Abstract
The discovery of small molecules that target the extracellular domain of prostate-specific membrane antigen (PSMA) has led to advancements in diagnostic imaging and the development of precision radiopharmaceutical therapies. In this review, we present the available existing data and highlight the key ongoing clinical evaluations of PSMA-based imaging in the management of primary, biochemically recurrent, and metastatic prostate cancer. We also discuss clinical studies that explore the use of PSMA-based radiopharmaceutical therapy (RPT) in metastatic prostate cancer and forthcoming trials that investigate PSMA RPT in earlier disease states. Multidisciplinary collaboration in clinical trial design and therapeutic administration is critical to the continued progress of this evolving radiotheranostics field.
Similar content being viewed by others
PSMA and prostate cancer
Prostate cancer (PCa) is the second most common cancer in men worldwide, and the fifth leading cause of death from cancer [1]. Prostate-specific membrane antigen (PSMA) is a type II transmembrane glutamate carboxypeptidase located on the prostate secretory-acinar epithelium, and is the most prostate-specific cell surface antigen yet identified [2,3,4,5,6]. PSMA was cloned as the target of the 7E11-C5 antibody [3], which was previously shown to bind the surface of prostate epithelial cells and the serum of prostate cancer patients [2]. PSMA has a relatively restricted normal expression, including salivary and lacrimal glands, proximal renal tubules, liver, etc. [7]. The majority of adenocarcinomas of the prostate demonstrate PSMA expression in the primary and metastatic lesions, and the level of PSMA expression is approximately 1000-fold higher than that of normal prostate tissue [8]. PSMA expression by immunohistochemistry has been correlated with de-differentiated, metastatic, or castrate-resistant disease [9, 10]. Since internalization occurs after binding of small-molecule ligands to PSMA, these molecules are ideal candidates for radio-ligand therapy [11].
PSMA PET imaging
Due to the low specificity and sensitivity of FDG positron emission tomography (PET) in prostate cancer [12], functional imaging of prostate malignancies has been explored using a variety of radiotracers targeting PSMA [13]. 7E11-C5 labeled with 111In (ProstaScint®) became the first Food and Drug Administration (FDA) approved SPECT imaging tracer in prostate cancer [14]. However, because 7E11-C5 only recognizes the intracellular portion of PSMA, it mainly identifies dead cells. Liu et al. [15] isolated the first monoclonal antibody to the extracellular domain of PSMA, the binding of which led to receptor dimerization and endocytosis. This led to the development of a humanized version of the antibody, J591, which demonstrated potential for molecular imaging in castrate-resistant prostate cancer [16]. Multiple small molecule ligands that bind to the extracellular domain of PSMA have also been developed, with shorter biological half-lives and improved tumor-to-background ratio. These include 68Ga-PSMA-11, 18F-DCFPyL, and 68Ga-PSMA-617. 68Ga-PSMA-11 (Locametz®) PET and 18F-DCFPyL (Pylarify®) PET gained FDA approval in 2020 and 2021, respectively. Other FDA approved PCa-specific PET tracers include carbon 11 (C-11) choline, which relies on aberrant choline metabolism in PCa, and 18F-fluorocyclobutane-1-carboxylic acid fluciclovine (Axumin®), which is an analog of L-leucine that is preferentially taken up by PCa and gliomas. In a network analysis comparing the diagnostic performance of radiotracers in recurrence PCa, small molecule inhibitors (PSMA-11, PSMA-1007, DCFPyL) were superior to choline-based tracers, however, there was no evidence that any one PSMA radiotracer had improved diagnostic characteristics compared with another [17]. As such, the appropriate use criteria for PSMA PET imaging refers to all PSMA PET tracers as interchangeable [12].
In a prospective trial comparing 68Ga-PSMA-11 PET to 18F-fluciclovine in patients with biochemical recurrence (BCR) and prostate specific antigen (PSA) ≤ 2 ng/mL, PSMA PET had a better detection rate at all regions with the exception of the prostate bed, where the two imaging modalities were similar in performance [18]. In head-to-head comparison of 68Ga-PSMA-11 PET with multiparametric magnetic resonance imaging MRI (mpMRI) in patients with intermediate- and high-risk disease, the overall cancer detection rate was 85% with PSMA PET vs. 83% with mpMRI [19].
Despite high detection rates, there are non-trivial rates of false negative and false positive. Up to 10% of prostate cancers do not express PSMA, including many neuroendocrine or dedifferentiated castrate-resistant prostate cancers (CRPC) [20]. False negatives also include the aforementioned PET detection limit of nodes ≤ 5 mm in size that may have microscopic burden of disease. Commonly seen false positives may be related to normal biodistribution of PSMA in the sympathetic ganglia. Brain tumors that have demonstrated PSMA uptake include meningioma, neurofibroma, and glioma [21, 22]. Pulmonary sarcoidosis and granulomatosis have also demonstrated mild uptake [23]. Ganglia are a common pitfall as they can mimic lymph nodes, however, the intensity of uptake, the shape, and the exact location along the sympathetic trunk may help distinguish them from nodal metastases [24]. PSMA uptake in benign bone lesions can be challenging due to propensity of prostate cancer to metastasize to the bone. PSMA uptake has been described in healing bone fractures [25], degenerative changes in Paget’s disease [26], and fibro-osseous lesions including hemangiomas and fibrous dysplasia [27].
Several reporting criteria exist in an effort to standardize PSMA PET interpretation: PSMA-RADS criteria [28], EANM criteria [29], and PROMISE criteria [30]. These are based on the amount of radiotracer uptake, on how typically radiotracer is distributed within the site, and on the presence of an anatomic correlate. External validation of the three proposed criteria have demonstrated good inter-reader, intra-reader, and inter-criteria reproducibility, with the lung nodules being the most frequent cause of disagreement [31]. This was incorporated into molecular imaging (MI)-RADS [32], which reports a PSMA expression score based on PSMA uptake relative to blood, liver, and parotid gland, which then informs a 5-point confidence score. Standardized imaging interpretation and reporting guidelines will facilitate data comparison between studies and improve reproducibility within clinical trials.
