Abstract
Background
Ketamine is an emerging third-line medication for refractory status epilepticus, a medical and neurological emergency requiring prompt and appropriate treatment. Owing to its pharmacological properties, ketamine represents a practical alternative to conventional anaesthetics.
Objective
The objective of this study was to assess the efficacy and safety of ketamine to treat refractory status epilepticus in paediatric and adult populations.
Methods
We conducted a literature search using the PubMed database, Cochrane Database of Systematic Reviews and ClinicalTrials.gov website.
Results
We found no results from randomised controlled trials. The literature included 27 case reports accounting for 30 individuals and 14 case series, six of which included children. Overall, 248 individuals (29 children) with a median age of 43.5 years (range 2 months to 67 years) were treated in 12 case series whose sample size ranged from 5 to 67 patients (median 11). Regardless of the status epilepticus type, ketamine was twice as effective if administered early, with an efficacy rate as high as 64% in refractory status epilepticus lasting 3 days and dropping to 32% when the mean refractory status epilepticus duration was 26.5 days. Ketamine doses were extremely heterogeneous and did not appear to be an independent prognostic factor. Endotracheal intubation, a negative prognostic factor for status epilepticus, was unnecessary in 12 individuals (10 children), seven of whom were treated with oral ketamine for non-convulsive status epilepticus.
Conclusions
Although ketamine has proven to be effective in treating refractory status epilepticus, available studies are hampered by methodological limitations that prevent any firm conclusion. Results from two ongoing studies (ClinicalTrials.gov identification number: NCT02431663 and NCT03115489) and further clinical trials will hopefully confirm the better efficacy and safety profile of ketamine compared with conventional anaesthetics as third-line therapy in refractory status epilepticus, both in paediatric and adult populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Available information about the efficacy of ketamine is unavoidably biased as it is only based on observational studies, most retrospective. |
The methodological limitations of the studies prevent any meaningful conclusions on comparative efficacy between ketamine and conventional anaesthetics. |
There is a need for clinical trials designed to assess the efficacy of ketamine as early third-line therapy, thus avoiding endotracheal intubation. |
The commonly held opinion that ketamine has a better safety profile than conventional anaesthetics needs additional evidence. |
The neuroprotective properties of ketamine in humans need to be assessed through analysis of plasma and cerebrospinal fluid biomarkers for neuroinjury and prospective neuropsychological and neuroimaging studies. |
1 Introduction
Status epilepticus (SE) is a life-threatening medical emergency and is traditionally defined as “an acute epileptic condition characterised by continuous seizures for at least 30 min, or by 30 min of intermittent seizures without full recovery of consciousness between seizures” [1]. Based on improved understanding of pathophysiology, there is now consensus that any seizure lasting longer than 5 min should be treated as SE [2]. Status epilepticus lasting longer than 120 min and not responding to first- and second-line treatments is defined as “refractory” (RSE) and requires intensive care unit admission [3]. Super-refractory SE is defined as SE that has continued or recurred despite therapy with general anaesthesia for 24 h or longer [3]. Based on the clinical features and severity, SE is distinguished as being either “convulsive” or “non-convulsive”, the former being the most common and harmful.
There is general consensus regarding the first and second lines of treatment for SE [4]. Although the same types of drugs are used in different countries, the algorithm/protocols may differ, even among institutions in the same country. At present, there is no definitive evidence or agreement to guide an optimal treatment choice for RSE [3, 5,6,7]. Refractory convulsive SE is generally treated with coma induction using high-dose midazolam (MDZ) or conventional anaesthetics such as thiopental, pentobarbital or propofol [5, 7]. Conversely, some concerns exist about the opportunity to use conventional anaesthetics in the less severe forms of non-convulsive SE (NCSE) with no impairment of consciousness [8, 9].
