Abstract
Ketamine is a noncompetitive N-methyl-D-aspartate antagonist with emerging evidence for use in medically refractory epilepsy. We describe the novel use of low-dose intravenous (IV) ketamine transitioning to enteral formulation in a patient with drug-resistant localization-related refractory epilepsy. We performed a National Library of Medicine (NLM) literature review using search terms “ketamine”, “low dose”, and “seizure” for similar cases, followed by an illustrative clinical case. Our NLM search engine methodology yielded 24 hits, none of which described use of low-dose ketamine for seizures. Anesthetic doses are used for status epilepticus, but we show that in a patient with postoperative worsening of his chronic seizure burden, low-dose IV ketamine can be used to avoid oversedation and intubation. We demonstrate that IV ketamine can be transitioned to oral regimen to shorten length of stay in the intensive care unit and hospital and has future CYP2B6 pharmacogenomic considerations for further dose individualization.
Similar content being viewed by others
Introduction
About 25% of all patients with epilepsy have drug-resistant epilepsy (DRE; also called medically refractory or pharmacoresistant epilepsy), and due to poor seizure control, they have an increased risk of early death, trauma, and psychosocial alterations, diminishing their quality of life [1]. DRE may show a temporary remission period, yet frequently relapse into periods of increased seizure burden, especially when provoked by physical stress, such as surgery [1]. Current Neurocritical Care Society guidelines recommend intravenous (IV) ketamine infusion as an alternative treatment for refractory status epilepticus in adults, and oral ketamine has been used in seven cases of nonconvulsive status epilepticus so far [2]. Few studies have been conducted using ketamine as an alternative agent to treat increased seizure burden in a patient with DRE.
Ketamine is an N-methyl-D-aspartate (NMDA) receptor antagonist not associated with cardiorespiratory depression or hypotension. Many studies have demonstrated the efficacy and safety of ketamine for treating status epilepticus [2,3,4,5,6]. Seizures can be acutely induced by blocking synaptic and voltage-gated inhibitory conductances or by activating synaptic and voltage-gated excitatory conductances [7, 8]. Antiepileptic drugs exert antiseizure effects by reducing the inflowing of positive ions (e.g., Na+ and Ca2+), increasing inhibitory neurotransmitter activity (i.e., γ-aminobutyric acid), and reducing excitatory neurotransmitter activity (i.e., glutamate, aspartate) [7]. Ketamine is a noncompetitive glutamate antagonist and acts at NMDA receptors. If used at sedative or anesthetic doses, it shows anticonvulsive properties [7]. We report a case of successful control of increased seizure frequency in a patient with DRE with low-dose IV ketamine infusion and gradual transition to enteral ketamine without recurrence of seizures or adverse events, and conduct a thorough literature review to find similar reports. Ketamine has cytochrome CYP2B6 predominant pharmacogenomic metabolism.
Patients and methods
National library of medicine search engine methodology
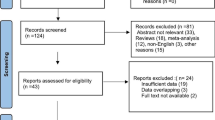
We searched the National Library of Medicine (NLM) using the search terms, “ketamine”, “seizure”, and “low dose”, for number of results using Boolean operator “AND” combination [9, 10]. We reviewed each NLM publication result for use in seizure and status epilepticus with ketamine.
Case presentation
A 21-year-old man with a 4-year history of multifocal DRE presented to Mayo Clinic for resection of a pilocytic astrocytoma that was felt to be one focus of his epilepsy. That same day, he experienced a tonic-clonic seizure for one minute that ceased spontaneously. He was on oral brivaracetam 100 mg twice daily, oral phenobarbital elixir 176 mg twice daily, and oral valproic acid elixir 145 mg in the morning and 80 mg in the evening. At that time, lacosamide 200 mg twice daily was added.
There were no immediate postoperative clinical or radiographic adverse effects, and he was discharged on postoperative day (POD) 3. He returned to the Emergency Department on POD 5 after experiencing 13 seizures at home. He continued to experience seizures for roughly 36 h after readmission, despite loading doses of IV lacosamide and IV fosphenytoin. The patient was then started on IV ketamine at 0.5 mg/kg/h (anesthetic dosing for refractory status epilepticus is 2.5–7.5 mg/kg/h). At this point, his clinical and electrographic seizures ceased.
On hospital day 5 of the patient’s readmission (POD 9), we began weaning the IV ketamine to transfer him out of from the neurocritical care unit, and replaced IV ketamine with oral ketamine, with the goal of achieving a comparable total daily dose. The starting oral dose was 50 mg in the morning and 100 mg in the evening. He experienced two focal seizures with tonic movements at the right upper limb. Over the next 4 days, the oral ketamine was titrated up to 500 mg twice daily as the IV ketamine was weaned off (Table 1). Other than two focal and three nonconvulsive seizures, he complained of cognitive slowing.