PSMA PET in initial diagnosis and staging
PRIMARY was the first prospective phase II trial that evaluated PSMA PET in the diagnosis of PCa in men with elevated PSA and/or abnormal physical exam. The addition of PSMA PET to MRI improved sensitivity (97% vs. 83%, p < 0.001) and negative predictive value (91% vs. 72%, p < 0.001) compared to MRI-guided biopsy alone [33]. The advantage of PSMA PET is most compelling in the setting of negative or equivocal MRI findings, where a positive or negative PSMA correlated with presence of absence of PCa in 90% of patients [33]. In a meta-analysis comparing imaging to histopathology at prostatectomy, the sensitivity and specificity of 68Ga-PSMA-11 at initial staging were 74% and 96%, respectively [34]. Zhang et al. demonstrated feasibility of using PSMA PET to guide targeted biopsies to detect clinically significant disease, with detection rates similar to that of systematic transrectal ultrasound-guided biopsies [35].
Maximum standardized uptake value (SUVmax) of PSMA PET has been shown to correlate with aggressive disease. In a review of over one thousand patients staged with PSMA PET prior to prostatectomy, with a median PSA of 6 ng/mL, the SUVmax correlated with grade group, where a SUVmax ≥ 11 improved the detection of ≥ Gleason grade group 3 (GG3) disease [36]. Higher PSMA avidity was associated with worse progression free survival (PFS) and worse BCR-free survival (BRFS) in patients with intermediate-risk disease (≤GG3) [37, 38]. On Cox regression analysis, PSMA intensity was associated with BRFS (HR per 5-unit increase = 1.10, 95% CI 1.01–1.19) by a magnitude that was similar to PSA (HR per 5-unit increase = 1.10, 95% CI 1.03–1.18), and independent of biopsy Gleason score [38].
Prospective trials have established the utility of PSMA PET in the staging of nodal and distant metastases in high-risk patients. In proPSMA, patients with at least one high-risk feature (PSA ≥ 20 ng/mL, ≥ GG3, or ≥ cT3) were randomized to upfront 68Ga-PSMA-11 vs. conventional imaging, and PET performed better at detecting any metastasis (nodal or distance) with a sensitivity of 85% (compared to 38% by conventional) and specificity of 98% (compared to 91% by conventional) [39]. In OSPREY, using histopathology as gold standard, the sensitivity of 18F-DCFPyL in detection of pelvic nodal metastasis in high-risk patients was comparable to conventional imaging, approximately 40%, but with 3-fold higher positive predictive values (PPV: 86.7% vs. 28.3%). Furthermore, sensitivity increased to 60% when lymph nodes were larger than 5 mm in the short-axis, reflecting the inherent spatial limitations of PET resolution [40].
Nodal staging of intermediate-risk patients was examined in the SALT and PEPPER, prospective studies that examined the diagnostic accuracy of 18F-DCFPyL and 68Ga-PSMA-11, respectively. Both trials enrolled patients with negative bone scans who were able to undergo extended pelvic lymph node dissection. Both agents demonstrated a similar sensitivity of 40% and a specificity of over 91% [41, 42]. As expected, the PPV of PSMA PET increased with increased pre-test probability. In the SALT study of patients with Memorial Sloan Kettering Cancer Center (MSKCC) nomogram probability of ≥ 8% risk of lymph-node metastases, the overall prevalence of lymph node metastasis was 14.5% and PSMA PET PPV was 54.8% [41]. In PEPPER, which required MSKCC nomogram probability of ≥ 10% risk of lymph-node metastases [42], the overall prevalence of nodal metastasis was 37.9% and PSMA PET PPV was 77%.
PSMA PET in biochemical recurrence
In the setting of biochemical recurrence (BCR), PSMA PET has a very high PPV of 99% [34], and remains high at 84% when assessed strictly by histopathologic validation with substantial inter-reader reproducibility [43]. The sensitivity of detection was highly dependent on PSA levels, with a detection rate of 63% at PSA < 2 ng/mL versus 94% when the PSA was > 2 ng/mL [34]. In a retrospective review of 200 patients with castrate-resistant disease judged non-metastatic by conventional imaging (M0 CRPC), with a median PSA of 5.3 ng/mL, 55% of those patients had PSMA-positive metastatic disease [44]. Whether earlier detection of metastatic disease will truly affect the natural history of the patient is unknown, and there is significant concern regarding the potential effects of stage migration and lead-time bias on clinical trials [45].
Table 1 lists trials of PSMA imaging agents in the biochemical recurrent and/or metastatic space include PROPER-ABX (NCT04239742), SPOTLIGHT (NCT04186845), PROfind (NCT03490032), and SECuRE (NCT04868604), and in newly diagnosed disease include LIGHTHOUSE (NCT04186819) and GuideView (NCT04838626). PRIMARY2 (NCT05154162) and PICture (NCT04487847) both investigate the potential added value of PSMA PET to mpMRI in patients with suspected cancer. These agents vary by the PET tracer and the PSMA-binding ligand. For instance, 68Ga can be made by an on-site 68Ge/68Ga generator, while 18F production requires a cyclotron. 18F-PSMA-1007 and 18F-rhPSMA-7.3 have the advantage of lower urinary excretion, which can improve the ability to detect lesions in close proximity to the urinary tract [46, 47]. Radiohybrid (rh) is a class of molecules where the PSMA ligand is connected to two separate binding sites for radiometals, where the radioactivity of each binding site is modulated by radioisotope exchange. The advantage is that a diagnostic and therapeutic pair of compounds will have similar biodistribution and pharmacokinetics [48].
Management implications using PSMA PET
Given its superior detection rate compared to conventional imaging, it is not surprising that PSMA PET results in a change in definitive radiation therapy (RT) volume. In a retrospective review from UCLA in intermediate- and high-risk patients without evidence of nodal or distant metastases by conventional imaging, with a median PSA of 13.9, 68Ga-PSMA-11 PET identified disease outside of standard RT fields in 37% of patients [49]. Similarly, in high- and very high-risk patients, with a median PSA of 16 ng/mL, RT was altered in 53% of patients [50]. Boost to an avid lymph node accounted for 24% of changes, followed by metastasis directed RT to bone metastases and extension of nodal clinical target volume (CTV) to include PSMA-avid nodes.