Status epilepticus results from the failure of inhibitory GABA-mediated mechanisms responsible for seizure termination and from the activation of excitatory glutamate-mediated mechanisms, which lead to abnormally prolonged seizures with consequent neuronal injury and death [10, 11]. In this scenario, N-methyl-d aspartate (NMDA)-receptor antagonist modulating molecules offer an attractive alternative in SE [12, 13]. Experimental models have demonstrated that ketamine (KE), a potent NMDA-receptor antagonist, controls prolonged SE while it is ineffective in its early phase [14]. It has also been demonstrated that the efficacy of KE is increased by the concomitant use of benzodiazepines [15, 16]. Ketamine is a phencyclidine derivative, with a chiral structure consisting of two optical isomers. Racemic KE is the most commonly used form and is a mixture of equal amounts of the two enantiomers (R)-KE and (S)-KE, the latter displaying analgesic and anaesthetic potency about three-fold superior to (R)-KE. Ketamine has a half-life of 2–3 h and is metabolised by cytochrome P450 3A and cytochrome P450 2B6 enzymes mainly owing to its active metabolite, norketamine. It is water and lipid soluble, reaching extensive distribution in the body. However, because of extensive first-pass metabolism, oral bioavailability is poor and vulnerable to pharmacokinetic drug interactions [17, 18].
The pharmacological profile is characterised by the so-called “dissociative anaesthetic state” described as a form of anaesthesia characterised by catalepsy, catatonia, analgesia and amnesia that does not necessarily cause loss of consciousness. These analgesic and anaesthetic properties of KE combined with its typical sympathomimetic effects are mediated by different sites of action. N-methyl-d aspartate-receptor antagonism is the most important neuropharmacological mechanism for the analgesic and anaesthetic effects and contributes to its neuroprotective action [19]. Analgesic state and dysphoric reactions are mediated by opiate receptors, whereas the enhancement of central peripheral monoaminergic transmission contributes to its sympathomimetic properties. Moreover, the inhibition of central and peripheral cholinergic transmission may contribute to the induction of the anaesthetic state and hallucinations, whereas the hyperpolarisation-activated cyclic nucleotide channels (HCN1 channels) contribute to the sedating actions of KE.
Compared with other drugs used for the treatment of SE, KE-induced respiratory depression is rare and this effect, such as the increase of bronchial secretions, can be prevented and reduced by the administration of a muscarinic antagonist such as atropine. The sympathomimetic properties of KE, in particular, subtend its vasopressor-sparing effect that reduces the need for vasoactive compounds to counteract hypotension, which is frequently seen with conventional intravenous anaesthetics commonly used in SE [18, 20]. Along with hallucinations and hypersalivation, nausea and vomiting are the most relevant adverse events reported with the use of KE [17, 18].
Based on its promising efficacy and good safety profile, KE may be considered as the anaesthetic agent of choice in specific situations and as an out-of-hospital treatment option of SE [18, 20, 21].
Ketamine is currently administered in patients with RSE only when conventional anaesthetics have failed [3]; however, based on its potential efficacy and good safety profile, more recent studies suggest [22] and recommend [23] an earlier administration. Here, we performed a systematic review of the literature on the efficacy and safety of KE in treating RSE in paediatric and adult populations.
2 Methods
We conducted a systematic review and reported it according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [24]. We performed a MEDLINE literature search using PubMed to identify all articles published as of February 2018 with the following research details: “ketamine”[MeSH Terms] OR “ketamine” [All Fields]) AND (“status epilepticus” [MeSH Terms] OR (“status” [All Fields] AND “epilepticus” [All Fields]) OR “status epilepticus” [All Fields]. We also searched the Cochrane Database of Systematic Reviews (keyword: “ketamine” OR “status epilepticus”) for related systematic reviews and the ClinicalTrials.gov website for ongoing clinical studies on RSE.
Titles and abstracts obtained through the literature search were screened for inclusion in the review using the following criteria: KE efficacy and safety as primary outcome in SE, both in the paediatric and adult populations; only studies published in English. Exclusion criteria were: preclinical studies, editorials, letters and non-English publications. After full-text reading of the accordingly selected papers, articles included in the review were further reviewed in their reference list for other publications relevant to the topic (secondary search).
The following data were extracted: study type and design, patient demographics, number of patients, type of SE (convulsive, non-convulsive and subtle SE, focal and generalised SE), aetiology of SE, dose, timing, duration and route of KE administration, prior and concomitant therapies, outcome defined as electrographic SE control and adverse events. Taking into account the different aetiologies of SE across ages, data on paediatric and adult populations were analysed separately. A systematic assessment of the available evidence was conducted in accordance with the GRADE methodology [25, 26]. A meta-analysis was not possible because of the lack of prospective randomised trials.
