasad S, Tripathi D, Rai MK, Aggarwal S, Mittal B, Agarwal V. Multidrug resistance protein-1 expression, function and polymorphisms in patients with rheumatoid arthritis not responding to methotrexate. Int J Rheum Dis. 2014;17:878–86.
Youssef DM, Elbehidy RM, Abdelhalim HS, Amr GE. Soluble interleukine-2 receptor and MDR1 gene expression levels as inflammatory biomarkers for prediction of steroid response in children with nephrotic syndrome. Iran J Kidney Dis. 2011;5:154–61.
Funaki S, Takahashi S, Wada N, Murakami H, Harada K. Multiple drug-resistant gene 1 in children with steroid-sensitive nephrotic syndrome. Pediatr Int. 2008;50:159–61.
Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–4.
Flens MJ, Zaman GJ, van der Valk P, Izquierdo MA, Schroeijers AB, Scheffer GL, et al. Tissue distribution of the multidrug resistance protein. Am J Pathol. 1996;148:1237–47.
Stride BD, Valdimarsson G, Gerlach JH, Wilson GM, Cole SP, Deeley RG. Structure and expression of the messenger RNA encoding the murine multidrug resistance protein, an ATP-binding cassette transporter. Mol Pharm. 1996;49:962–71.
Peng KC, Cluzeaud F, Bens M, Duong Van Huyen JP, Wioland MA, Lacave R, et al. Tissue and cell distribution of the multidrug resistance-associated protein (MRP) in mouse intestine and kidney. J Histochem Cytochem. 1999;47:757–68.
Valera ET, Scrideli CA, Queiroz RG, Mori BM, Tone LG. Multiple drug resistance protein (MDR-1), multidrug resistance-related protein (MRP) and lung resistance protein (LRP) gene expression in childhood acute lymphoblastic leukemia. Sao Paulo Med J. 2004;122:166–71.
den Boer ML, Pieters R, Kazemier KM, Janka-Schaub GE, Henze G, Veerman AJ. The modulating effect of PSC 833, cyclosporin A, verapamil and genistein on in vitro cytotoxicity and intracellular content of daunorubicin in childhood acute lymphoblastic leukemia. Leukemia. 1998;12:912–20.
Singh H, Prasad N, Misra DP, Jaiswal AK, Agarwal V. P-glycoprotein and/or Histone Deacetylase 2 Regulates Steroid Responsiveness in Childhood Nephrotic Syndrome. Indian. J Rheumatol. 2020;15:5–10.
Chowdhary VR. When doing the right thing is wrong–drug efflux pumps in steroid-resistant nephrotic syndrome. Indian J Rheumatol. 2020;15:1.
Cizmarikova M, Podracka L, Klimcakova L, Habalova V, Boor A, Mojzis J, et al. MDR1 polymorphisms and idiopathic nephrotic syndrome in Slovak children: preliminary results. Med Sci Monit. 2015;21:59–68.
Belliard AM, Tardivel S, Farinotti R, Lacour B, Leroy C. Effect of hr-IL2 treatment on intestinal P-glycoprotein expression and activity in Caco-2 cells. J Pharm Pharm. 2002;54:1103–9.
Gulati S, Prasad N, Sharma RK, Kumar A, Gupta A, Baburaj VP. Tacrolimus: a new therapy for steroid-resistant nephrotic syndrome in children. Nephrol Dial Transpl. 2008;23:910–3.
Kim HG, Hien TT, Han EH, Hwang YP, Choi JH, Kang KW, et al. Metformin inhibits P-glycoprotein expression via the NF-kappaB pathway and CRE transcriptional activity through AMPK activation. Br J Pharm. 2011;162:1096–108.
Acknowledgements
We acknowledge the research grant of Indian Council of Medical Research for the completion of the project GIA/40/2014-DHR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Prasad, N., Singh, H., Jaiswal, A. et al. Overexpression of P-glycoprotein and MRP-1 are pharmacogenomic biomarkers to determine steroid resistant phenotype in childhood idiopathic nephrotic syndrome. Pharmacogenomics J 21, 566–573 (2021). https://doi.org/10.1038/s41397-021-00233-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-021-00233-9
- Springer Nature Limited