These symptoms resolved after we spread out the total daily dosing into 300 mg three times a day on POD 14. He was discharged on POD 16 and remained seizure-free on an oral regimen of ketamine 300 mg three times daily, phenytoin 150 mg three times daily, phenobarbital elixir 176 mg twice daily, and brivaracetam 100 mg twice daily.
Results
Literature search parameters
The NLM search engine methodology yielded only 24 results, of which none were related to human seizures, status epilepticus, or low-dose ketamine use in this indication. Interestingly, low-dose ketamine was reported for depression and analgesia, but not for seizures. A subsequent search of terms, “ketamine”, “pharmacogenomics”, and “seizure”, with combination Boolean “AND”, yielded 0 results. Therefore, the original case presentation appears to represent the first case of low-dose ketamine for status epilepticus and avoided intubation in this case.
Discussion
Ketamine is a noncompetitive NMDA receptor antagonist that inhibits the excitatory effects elicited by glutamate in the central nervous system and promotes activity at cholinergic, muscarinic, monoaminergic, and opioid receptors. Research in animal models show that seizure progression may result by downregulation of the γ-aminobutyric acid-A receptors (neuroinhibitory pathway) and upregulation of the NMDA receptors (neuroexcitatory pathway), making the seizures more refractory and resistant to standard therapy (benzodiazepines and barbiturates) and more responsive to ketamine. Therefore, the effectiveness of benzodiazepines, barbiturates, and propofol may diminish, and seizure breakthrough may occur.
In comparison to other drugs, ketamine has shown more favorable hemodynamic effects and minimal respiratory depression, meaning patients can be maintained on high doses without the need of mechanical ventilation [2, 7]. Furthermore, ketamine’s effects on hemodynamics are often favorable, with increases in blood pressure, heart rate, and cardiac output, which may have vasopressor-sparing effects.
Due to its activity at cholinergic, muscarinic, monoaminergic, and opioid receptors, ketamine can cause some adverse effects that are usually dose dependent. There can be agitation with auditory and visual hallucinations, conscious vivid dreams (more likely to occur in adult and female patients), tachyarrhythmias, and hypersalivation. It is shown that the concomitant administration of benzodiazepines or propofol may lessen the severity of those effects. Hypersalivation can lead to respiratory distress, but anticholinergic medications can prevent this adverse effect.
Ketamine is lipid soluble, which leads to an onset of action with maximal effect in one minute and a quick redistribution into peripheral compartments. It has a half-life of two to three hours and undergoes hepatic metabolism to produce an active metabolite (norketamine). Because of its quick redistribution, it requires continuous infusion to maintain adequate central nervous system levels. Its initial bolus dose is 1–2 mg/kg IV slow push, and continuous IV dosing is 0.5–7.5 mg/kg/h. High-volume infusions are often required due to the lack of stability information to support IV infusions with concentrations greater than 2 mg/mL. There is minimal data in the literature to support concentrations up to 10 mg/mL. Ketamine infusions can be titrated by 0.5 mg/kg/h every 10 min to allow for quick titration in patients with refractory status epilepticus. To transition from IV ketamine to per oral, the medication has to be prepared as an oral suspension with a target dose of 500–2000 mg daily in two to four doses. Because oral ketamine is not commercially available, it has to be compounded in a pharmacy using a ketamine base powder.
An important consideration for ketamine is its pharmacogenomic metabolism, since there are genetic variations of the CYP2B6 genotype (*1 and *6), which may result in different levels of ketamine plasma concentrations [11,12,13]. Dinis-Oliveira [11] found that patients with *1/*1 genotypes and chronic pain or cancer may have increased clearance of ketamine, compared with decreased clearance from patients with the *1/*6, and especially,*6/*6 genotypes. The authors hypothesized that the higher plasma concentrations (seen with genotypes *1/*6, *6/*6, *1/*6) obtained may prompt higher incidence of ketamine adverse effects. However, in a subsequent study, Li et al. [12] measured clearance of plasma and urine metabolites and found no significant difference in clearance rates between the CYP2B6 genotypes. Variation between the studies included route and dose of ketamine administration (subcutaneous vs. oral), absence/presence of medical conditions, and limitation of substances prior to participation [11, 12]. The opposing outcomes in research may suggest that plasma concentration is dependent on dose and route, as well as patient factors.