3 Results
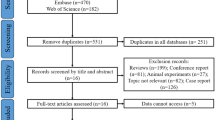
The search strategy yielded 135 MEDLINE abstracts, no Cochrane systematic review and two ongoing clinical trials on KE use in refractory convulsive SE in children (ClinicalTrials.gov identification number: NCT02431663) and adults (ClinicalTrials.gov identification number: NCT03115489). Eighty-six full texts were analysed and 63 included in the review. A further nine articles emerged from the secondary search and a total of 72 articles contributed to this review. Results of the search strategy across all databases and other sources are summarised in Fig. 1.
No randomised controlled trials were available and the current evidence consisted of 27 case reports accounting for 30 individuals and 14 case series, 6 of which included children. Most of the studies were retrospective and three were companion publications expanding on the original data set of Meyer Children’s Hospital [22, 27, 28]. For this reason, only one case series [22] was considered in the data analysis of the current review. Table 1 shows the characteristics of the selected case series by study design and population (adults vs. children). A total of 248 individuals (29 children) with a median age of 43.5 years (range 2 months to 67 years) were treated in 12 case series with a sample size ranging from 5 to 67 individuals (median 11). Table 2 reports the GRADE assessment for case series only.
3.1 Adults
A total of 219 adults (median age 54.5 years, range 24–67 years) were treated in eight case series [29,30,31,32,33,34,35,36] with a sample size ranging from 7 to 67 individuals (median 11). In 16 case reports [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52], 19 individuals and 20 RSE episodes were treated with KE (Tables 3, 4). Infections and anoxia were the most frequent aetiologies [30, 33, 35, 36]. In more than half of 60 RSE episodes described by Gaspard et al. [31] the aetiology remained unknown. The type of RSE was not specified in four out of eight case series and NCSE was the type of SE most commonly treated with KE, both in case series and in case reports (Tables 3, 4). The mean duration of SE prior to KE administration was highly heterogeneous, regardless of SE type, and ranged from 24 h to 26.5 days in case series (Table 3) and from 12 h to 5 months in case reports (Table 4).
Considering both case reports and case series, KE was always administered after conventional anaesthetics, with the exception of the patient described by Pizzi et al. [50] while propofol was the most common third-line treatment administered. Benzodiazepines, especially MDZ, were the most commonly used drugs in add-on. Ketamine dosage ranged from 0.07 to 15 mg/kg/h. The duration of KE infusion ranged from 6 h to 29 days. The proportion of individuals where KE was effective (resolution of RSE) ranged from 11% in the study by Gosselin-Lefebvre et al. [32] which enrolled nine patients, to 100% in the case series described by Synowiec et al. [33] which included 11 individuals. Considering all RSE episodes, 156/222 (70.3%) were controlled by KE administration. Electroencephalography (EEG) features were not specified in the majority of the case series and a burst-suppression pattern was only observed in three out of seven patients as reported by Bleck et al. [29] as well as in three case reports [44, 47, 52]. Diffuse slowing and diffuse beta activity were EEG patterns observed in RSE episodes in which KE was effective. Adverse events, including shock, sepsis, renal failure, pneumonia and acidosis were only reported only in the series of Gaspard et al. [31] Cerebellar atrophy and cardiac arrest were documented by Ubogu et al. [38] and Koffman et al. [51] Endotracheal intubation was avoided in two patients in whom KE was effective [42, 50].
3.2 Children
A total of 29 children (age range 2 months to 18 years) accounting for 35 RSE episodes were treated in four case series [22, 53,54,55] with a sample size ranging from five to 13 individuals (median 5.5). Eleven patients treated with KE were also documented in corresponding case reports [56,57,58,59,60,61,62,63,64,65,66]. In case series, epileptic encephalopathy was the most frequent underlying condition (Table 5). Heterogeneous aetiologies were documented in case reports (Table 6). The type of RSE was not specified in two of the four case series, although convulsive SE was the most common form treated with KE, both in case series and in case reports (Tables 5, 6). The mean duration of SE prior to KE administration was highly heterogeneous, regardless of the RSE type, and ranged from 5 h to 26 days in case series and from 10 to 73 days in case reports.