The CONDOR trial investigated 18F-DCFPyL in men with biochemical recurrence after prostatectomy or prior radiation with negative or equivocal conventional imaging and showed that almost 64% of patients had a change in intended management based on 18F-DCFPyL findings [51]. In the salvage setting, about 20–30% of patients will have at least one PSMA PET lesion that is not covered by traditional salvage RT fields. In a retrospective review of patients with BCR after prostatectomy and PSA < 2.0 ng/mL (median PSA of 0.4 ng/mL), 30% of patients were found to have at least one PSMA PET-avid lesion outside salvage RT fields [52]. In a single arm prospective trial analyzing diagnostic sensitivity of 68Ga-PSMA-11 in patients with BCR after prostatectomy, over half of which did not have conventional imaging, PET imaging findings resulted in a change in the intended management in 68% of patients [53]. In a post-hoc analysis of this trial in patients with a PSA < 1.0 ng/mL, almost half (49%) had a 68Ga-PSMA-11 positive lesion, and 52% of those PSMA PET-avid lesions were outside the consensus RT volumes [54]. The majority of these (64%) were extra-pelvic lesions, and the remaining (36%) were within the pelvis but still outside the consensus volumes [54].
It remains to be determined whether changes in management resulting from PSMA PET information will translate to oncologic benefit. Furthermore it is uncertain whether M1 disease defined by conventional imaging reflects the same disease state and trajectory as M1 disease on molecular imaging. Table 2 summarizes select ongoing clinical trials that are investigating whether changes in management by molecular imaging translate into oncologic benefit: EMPIRE II (NCT03762759), PSMA SRT (NCT03582774), PSMA-PETgRT (NCT03525288) [55], NCT04794777, INDICATE (NCT04423211), and PATRON (NCT04557501).
PSMA PET in oligometastatic disease
The use of PSMA PET in oligometastatic disease, based on a pragmatic cut-off of 3–5 lesions, was explored in PSMA MRgRT, where patients with BCR and M0 disease by conventional imaging underwent metastasis directed therapy (MDT) to all sites of PSMA-avid disease without receiving concurrent hormonal therapy. The median time to PSA progression was 17.7 months and the median time to starting ADT was not reached [56]. Furthermore, complete metabolic response on PSMA PET at 4 months post MDT was prognostic for biochemical control. Similar results were observed in STOMP, where choline (11C or 18F) PET directed MDT improved median ADT-free survival to 21 months compared to 13 months with observation (p = 0.11) [57]. The 5-year ADT free survival was 34% vs. 8% (p = 0.06) and there were no reported CTCAE Gr ≥ 2 toxicities [58]. In contrast, ORIOLE enrolled participants with metastatic disease based on conventional imaging, and still demonstrated decreased from progression at 6 month of 61% vs. 19%, favoring MDT (p = 0.005) [59]. Sixteen of the 36 patients had baseline PSMA-PET imaging prior to treatment, which were blinded to the investigative team. Post hoc analysis noted that total consolidation of PSMA-avid lesions improved PFS (HR 0.26, p = 0.0055) and decreased the incidence of new metastases at 6 months from 63% with subtotal consolidation to 16% with total consolidation (p = 0.006) [59]. In the updated pooled analysis of STOMP and ORIOLE, MDT improved median PFS to 11.9 months compared with 5.9 months for observation (HR 0.44, p < 0.001) [60]. Notably, patients with a high-risk mutational signature (pathogenic somatic mutations in ATM, BRCA1/2, RB1, or TP53) derived a greater PFS benefit from MDT relative to no MDT (HR 0.05, p < 0.01) compared to those without the high-risk mutations (HR 0.42, p = 0.01) [60].
PSMA ligands and radiopharmaceutical opportunities
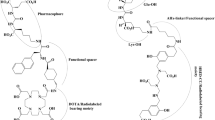
Multiple small molecule PSMA-targeted radiopharmaceuticals with beta or alpha-emitting radionuclides have been developed for prostate cancer therapy, including the recently FDA-approved 177Lu-PSMA-617 (177Lu vipivotide tetraxetan: Pluvicto®). Lutetium-177 has a convenient physical half-life of 6.7 days and emits β particles (maximum energy 497 keV) in the therapeutic range with a relatively low proportion of γ emission (113 keV at 6% and 208 keV at 11%). Its maximal tissue penetration of < 2 mm significantly reduces bystander dose to adjacent normal tissues. As a result of these favorable characteristics, 177Lu has emerged as a promising therapeutic radionuclide for multiple targets. Binding of the PSMA receptor induces dimerization and internalization of the complex, effectively trapping the radionuclide within the prostate cancer cell, where it subsequently induces double-strand DNA (dsDNA) damage leading to apoptosis (Fig. 1). Notably, other therapies that target PSMA are also in development, including radionuclide-conjugated antibodies, antibody-drug conjugates, T-cell recruiting bispecific agents, and cellular approaches with PSMA-directed chimeric antigen receptor (CAR)-T cells [61].
PSMA-617 and PSMA-I&T are low-molecular weight ligands (in black) with high binding affinity to PSMA, connected to the radionuclide by a linker (in red). Once bound, receptor dimerization can lead to internalization of the receptor-ligand complex. This allows concentration of the radionuclide, 177Lu, within the cell. Ionizing radiotherapy may produce DNA double-strand breaks in the cell and neighboring cells (up to ~2 mm), ultimately leading to cell death.
Clinical data supporting the use of 177Lu-PSMA-617 RPT
Metastatic castrate-resistant prostate cancer (mCRPC) remains an incurable disease. Figure 2 outlines the current landscape of treatment options, which includes conventional chemotherapy, such as docetaxel and cabazitaxel, additional androgen receptor signaling inhibitor (ARSI), such as abiraterone and enzalutamide, bone-seeking radiopharmaceuticals such as radium-223 dichloride, and most recently, 177Lu-PSMA-617 radioligand therapy.
SOC regimens are outlined in gray boxes, ongoing Phase 2 and 3 trials are highlighted in light-blue boxes and italicized. ECLIPSE uses 7.4GBq of 177Lu-PSMA-I&T, while SPLASH uses 6.8GBq of 177Lu -PNT2002. ARSI Androgen receptor signaling inhibitors, dMMR Deficient mismatch repair, HRR Homologous recombination repair, MSI Microsatellite instability, SOC Standard of care. *Progression on previous treatment with one ARSI (abiraterone or enzalutamide or darolutamide or apalutamide) **an ARSI that has not been previously tried.