Ketamine in nonconvulsive status epilepticus was utilized in seven case reports, five of which were pediatric patients [2]. Its use for acute seizure relapse in DRE is very recent and has not been reported. Most articles and guidelines use only a combination of antiepileptic drugs, which can be associated with nonmedical therapy, such as ketogenic diet and vagal nerve stimulation to treat medically refractory epilepsy [14]. The importance of CYP2B6 inducers with ketamine use should be considered, such as phenobarbital, since it could reduce the effectiveness of ketamine [15].
Conclusions
Low-dose IV ketamine (0.25–0.5 mg/kg/h) can be used for acute provoked worsening of DRE, and may avoid unwanted cardiac and respiratory depression. It can also be converted to enteral formulation and potentially shorten intensive care unit length of stay. However, to compare effectiveness of ketamine, a multicenter trial would need to compare ketamine to usual status epilepticus management to see if ketamine achieves similar patient outcomes and seizure control. Future studies of ketamine should also consider a precision medicine approach, such as pharmacogenomics of CYP2B6*1 and *6 genotype drug metabolism, since CYP inducers, such as phenobarbital, are commonly used in the status epilepticus population, which could lower ketamine effective dose. Furthermore, future studies should consider other patient-specific factors, routes of dosing (enteral vs. IV), and potential CYP drug–drug interactions.
References
Lopez Gonzalez FJ, Rodriguez Osorio X, Gil-Nagel Rein A, Carreno Martinez M, Serratosa Fernandez J, Villanueva Haba V, et al. Drug-resistant epilepsy: definition and treatment alternatives. Neurologia. 2015;30:439–46.
Pizzi MA, Kamireddi P, Tatum WO, Shih JJ, Jackson DA, Freeman WD. Transition from intravenous to enteral ketamine for treatment of nonconvulsive status epilepticus. J Intensive Care. 2017;5:54.
Fang Y, Wang X. Ketamine for the treatment of refractory status epilepticus. Seizure. 2015;30:14–20.
Shrestha GS, Joshi P, Chhetri S, Karn R, Acharya SP. Intravenous ketamine for treatment of super-refractory convulsive status epilepticus with septic shock: a report of two cases. Indian J Crit Care Med. 2015;19:283–5.
Synowiec AS, Singh DS, Yenugadhati V, Valeriano JP, Schramke CJ, Kelly KM. Ketamine use in the treatment of refractory status epilepticus. Epilepsy Res. 2013;105:183–8.
Yeh PS, Shen HN, Chen TY. Oral ketamine controlled refractory nonconvulsive status epilepticus in an elderly patient. Seizure. 2011;20:723–6.
Perks A, Cheema S, Mohanraj R. Anaesthesia and epilepsy. Br J Anaesth. 2012;108:562–71.
Staley K. Molecular mechanisms of epilepsy. Nat Neurosci. 2015;18:367–72.
US National Library of Medicine. Introduction to Boolean logic. https://www.nlm.nih.gov/bsd/disted/pubmedtutorial/020_350.html. Accessed Oct 2018.
US National Library of Medicine. PubMed search: ketamine AND low dose AND seizure. 2018. https://www.ncbi.nlm.nih.gov/pubmed/?term=ketamine+AND+low+dose+AND+seizure. Accessed Oct 2018.
Dinis-Oliveira RJ. Metabolism and metabolomics of ketamine: a toxicological approach. Forensic Sci Res. 2017;2:2–10.
Li Y, Jackson KA, Slon B, Hardy JR, Franco M, William L, et al. CYP2B6*6 allele and age substantially reduce steady-state ketamine clearance in chronic pain patients: impact on adverse effects. Br J Clin Pharm. 2015;80:276–84.
Rosati A, De Masi S, Guerrini R. Ketamine for refractory status epilepticus: a systematic review. CNS Drugs. 2018;32:997–1009.
Krauss GL, Sperling MR. Treating patients with medically resistant epilepsy. Neurol Clin Pract. 2011;1:14–23.
US Food & Drug Administration. Drug development and drug interactions: table of substrates, inhibitors and inducers. 2017. https://www.fda.gov/drugs/developmentapprovalprocess/developmentresources/druginteractionslabeling/ucm093664.htm. Accessed Oct 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Borsato, G.S., Siegel, J.L., Rose, M.Q. et al. Ketamine in seizure management and future pharmacogenomic considerations. Pharmacogenomics J 20, 351–354 (2020). https://doi.org/10.1038/s41397-019-0120-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-019-0120-2
- Springer Nature Limited
This article is cited by
-
Ontology-based identification and prioritization of candidate drugs for epilepsy from literature
Journal of Biomedical Semantics (2022)
-
The Double-Edged Sword of Seizures and Nonconvulsive Status Epilepticus on Aneurysmal Subarachnoid Hemorrhage Outcomes
Neurocritical Care (2022)