Both in case reports and case series, KE was always administered after conventional anaesthetics, with MDZ the third-line treatment most commonly employed. Ketamine dosage ranged from 0.04 to 10 mg/kg/h. The duration of KE infusion ranged from 1 to 21 days. The proportion of individuals in whom KE was effective (resolution of RSE) ranged from 20% in the study by Al-Otaibi et al. [55] which enrolled five patients, to 100% in the case series described by Mewasingh et al. [53] which included five NCSE children treated with oral KE. Of the 19 refractory convulsive SE episodes treated by Ilvento et al. [22] KE was effective in 14 (74%). Overall, 28/46 (61%) of RSE episodes were controlled by KE administration.
A burst-suppression pattern was observed during the initial bolus of 3 mg/kg in most responders in the case series of Ilvento et al. [22] and was followed by diffuse theta and beta activity in four children; endotracheal intubation was avoided in two of them. Mewasingh et al. [53] documented diffuse theta activity in five children who were treated orally. Adverse events were only reported in the series of Ilvento et al. [22] and consisted of a slight increase of saliva secretion in all patients and a transient mild increase of liver enzymes in four out of 13 children. Endotracheal intubation was unnecessary in the five individuals with NCSE treated with oral KE [53] and in 5 out of 13 children with refractory convulsive SE [22].
4 Discussion
Ketamine has proven effective in treating both convulsive and non-convulsive RSE in the adult and paediatric populations. In the case series with the largest sample, resolution of RSE was obtained in 74% of 19 patients with SE prospectively followed [22] and in 91% of 67 adults in a retrospective review of medical records [35]. The available information on the efficacy of KE is biased by the design of the available studies that is always observational, mostly retrospective. Indeed, only single-arm studies without a control group were available, and only two paediatric case series out of four and none in the adult population had a prospective design [22, 53].
In adults, the efficacy of KE was higher in convulsive SE compared with NCSE in the two largest series [31, 36]. Evidence of the efficacy of KE in paediatric NCSE was reported only in five children successfully treated with oral administration after a mean RSE duration of 4 days [53]. Regardless of SE type, KE was twice as effective if given early, with efficacy dropping from 64% in the 42 patients with RSE lasting 3 days [36] to 32% when the mean duration of 60 RSE was 26.5 days [31]. A similarly good efficacy was observed in children, with a response rate of 74% in the 19 refractory convulsive SE episodes treated after a mean RSE duration of 7 days [22].
Data from experimental models suggest the efficacy of KE in the treatment of SE when the drug is administered not too early (after 15 min) but at least 1 h after the onset of symptoms [14]. Likewise, the increased efficacy of KE when administered in the early stages of SE is confirmed even in the clinical setting, although the definition “early” is widely heterogeneous in different studies, ranging from a few hours to some days after onset.
Timing in KE administration and convulsive SE both appear to be the most relevant predictive determinants of the efficacy of KE in adults and children, while KE dosage, which was extremely heterogeneous throughout the studies, does not seem to be an independent prognostic factor, both in case series and in individual case reports. Moreover, timing and modalities of KE dosage titration were not reported in most articles, making it impossible to estimate the timing of SE control after the start of KE treatment.
The missing information, mainly owing to the retrospective design of the studies and the absence of centre-specific and standardised protocols for the treatment of SE, represents the most important limitation to assess the possible advantages from the use of KE in this clinical scenario.
Benzodiazepines, and especially MDZ, were the drugs most commonly used in add-on but no conclusions or speculations about a possible synergic effect, as documented in animal models [15, 16], were possible in this review because of a lack of detailed information.
As expected, in most instances, the delay using KE was related to prior administration of conventional anaesthetics, such as MDZ, thiopental, pentobarbital and/or propofol. Experimental models suggest that, with continuing seizures, inhibitory GABAA receptors are internalised in clathrin-coated vesicles, and excitatory NMDA receptors are mobilised to the membrane [10, 11]. However, the mechanisms underlying the refractoriness of SE are likely to be multifactorial and more complex as suggested by preclinical evidence indicating that polytherapy is more effective than monotherapy [67]. Conventional anaesthetics, all acting on GABAA receptors will be, therefore, less active, prompting administration of higher doses, which will in turn enhance their untoward effects, especially hypotension [20]. Ketamine represents an attractive alternative for SE, also in relation to its sympatico-mimetic action [12]. Owing to its pharmacological properties, KE use does not necessarily require amine administration or mechanical ventilation. Nevertheless, in the largest adult case series, a higher use of vasopressors was reported in association with KE compared with earlier treatments [31, 35]. Additional evidence is needed to confirm the safety profile of KE and the real feasibility in avoiding amine administration.