The LuPSMA trial was the first prospective phase 2 study to evaluate 177Lu-PSMA-617 in 30 patients with mCRPC that had progression on standard treatment, demonstrated PSMA avid disease on PSMA PET imaging and, notably, and did not harbor discordant 18F-FDG-avid disease that was not PSMA-positive. Patients received up to 4 cycles of 177Lu-PSMA-617 at a mean of 7.5 GBq each. The primary endpoint of a ≥ 50% decline in PSA was achieved in 57% of patients [62]. Importantly, all patients who reported pain at baseline noted decrease in pain at all timepoints. Based on these clinically meaningful improvements, TheraP was developed to test 177Lu-PSMA-617 against cabazitaxel in a randomized fashion in patients with mCRPC who had progression on docetaxel. In this 200-patient trial, PSA response of ≥ 50% was achieved in 66% vs. 37% (p < 0.001) of patients who received 177Lu-PSMA-617 vs. cabazitaxel, respectively [63]. While the median PFS was similar between both arms at 5.1 months, the 12-month PFS was 19% vs. 3% (HR 0.63, 95% CI 0.46–0.86), favoring 177Lu-PSMA-617 [63]. At a median follow-up of 36 months, the restricted mean survival time was similar between both arms (19.1 months vs. 19.6 months) [64].
In 2021, the VISION phase III trial testing 177Lu-PSMA-617 was completed. VISION randomized 831 patients to 177Lu-PSMA-617 (7.4 GBq) plus standard of care (SOC) vs. SOC alone [65]. Of the 1003 patients who underwent 68Ga-PSMA-11 imaging, 87% had at least one PSMA positive lesion and were eligible for randomization. While patients with PSMA non-avid lesions were not eligible (required SUVmax ≥ liver), FDG PET was not required, which may explain why response rates were lower in VISION than compared to LuPSMA and TheraP. This was a pre-treated population, with virtually all having previously received docetaxel and ∼40% receiving two prior regimens of ARSI. Nevertheless, the addition of 177Lu-PSMA-617 to SOC demonstrated superior radiographic progression-free survival (rPFS) of 8.7 months vs. 3.4 months with SOC alone (HR 0.4, 95% CI 0.29–0.57). Median overall survival (OS) also favored the 177Lu-PSMA-617 arm at 15.3 months vs. 11.3 months with SOC alone (HR 0.62, 95% CI 0.52–0.74) [65]. Of note, while rPFS was evaluated in the set of 581 patients enrolled after corrective measures to reduce drop-out in the SOC arm, the OS benefit persisted in the entire cohort. Brief pain inventory (BPI-SF) and patient reported outcomes (EQ-5D, FACT-P) also favored the 177Lu-PSMA-617 arm. Results from VISION led to the Food and Drug Administration (FDA) approval of 177Lu-PSMA-617 in March 2022 for the treatment of PSMA-positive mCRPC (by 68Ga-PSMA-11) having previously been treated with ARSI and taxane-based chemotherapy [66].
Ongoing phase 2 and 3 trials are evaluating 177Lu-PSMA therapy in earlier line and earlier stage prostate cancer. This includes studies in men with chemo-naïve metastatic castrate resistant prostate cancer (SPLASH NCT04647526; PSMAFore NCT04689828; ENZA-P NCT04419402, ECLIPSE NCT05204927), metastatic castration-sensitive prostate cancer (UpFrontPSMA NCT04343885, PSMAddition NCT04720157), oligometastatic castration-sensitive prostate cancer (Bullseye NCT04443062; LUNAR NCT05496959), and locoregionally advanced or high-risk prostate cancer (LuTectomy NCT04430192; PROQURE-1 NCT05162573) (Fig. 2). Of note, 177Lu-PSMA-I&T (in ECLIPSE) and 177Lu-PNT2002 (in SPLASH) differ in formulations, but both contain the same urea-binding motif, linker and DOTAGA chelator [67].
In high-risk prostate cancer, Globan et al. [68] demonstrated safety and feasibility of administering up to 3 doses of neoadjuvant 177Lu-PSMA-I&T at 7.4 GBq per dose, given every two weeks, followed by surgery 4 weeks after the last dose. There were no Common Terminology Criteria for Adverse Events (CTCAE) Gr > 3 events during 177Lu-PSMA treatment and no intraoperative complications. Positive margins were identified in 53% of patients. In the phase 1 portion of LuTectomy, 10 patients were received 1 dose of 5 GBq of 177Lu-PSMA-617, proceeded by surgery 6 weeks later [69]. Preliminary results presented at the 2022 European Association of Urology showed a median absorbed dose of 48 Gy in the prostate and 50 Gy in the lymph nodes, with no CTCAE Gr ≥ 1 adverse events or Clavien-Dindo Gr ≥ 3 events. There were no cases of pathologic complete response or minimal residual disease in that study.
Biomarkers to predict benefit from 177Lu-PSMA
Heterogeneity of PSMA receptor activity (i.e. lack of dimerization and internalization), and varying levels of PSMA expression on tumor cells may account for the lack of response in some patients. Furthermore, differences in intrinsic cellular DNA-repair capabilities may also contribute to primary resistance. Therefore, biomarkers are needed to predict response to 177Lu-PSMA RPT. From the LuPSMA trial, tumor SUVmean was found to be positively correlated to whole-body tumor dose determined by single photon emission computed tomography (SPECT) dosimetry of 177Lu. Patients who achieved at least a 50% decline in PSA at 12 weeks had a median whole-body absorbed tumor dose of 14.1 Gy, compared to a whole-body tumor dose of 9.6 Gy in those who experienced less than a 50% PSA decline [70]. PSMA PET surrogate calculations from TheraP showed that a SUVmean ≥ 10 predicted a much greater odds of PSA response in patients who received 177Lu-PSMA-617 compared to cabazitaxel, with an odds ratio of 12.19 for SUVmean ≥ 10 vs. 2.22 for SUVmean < 10 (p = 0.039). The PSMA PET SUVmean did not predict for response to cabzitaxel [71]. Similarly, results from VISION found whole-body tumor SUVmean was associated with improved PFS (HR 0.86, 95% CI 0.82–0.91) and OS (HR 0.88, 95% CI 0.84–0.91) on multivariate analysis [72].
Data from the outcomes of RESIST-PC (study closed early due to sponsorship transfer) [73] and LuPSMA [62] were combined to develop a nomogram to predict outcomes after treatment with 177Lu-PSMA-617, which was both internally and externally validated [74]. Clinical characteristics that were predictive for both OS and PSA-progression-free survival included time since diagnosis (years), chemotherapy status (yes or no), tumor SUVmean (continuous variable), bone metastasis (present or absent), and liver metastasis (present or absent). The total number of lesions (< 20 or ≥ 20) was a predictor for OS, while pelvic nodal metastasis (present or absent) was a predictor for PSA-PFS. With a modest C-index of 0.71, an optimal cutoff score was used to stratify patients into low-risk vs. high-risk, where median OS was 24.9 months vs. 7.9 months (p < 0.0001) and PSA-PFS was 6.6 months vs. 2.5 months (p = 0.022), respectively [74]. While prospective validation is needed, these nomograms that are predictive of outcomes after 177Lu-PSMA in patients with mCRPC serve to guide individual clinical decision making.
The cytotoxic effects of radiation stems from either direct action (direct breakage of DNA atomic bonds) or indirect action (through ionization of water and creation of free radicals to induce DNA damage). The proportion of direct versus indirect effects is related to the linear energy transfer (LET), where alpha particles (e.g., 223Ra) have a much higher LET than beta particles (e.g. 177Lu). Higher LET will create more double-stranded breaks (DSBs), which are the main lethal event in inducing cell death, and is less dependent on the cell cycle phase and presence of hypoxia. Therefore, the type of radiation utilized and the integrity of genes that mediate the DNA damage response (DDR) pathway will influence the cytotoxic responsiveness to radiation. Aberrations in DSB repair mechanisms are increasingly recognized in mCRPC patients, where the prevalence of DDR germline and somatic mutations range from 8% to 12% [75, 76] and 20% to 25% [77], respectively. In a retrospective single-institution review of 28 mCRPC patients who received 223Ra, 80% of patients with HR mutations experienced a ≥ 30% decline in alkaline phosphatase (ALP) compared to 39% patients without HR mutations (p = 0.04) [78]. While there were no differences in PSA response, there was a trend towards improved median OS in patients who were HR-deficient (36.9 months vs. 19 months, p = 0.11) [78]. This is consistent with data suggesting that ALP decline was prognostic for OS independently of PSA changes in mCRPC patients with bone metastases who received chemotherapy [79]. Likewise, a multicenter cohort review noted that the presence of DDR aberrations in patients receiving 223Ra was associated with a longer median OS compared to those without mutations (36.3 months vs. 17 months, p = 0.01) [80]. Preliminary results from the prospective observational biomarkers study, PROPRADIUM (NCT02925702), also demonstrated improved ALP responses (> 30% decline in ALP at 12 weeks) in patients with germline HR-mutations (75% vs. 43%, p = 0.036), with a similar trend towards improvement in median OS (14.4 months vs. 10.6 months, p = 0.066) [81]. While there was one case report of extraordinary PSA response in a mCRPC patient with germline DDR mutations following 177Lu-PSMA-617 [82], a predefined retrospective review of 40 patients (42.5% of whom were DDR-deficient) did not identify any associations between pathogenic DDR aberrations and responsiveness to PSMA-RPT, regardless of the radionuclide used (177Lu or 225Ac) [83]. However, since only 7/40 patients received 225Ac by itself, there was unlikely to be sufficient power to determine whether patients with damage repair deficiencies benefited more from an alpha emitter. This “synthetic lethality” hypothesis that defects in mechanisms of DNA repair would render a tumor more susceptible to high-LET radiation is prospectively explored in NCT04489719. An excellent review of genomic biomarkers utilized in radiotherapy can be found here [84].
Radiation dosimetry of 177Lu-PSMA-617
Patient-specific dosimetry is not standardized for 177Lu-PSMA-617. Patients in VISION received a fixed dose of 7.4 GBq (200 mCi) per cycle. In TheraP, administered dose per cycle was between 6.0–8.5 GBq, adjusted based on tumor burden, patient’s weight, and renal function. The dose of 177Lu-PSMA used was informed by safety data from 177Lu-DOTATATE [85] and applying external beam radiation therapy (EBRT) absorbed dose constraints on bone marrow and kidney. While the convenience of a fixed dose has allowed ease of RPT integration into clinical workflow, patient-specific dosimetry using SPECT to directly image 177Lu could inform modification of the injected activity in order to increase the therapeutic index [86].
Current dosimetric normal organ constraints are primarily based on toxicity data from EBRT [87, 88]. However, there are critical differences between EBRT and RPT. First, while EBRT is generally prescribed as a dose to a point or volume, RPT is usually prescribed as an activity per injection, body weight, or surface area. Second, treatment with 177Lu-PSMA is greatly protracted, with 6 weeks between each dose. This is opposed to daily treatment with EBRT where there is normally a 24-hour interval in between each treatment. Kidneys have a low α/β ratio (∼2.6) [89], and as such, are very sensitive to the dose per fraction [90]. Third, 177Lu-PSMA is continuous therapy at a low and exponentially decreasing dose-rate due to source decay over time. This is in contrast to the relatively high dose-rate of EBRT. Fourth, 177Lu-PSMA uptake in areas of disease may be heterogeneous and vary by metastatic site or patient, and normal tissue uptake and corresponding dose are also variable. Violet et al. [70] found an inverse correlation between parotid dose and total volume of disease, suggesting a “sink effect”, in which the higher the burden of disease the more 177Lu-PSMA-617 is removed from circulation, thus resulting in less of the dose reaching normal organs.
There has been very limited acute renal toxicity observed in TheraP and VISION, as well as in most retrospective studies of 177Lu-PSMA. In VISION, the CTCAE Gr 3–4 renal effects – defined as any increase in blood creatinine or blood urea, acute kidney injury, proteinuria, or decreased urine output – were not statistically different between the two arms, at 3.4% in the 177Lu-PSMA-617 plus SOC vs. 2.9% in the SOC arm [65]. However, late radiation nephrotoxicity was not yet investigated in these trials, and recent studies have indeed demonstrated late radiation nephropathy in patients receiving excess cycles of 177Lu-PSMA [91].