Ketamine has neither cardiac nor respiratory depressant properties, therefore, its use does not routinely require endotracheal intubation. This is considerably advantageous as intubation represents per se a negative prognostic factor of increased morbidity and mortality in critically ill adults and children [20, 68, 69]. The risk-benefit profile for conventional anaesthetics is questionable and their use seems to be unjustified especially in NCSE where endotracheal intubation is unnecessary, or when consciousness impairment does not occur [9]. The good safety profile of KE is furthermore documented by the paucity of adverse events emerging from both case series and case reports in adults and in children.
An EEG burst-suppression pattern is typically observed in patients treated with conventional anaesthetics and represents, along with seizure control, the goal of treatment. The clinical efficacy of KE is associated with a more heterogeneous EEG pattern in which diffuse slowing and diffuse beta activity should be considered the targets to achieve and retain on a par with the burst-suppression pattern.
Neuroprotection from glutamate-induced neurotoxicity is another potential advantage associated with KE use [70,71,72]. This hypothesis was substantiated by the ongoing KIND trial, examining the ability of subanaesthetic doses of KE to improve outcome and mitigate neuronal injury [as assessed using 3 Tesla magnetic resonance imaging and analysis of plasma and cerebrospinal fluid neuroinjury biomarkers in patients with grade I–IV subarachnoid haemorrhage (ClinicalTrials.gov identification number: NCT02636218)].
Finally, preliminary evidence is available about immunomodulatory and anti-inflammatory properties of KE, which seem to contribute to its anti-epileptogenic activity [73] and to a more favourable outcome [74]. Appropriately designed studies are needed to confirm this possible additional beneficial effect of KE in SE.
A systematic prospective collection of data accounts for more solid clinical, EEG and therapeutic information. Therefore, a targeted clinical database should be established to attempt to answer key questions such as: (1) is KE efficacy increased by add-on benzodiazepines use? (2) does KE require amine use and if, when? (3) is there any specific or more frequently observed EEG pattern in responders? (4) how long is KE infusion to be continued in responders? and (5) does KE have a neuroprotective action and can it be assessed by neuro-injury biomarker sampling in plasma and cerebrospinal fluid and prospective neuropsychological and neuroimaging evaluations?
5 Conclusion
As reported in a recent editorial by Dorandeu [75], despite the poor quality of the available evidence on the efficacy and safety of KE, data are encouraging and support the interest in developing future specifically designed clinical trials to investigate its role in the early stages of SE. The less pronounced hypotensive and respiratory depressive effects of KE and the potentially favourable risk/benefit profile compared with conventional anaesthetics, as well as the plausible neuroprotective effect, draw a concrete possibility of a future widespread application. We are confident that the two specifically designed ongoing trials will provide unbiased evidence on the efficacy and safety of KE in this particular scenario.
References
Commission on Classification and Terminology of the International. League Against Epilepsy. Proposal for revised clinical and electrographic classification of epileptic seizures. Epilepsia. 1981;22:489–501.
Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus: report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56:1515–23.
Shorvon S, Ferlisi M. The treatment of super-refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain. 2011;134:2802–18.
Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23.
Abend NS, Duglas DT. Treatment of refractory status epilepticus: literature review and a proposed protocol. Pediatr Neurol. 2008;38:377–80.
Sofou K, Kristjansdòttir R, Papachatzakis N, Ahmadzadeh A, Uvebrant P. Management of prolonged seizures and status epilepticus in childhood: a systematic review. J Child Neurol. 2009;24:918–26.
Fernandez A, Claassen J. Refractory status epilepticus. Curr Opin Crit Care. 2012;18:127–31.
Leitinger M, Beniczky S, Rohracher A, et al. Salzburg consensus criteria for non-convulsive status epilepticus: approach to clinical application. Epilepsy Behav. 2015;49:158–63.
Meierkord H, Holtkamp M. Non-convulsive status epilepticus in adults: clinical forms and treatment. Lancet Neurol. 2007;6:329–39.
Wasterlain CG, Chen JW. Mechanistic and pharmacologic aspects of status epilepticus and its treatment with new antiepileptic drugs. Epilepsia. 2008;49(Suppl. 9):63–73.
Naylor DE. Glutamate and GABA in the balance: convergent pathways sustain seizures during status epilepticus. Epilepsia. 2010;5(Suppl. 3):106–9.
Zeiler FA, Teitelbaum J, Gillman LM, West M. NMDA antagonists for refractory seizures. Neurocrit Care. 2014;20:502–13.
Dorandeu F, Dhote F, Barbier L, Baccus B, Testylier G. Treatment of status epilepticus with ketamine, are we there yet? CNS Neurosci Ther. 2013;19:411–27.
Borris DJ, Bertram EH, Kapur J. Ketamine controls prolonged status epilepticus. Epilepsy Res. 2000;42:117–22.
Martin BS, Kapur J. A combination of ketamine and diazepam synergistically controls refractory status epilepticus induced by cholinergic stimulation. Epilepsia. 2008;49:248–55.
Niquet J, Baldwin R, Norman K, Suchomelova L, Lumley L, Wasterlain CG. Midazolam-ketamine dual therapy stops cholinergic status epilepticus and reduces Morris water maze deficits. Epilepsia. 2016;57:1406–15.
Zanos P, Moaddel R, Morris PJ, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70:621–60.
Craven R. Ketamine. Anaesthesia. 2007;62:48–53.
Shibuta S, Varathan S, Mashimo T. Ketamine and thiopental sodium: individual and combined neuroprotective effects on cortical cultures exposed to NMDA or nitric oxide. Br J Anaesth. 2006;97:517–24.
Schmutzhard E, Pfausler B. Complications of the management of status epilepticus in the intensive care unit. Epilepsia. 2011;52(Suppl. 8):39–41.
Dorandeu F, Barbier L, Dhote F, Testylier G, Carpentier P. Ketamine combinations for the field treatment of soman-induced self-sustaining status epilepticus: review of current data and perspectives. Chem Biol Interact. 2013;203:154–9.
Ilvento L, Rosati A, Marini C, L’Erario M, Mirabile L, Guerrini R. Ketamine in refractory convulsive status epilepticus in children avoids endotracheal intubation. Epilepsy Behav. 2015;49:343–6.
Zeiler FA, West M. Ketamine for status epilepticus: Canadian physician views and time to push forward. Can J Neurol Sci. 2015;42:132–4.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Guyatt GH, Oxman AD, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6.
Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ, et al. Rating quality of evidence and strength of recommendations: what is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336:995–8.
Rosati A, L’Erario M, Ilvento L, Pisano T, Mirable L, Guerrini R. An ongoing open-label uncontrolled study of the efficacy and safety of ketamine in children with refractory status epilepticus. Epilepsia. 2013;54(Suppl. 3):17.
Rosati A, L’Erario M, Ilvento L, Cecchi C, Pisano T, Mirabile L, et al. Efficacy and safety of ketamine in refractory status epilepticus in children. Neurology. 2012;79:2355–8.
Bleck TP, Quigg MS, Nathan BR, Smith TL, Kapur J. Electroencephalographic effects of ketamine treatment for refractory status epilepticus. Epilepsia. 2002;43(Suppl. 7):282.
Singh D, Kelly K, Rana S, Valeriano J. Use of ketamine in treating refractory status epilepticus. Epilepsia. 2009;50(Suppl. 11):63.
Gaspard N, Foreman B, Judd LM, et al. Intravenous ketamine for the treatment of refractory status epilepticus: a retrospective multicenter study. Epilepsia. 2013;54:1498–503.
Gosselin-Lefebvre S, Rabinstein A, Rossetti A, Savard M. Ketamine usefulness in refractory status epilepticus: a retrospective multicenter study. Can J Neurol Sci. 2013;40(Suppl. 1):S31.
Synowiec AS, Singh DS, Yenugadhati V, et al. Ketamine use in the treatment of refractory status epilepticus. Epilepsy Res. 2013;105:183–8.
Basha MM, Alqallaf A, Shah AK. Drug-induced EEG pattern predicts effectiveness of ketamine in treating refractory status epilepticus. Epilepsia. 2015;56:e44–8.
Sabharwal V, Ramsay E, Martinez R, Shumate R, Khan F, Dave H, Iwuchukwu I, McGrade H. Propofol-ketamine combination therapy for effective control of super-refractory status epilepticus. Epilepsy Behav. 2015;52:264–6.