Dosimetry studies using SPECT will be particularly critical in studies investigating dose escalation, re-treatment with RPTs, predictive variables for RPT biodistribution such as tumor burden, and combinations of external beam and RPT. In VISION, 15% of patients receiving 177Lu-PSMA also received concurrent palliative EBRT, without noticeable added toxicity [65].
Alpha emitters
As a calcium analogue, 223Ra directly targets the bone mineral hydroxyapatite, which is present and incorporated at the highest rate in areas of increased bone turnover, such as osteoblastic bone metastases. In contrast, the target of 177Lu-PSMA is the transmembrane receptor PSMA, which is overexpressed on prostate cancer cells. Therefore, while 223Ra localizes only to the bone, 177Lu-PSMA will target any cell with PSMA expression, including nodal or visceral metastases. 223Ra is an alpha emitter, and thus has an exquisitely short radiation path length of up to 0.05 mm. In contrast, 177Lu is a beta emitter with a path length > 10 times longer. These properties each have advantages and disadvantages. A shorter path length can reduce bystander normal tissue damage but may leave some tumor cells under-dosed with alpha emitting RPT. Other bone-targeting agents include β-emitters such as 32P, 153Sm, and 90Sr, which have historically been used for palliation of pain, but their deeper tissue penetrance and consequent hematologic toxicities have limited their utility [92].
223Ra dichloride (Xofigo®) gained FDA approval in 2013 for the treatment of men with symptomatic mCRPC bone metastases without known visceral metastases [93] (Table 3). This was based on the pivotal ALSYMPCA randomized phase III trial demonstrating an overall survival (OS) benefit (median OS of 11.3 m to 14.9 m, p < 0.001) [94]. Small institutional studies have shown that with more than 5 FDA approved lines of therapy in the mCRPC setting, success from 223Ra after multiple lines of therapy is likely suboptimal due to the probability of a greater extent of non-osseous disease and inability to complete all therapy [95]. Furthermore, the phase III ERA 223 trial testing the combination of 223Ra with abiraterone acetate plus prednisolone noted an absolute increase of 18% in the incidence of fractures in the combination therapy arm compared to abiraterone alone (29% versus 11%, respectively), where 79% of these fractures occurred at sites without bone metastases (i.e. non-pathologic fractures) [96]. Post-hoc analysis revealed that fractures were less common in patients taking bone health agents (BHA). As a result, the ongoing PEACE III trial (NCT02194842), which randomizes patients to enzalutamide with or without 223Ra, mandated the use of preventative BHA and noted a reduction of fractures to < 4% on both arms since implementation of BHA [97].
There are key differences in the patient populations between VISION and ALSYMPCA: 1) virtually all patients had previous exposure to docetaxel as was mandated by the VISION trial, in contrast to 57% in ALSYMPCA; 2) visceral metastases (lung and/or liver) were identified in 24% of patients on VISION using PSMA PET, while ALSYMPCA required no evidence of visceral metastases by conventional imaging.
Therefore, based on current evidence, situations that may be more appropriate for using 223Ra over 177Lu-PSMA-617 include patients with low or absent PSMA PET SUV expression, discordant FDG-positive disease on FDG-PET, and/or bone-predominant/only disease. Conversely, the presence of visceral disease or significant burden of nodal disease may favor 177Lu-PSMA-617 provided that these lesions express PSMA avidly. Table 4 provides a comparison between the two RPTs. A detailed review of 223Ra including mechanism of action [98] and its use in the treatment of bone metastases in mCRPC can be found here [99].
Alpha labeled PSMA agents include 213Bi, 225Ac, 211At, and 227Th. In a meta-analysis of 256 mCRPC patients treated with 225Ac-PSMA agents, a ≥ 50% decline in serum PSA was achieved in 62.8% patients, with estimated median PFS and OS of 9.1 months and 12.8 months, respectively [100]. While CTCAE Gr ≥ 3 xerostomia was limited to 1.2%, Gr 1–2 xerostomia ranged from 36% to 100% and was a major reason for treatment discontinuation [100]. On-going phase I/II studies evaluating small molecule PSMA-targeted alpha therapy include 227Th-PSMA (NCT03724747 [101]), 225Ac-PSMA-617 (NCT04597411), and TATCIST (225Ac-PSMA-I&T, NCT05219500).
Conclusions
PSMA PET has superior diagnostic accuracy compared to conventional imaging in both the initial staging of prostate cancer and in the setting of disease recurrence, with improved sensitivity at higher PSA values relative to lower PSA. Integration of PSMA PET imaging into radiation therapy decision-making and planning may improve biochemical EFS, and consolidation of all PSMA PET-avid lesions in oligometastatic disease may increase PFS and reduce the incidence of new metastases. Ongoing clinical trials will explore whether changes in clinical decision-making will translate into oncologic benefit. PSMA-targeted therapy with 177Lu-PSMA-617 is effective and well-tolerated in heavily pre-treated patients with PSMA-expressing mCRPC. PSMA PET SUV is prognostic for treatment response (SUVmean ≥ 10). Additional biomarkers are needed to predict therapy response and to enable better patient selection. The field of RPT in oncology is expanding, and embracing a multi-disciplinary approach involving radiation oncology, medical oncology, and nuclear medicine is important in the comprehensive strategic care of patients with advanced prostate cancer.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987;7:927–35.
Israeli RS, Powell CT, Fair WR, Heston WD. Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res. 1993;53:227–30.
Israeli RS, Powell CT, Corr JG, Fair WR, Heston WD. Expression of the prostate-specific membrane antigen. Cancer Res. 1994;54:1807–11.
Troyer JK, Beckett ML, Wright GL Jr. Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int J Cancer. 1995;62:552–8.
Wright GL Jr., Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol. 1995;1:18–28.
Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–5.
Sokoloff RL, Norton KC, Gasior CL, Marker KM, Grauer LS. A dual-monoclonal sandwich assay for prostate-specific membrane antigen: levels in tissues, seminal fluid and urine. Prostate. 2000;43:150–7.
Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52:637–40.