Höfler J, Rohracher A, Kalss G, Zimmermann G, Dobesberger J, Pilz G, Leitinger M, Kuchukhidze G, Butz K, Taylor A, Novak H, Trinka E. (S)-Ketamine in refractory and super-refractory status epilepticus: a retrospective study. CNS Drugs. 2016;30:869–76.
Walker MC, Howard RS, Smith SJ, et al. Diagnosis and treatment of status epilepticus on a neurological intensive care unit. QJM. 1996;89:913–20.
Ubogu EE, Sagar SM, Lerner AJ, et al. Ketamine for refractory status epilepticus: a case of possible ketamine induced neurotoxicity. Epilepsy Behav. 2003;4:70–5.
Robakis TK, Hirsch LJ. Literature review, case report, and expert discussion of prolonged refractory status epilepticus. Neurocrit Care. 2006;4:35–46.
Prüss H, Holtkamp M. Ketamine successfully terminates malignant status epilepticus. Epilepsy Res. 2008;82:219–22.
Hsieh CY, Sung PS, Tsai JJ, et al. Terminating prolonged refractory status epilepticus using ketamine. Clin Neuropharmacol. 2010;33:165–7.
Yeh PS, Shen HN, Chen TY. Oral ketamine controlled refractory nonconvulsive status epilepticus in an elderly patient. Seizure. 2011;20:723–6.
Kramer AH. Early ketamine to treat refractory status epilepticus. Neurocrit Care. 2012;16:299–305.
Zeiler FA, Kaufmann AM, Gillman LM, et al. Ketamine for medically refractory status epilepticus after elective aneurysm clipping. Neurocrit Care. 2013;19:119–24.
Esaian D, Joset D, Lazarovits C, Dugan PC, Fridman D. Ketamine continuous infusion for refractory status epilepticus in a patient with anticonvulsant hypersensitivity syndrome. Ann Pharmacother. 2013;47:1569–76.
Shrestha GS, Joshi P, Chhetri S, et al. Intravenous ketamine for treatment of super-refractory convulsive status epilepticus with septic shock: a report of two cases. Indian J Crit Care Med. 2015;19:283–5.
McGinn KA, Bishop L, Sarwal A. Use of ketamine in barbiturate coma for status epilepticus. Clin Neuropharmacol. 2016;39:62–5.
Dillien P, Ferrao Santos S, van Pesch V, Suin V, Lamoral S, Hantson P. New-onset refractory status epilepticus: more investigations, more questions. Case Rep Neurol. 2016;8:127–33.
Al-Busaidi M, Burad J, Al-Belushi A, Gujjar A. Super refractory status epilepticus in Hashimoto’s encephalopathy. Oman Med J. 2017;32:247–50.
Pizzi MA, Kamireddi P, Tatum WO, Shih JJ, Jackson DA, Freeman WD. Transition from intravenous to enteral ketamine for treatment of nonconvulsive status epilepticus. J Intensive Care. 2017;5:54.
Koffman L, Yan Yiu H, Farrokh S, Lewin J, Geocadin R, Ziai W. Ketamine infusion for refractory status epilepticus: a case report of cardiac arrest. J Clin Neurosci. 2018;47:149–51.
Mutkule DP, Rao SM, Chaudhuri JR, Rajasri K. Successful use of ketamine for burst suppression in super refractory status epilepticus following substance Abuse. Indian J Crit Care Med. 2018;22:49–50.
Mewasingh LD, Sekhara T, Aeby A, Christiaens FJC, Dan B. Oral ketamine in paediatric non-convulsive status epilepticus. Seizure. 2003;12:483–9.
Kravljanac R, Nikolic LJ, Djuric M, Jovic N, Jankovic B. Treatment of status epilepticus in children: 15-year single center experience. Acta Paediatr. 2010;99(Suppl. 462):107.
Al-Otaibi AD, McCoy B, Cortez M, Hutchison JS, Hahn CS. The use of ketamine in refractory status epilepticus. Can J Neurol Sci. 2010;37(3 Suppl. 1):S69.
Sheth RD, Gidal BE. Refractory status epilepticus: response to ketamine. Neurology. 1998;51:1765–6.