Minner S, Wittmer C, Graefen M, Salomon G, Steuber T, Haese A, et al. High level PSMA expression is associated with early PSA recurrence in surgically treated prostate cancer. Prostate. 2011;71:281–8.
Haberkorn U, Eder M, Kopka K, Babich JW, Eisenhut M. New Strategies in Prostate Cancer: Prostate-Specific Membrane Antigen (PSMA) Ligands for Diagnosis and Therapy. Clin Cancer Res. 2016;22:9–15.
Jadvar H. Imaging evaluation of prostate cancer with 18F-fluorodeoxyglucose PET/CT: utility and limitations. Eur J Nucl Med Mol Imaging. 2013;40:S5–10.
Bednarova S, Lindenberg ML, Vinsensia M, Zuiani C, Choyke PL, Turkbey B. Positron emission tomography (PET) in primary prostate cancer staging and risk assessment. Transl Androl Urol. 2017;6:413–23.
Sodee DB, Conant R, Chalfant M, Miron S, Klein E, Bahnson R, et al. Preliminary imaging results using In-111 labeled CYT-356 (Prostascint) in the detection of recurrent prostate cancer. Clin Nucl Med. 1996;21:759–67.
Liu H, Moy P, Kim S, Xia Y, Rajasekaran A, Navarro V, et al. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997;57:3629–34.
Bander NH, Trabulsi EJ, Kostakoglu L, Yao D, Vallabhajosula S, Smith-Jones P, et al. Targeting metastatic prostate cancer with radiolabeled monoclonal antibody J591 to the extracellular domain of prostate specific membrane antigen. J Urol. 2003;170:1717–21.
Alberts IL, Seide SE, Mingels C, Bohn KP, Shi K, Zacho HD, et al. Comparing the diagnostic performance of radiotracers in recurrent prostate cancer: a systematic review and network meta-analysis. Eur J Nucl Med Mol imaging. 2021;48:2978–89.
Calais J, Ceci F, Eiber M, Hope TA, Hofman MS, Rischpler C. et al. 18)F-fluciclovine PET-CT and (68)Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019;90:1286–94.
Sonni I, Felker ER, Lenis AT, Sisk AE, Bahri S, Allen-Auerbach M, et al. Head-to-Head Comparison of (68)Ga-PSMA-11 PET/CT and mpMRI with a Histopathology Gold Standard in the Detection, Intraprostatic Localization, and Determination of Local Extension of Primary Prostate Cancer: Results from a Prospective Single-Center Imaging Trial. J Nucl Med. 2022;63:847–54.
Tosoian JJ, Gorin MA, Rowe SP, Andreas D, Szabo Z, Pienta KJ, et al. Correlation of PSMA-targeted 18F-DCFPyL PET/CT findings with immunohistochemical and genomic data in a patient with metastatic neuroendocrine prostate cancer. Clin Genitourin Cancer. 2017;15:e65–8.
Bertagna F, Albano D, Cerudelli E, Gazzilli M, Giubbini R, Treglia G. Potential of radiolabeled PSMA PET/CT or PET/MRI diagnostic procedures in gliomas/glioblastomas. Curr Radiopharmaceuticals. 2020;13:94–98.
Bilgin R, Ergül N, Çermik TF. Incidental meningioma mimicking metastasis of prostate adenocarcinoma in 68Ga-labeled PSMA ligand PET/CT. Clin Nucl Med. 2016;41:956–8.
Hermann RM, Djannatian M, Czech N, Nitsche M. Prostate-specific membrane antigen PET/CT: False-positive results due to sarcoidosis. Case Rep. Oncol. 2016;9:457–63.
de Galiza Barbosa F, Queiroz MA, Nunes RF, Costa LB, Zaniboni EC, Marin JFG, et al. Nonprostatic diseases on PSMA PET imaging: a spectrum of benign and malignant findings. Cancer Imaging. 2020;20:23.
Rauscher I, Krönke M, König M, Gafita A, Maurer T, Horn T, et al. Matched-pair comparison of 68Ga-PSMA-11 PET/CT and 18F-PSMA-1007 PET/CT: frequency of pitfalls and detection efficacy in biochemical recurrence after radical prostatectomy. J Nucl Med. 2020;61:51–7.
Sasikumar A, Joy A, Nanabala R, Pillai M, Hari T. 68Ga-PSMA PET/CT false-positive tracer uptake in Paget disease. Clin Nucl Med. 2016;41:e454–5.
Ribeiro AMB, Lima ENP, Rocha MM. Fibrous dysplasia as a possible false-positive finding in 68Ga-labeled prostate-specific membrane antigen positron emission tomography/computed tomography study in the follow-up of prostate cancer. World J Nucl Med. 2019;18:409–12.
Rowe SP, Pienta KJ, Pomper MG, Gorin MA. PSMA-RADS version 1.0: A step towards standardizing the interpretation and reporting of PSMA-targeted PET imaging studies. Eur Urol. 2018;73:485.
Fanti S, Minozzi S, Morigi JJ, Giesel F, Ceci F, Uprimny C, et al. Development of standardized image interpretation for 68Ga-PSMA PET/CT to detect prostate cancer recurrent lesions. Eur J Nucl Med Mol Imaging. 2017;44:1622–35.
Eiber M, Herrmann K, Calais J, Hadaschik B, Giesel FL, Hartenbach M, et al. Prostate cancer molecular imaging standardized evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. 2018;59:469–78.
Toriihara A, Nobashi T, Baratto L, Park S, Hatami N, Duan H, et al. Comparison of three interpretation criteria of 68Ga-PSMA PET based on inter-and intra-reader agreement. Soc Nuclear Med. 2020;61:533–9.
Ceci F, Oprea-Lager DE, Emmett L, Adam JA, Bomanji J, Czernin J, et al. E-PSMA: The EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur J Nucl Med Mol Imaging. 2021;48:1626–38.
Emmett L, Buteau J, Papa N, Moon D, Thompson J, Roberts MJ, et al. The additive diagnostic value of prostate-specific membrane antigen positron emission tomography computed tomography to multiparametric magnetic resonance imaging triage in the diagnosis of prostate cancer (PRIMARY): a prospective multicentre study. Eur Urol. 2021;80:682–9.