Kramer U, Shorer Z, Ben-Zeev B, Lerman-Sagie T, Goldberg-Stern H, Lahat E. Severe refractory status epilepticus owing to presumed encephalitis. J Child Neurol. 2005;20:184–7.
Elting JW, Naalt JV, Fock JM. Mild hypothermia for refractory focal status epilepticus in an infant with hemimegalencephaly. Eur J Paediatr Neurol. 2010;14:452–5.
Andrade C, Franca S, Sampaio M, et al. Successful use of ketamine in paediatric super-refractory status epilepticus—case report. Epilepsia. 2012;53:98.
Tarocco A, Ballardini E, Garani G. Use of ketamine in a newborn with refractory status epilepticus: a case report. Pediatr Neurol. 2014;51:154–6.
Horino A, Kawawaki H, Fukuoka M, et al. A case of succinic semialdehyde dehydrogenase deficiency with status epilepticus and rapid regression. Brain Dev. 2016;38:866–70.
Mirás Veiga A, Moreno DC, Menéndez AI, et al. Effectiveness of electroconvulsive therapy for refractory status epilepticus in febrile infection-related epilepsy syndrome. Neuropaediatrics. 2017;48:45–8.
Chiusolo F, Diamanti A, Bianchi R, et al. From intravenous to enteral ketogenic diet in PICU: a potential treatment strategy for refractory status epilepticus. Eur J Paediatr Neurol. 2016;20:843–7.
Li D, Yuan H, Ortiz-Gonzalez XR, et al. GRIN2D recurrent de novo dominant mutation causes a severe epileptic encephalopathy treatable with NMDA receptor channel blockers. Am J Hum Genet. 2016;99:802–16.
Aroor S, Shravan K, Mundkur SC, Jayakrishnan C, Rao SS. Super-refractory status epilepticus: a therapeutic challenge in paediatrics. J Clin Diagn Res. 2017;11:SR01–4.
Caputo D, Iorio R, Vigevano F, Fusco L. Febrile infection-related epilepsy syndrome (FIRES) with super-refractory status epilepticus revealing autoimmune encephalitis due to GABAAR antibodies. Eur J Paediatr Neurol. 2018;22:182–5.
Löscher W. Single versus combinatorial therapies in status epilepticus: novel data from preclinical models. Epilepsy Behav. 2015;49:20–5.
Griesdale DE, Bosma TL, Kurth T, Isac G, Chittock DR. Complications of endotracheal intubation in the critically ill. Int Care Med. 2008;34:1835–42.
Carroll CL, Spinella PC, Corsi JM, Stoltz P, Zucker AR. Emergent endotracheal intubations in children: be careful if it’s late when you intubate. Pediatr Crit Care Med. 2010;11:343–8.
Fujikawa DG. Neuroprotective effect of ketamine administered after status epilepticus onset. Epilepsia. 1995;36:186–95.
Cunha AO, Mortari MR, Liberato JL, dos Santos WF. Neuroprotective effects of diazepam, carbamazepine, phenytoin and ketamine after pilocarpine-induced status epilepticus. Basic Clin Pharmacol Toxicol. 2009;104:470–7.
Dorandeu F, Baille V, Mikler J, et al. Protective effects of S+ ketamine and atropine against lethality and brain damage during soman-induced status epilepticus in guinea-pigs. Toxicology. 2007;234:185–93.
Vargas-Sánchez K, Mogilevskaya M, Rodríguez-Pérez J, Rubiano MG, Javela JJ, González-Reyes RE. Astroglial role in the pathophysiology of status epilepticus: an overview. Oncotarget. 2018;9(42):26954–76.
Loix S, De Kock M, Henin P. The anti-inflammatory effects of ketamine: state of the art. Acta Anaesthesiol Belg. 2011;62:47–58.
Dorandeu F. Ketamine for the treatment of (super) refractory status epilepticus? Not quite yet. Expert Rev Neurother. 2017;17:419–21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were received for the preparation of this study.
Conflict of interest
Anna Rosati, Salvatore De Masi and Renzo Guerrini have no conflicts of interest that are directly relevant to the contents of this study.
Rights and permissions
About this article
Cite this article
Rosati, A., De Masi, S. & Guerrini, R. Ketamine for Refractory Status Epilepticus: A Systematic Review. CNS Drugs 32, 997–1009 (2018). https://doi.org/10.1007/s40263-018-0569-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-018-0569-6