Hope TA, Goodman JZ, Allen IE, Calais J, Fendler WP, Carroll PR. Metaanalysis of (68)Ga-PSMA-11 PET Accuracy for the Detection of Prostate Cancer Validated by Histopathology. J Nucl Med. 2019;60:786–93.
Zhang LL, Li WC, Xu Z, Jiang N, Zang SM, Xu LW. et al.(68)Ga-PSMA PET/CT targeted biopsy for the diagnosis of clinically significant prostate cancer compared with transrectal ultrasound guided biopsy: A prospective randomized single-centre study. Eur J Nucl Med Mol Imaging. 2021;48:483–92.
Raveenthiran S, Yaxley WJ, Franklin T, Coughlin G, Roberts M, Gianduzzo T, et al. Findings in 1,123 Men with Preoperative (68)Ga-Prostate-Specific Membrane Antigen Positron Emission Tomography/Computerized Tomography and Multiparametric Magnetic Resonance Imaging Compared to Totally Embedded Radical Prostatectomy Histopathology: Implications for the Diagnosis and Management of Prostate Cancer. J Urol. 2022;207:573–80.
Roberts MJ, Morton A, Donato P, Kyle S, Pattison DA, Thomas P, et al. 68Ga-PSMA PET/CT tumour intensity pre-operatively predicts adverse pathological outcomes and progression-free survival in localised prostate cancer. Eur J Nucl Med Mol imaging. 2021;48:477–82.
Roberts MJ, Morton A, Papa N, Franklin A, Raveenthiran S, Yaxley WJ, et al. Primary tumour PSMA intensity is an independent prognostic biomarker for biochemical recurrence-free survival following radical prostatectomy. Eur J Nucl Med Mol Imaging. 2022;49:3289–94.
Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395:1208–16.
Pienta KJ, Gorin MA, Rowe SP, Carroll PR, Pouliot F, Probst S, et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with (18)F-DCFPyL in Prostate Cancer Patients (OSPREY). J Urol. 2021;206:52–61.
Jansen BHE, Bodar YJL, Zwezerijnen GJC, Meijer D, van der Voorn JP, Nieuwenhuijzen JA, et al. Pelvic lymph-node staging with (18)F-DCFPyL PET/CT prior to extended pelvic lymph-node dissection in primary prostate cancer - the SALT trial. Eur J Nucl Med Mol Imaging. 2021;48:509–20.
van Kalmthout LWM, van Melick HHE, Lavalaye J, Meijer RP, Kooistra A, de Klerk JMH, et al. Prospective Validation of Gallium-68 Prostate Specific Membrane Antigen-Positron Emission Tomography/Computerized Tomography for Primary Staging of Prostate Cancer. J Urol. 2020;203:537–45.
Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5:856–63.
Fendler WP, Weber M, Iravani A, Hofman MS, Calais J, Czernin J, et al. Prostate-specific membrane antigen ligand positron emission tomography in men with nonmetastatic castration-resistant prostate cancerdisease burden by PSMA-PET in nmCRPC. Clin Cancer Res. 2019;25:7448–54.
Schöder H, Hope TA, Knopp M, Kelly WK, Michalski JM, Lerner SP, et al. Considerations on integrating prostate-specific membrane antigen positron emission tomography imaging into clinical prostate cancer trials by national clinical trials network cooperative groups. J Clin Oncol. 2022;40:1500–5.
Dietlein F, Kobe C, Hohberg M, Zlatopolskiy BD, Krapf P, Endepols H, et al. Intraindividual comparison of 18F-PSMA-1007 with renally excreted PSMA ligands for PSMA PET imaging in patients with relapsed prostate cancer. J Nucl Med. 2020;61:729–34.
Langbein T, Wang H, Rauscher I, Kroenke M, Knorr K, Wurzer A, et al. Utility of 18F-rhPSMA-7.3 PET for imaging of primary prostate cancer and preoperative efficacy in N-staging of unfavorable intermediate-to very high-risk patients validated by histopathology. J Nucl Med. 2022;63:1334–42.
Wurzer A, Di Carlo D, Schmidt A, Beck R, Eiber M, Schwaiger M, et al. Radiohybrid ligands: A novel tracer concept exemplified by 18F-or 68Ga-labeled rhPSMA inhibitors. J Nucl Med. 2020;61:735–42.
Calais J, Fendler WP, Eiber M, Gartmann J, Chu FI, Nickols NG, et al. Impact of (68)Ga-PSMA-11 PET/CT on the management of prostate cancer patients with biochemical recurrence. J Nucl Med. 2018;59:434–41.
Wu SY, Boreta L, Shinohara K, Nguyen H, Gottschalk AR, Hsu I-C, et al. Impact of staging 68Ga-PSMA-11 PET scans on radiation treatment plansin patients with prostate cancer. Urology. 2019;125:154–62.
Morris MJ, Rowe SP, Gorin MA, Saperstein L, Pouliot F, Josephson D, et al. Diagnostic Performance of (18)F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin Cancer Res. 2021;27:3674–82.
Boreta L, Gadzinski AJ, Wu SY, Xu M, Greene K, Quanstrom K, et al. Location of Recurrence by Gallium-68 PSMA-11 PET scan in prostate cancer patients eligible for salvage radiotherapy. Urology. 2019;129:165–71.
Fendler WP, Ferdinandus J, Czernin J, Eiber M, Flavell RR, Behr SC, et al. Impact of (68)Ga-PSMA-11 PET on the Management of Recurrent Prostate Cancer in a Prospective Single-Arm Clinical Trial. J Nucl Med. 2020;61:1793–9.
Calais J, Czernin J, Cao M, Kishan AU, Hegde JV, Shaverdian N, et al. 68Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/mL: impact on salvage radiotherapy planning. J Nucl Med. 2018;59:230–7.
Menard C, Delouya G, Wong P, Beauchemin M, Barkati M, Taussky D, et al. Randomized controlled trial of PSMA PET/CT guided intensification of radiotherapy for prostate cancer: Detection rates and impact on radiotherapeutic management. Int J Radiat Oncol, Biol, Phys. 2020;108:S18.
Glicksman RM, Metser U, Vines D, Valliant J, Liu Z, Chung PW, et al. Curative-intent metastasis-directed therapies for molecularly-defined oligorecurrent prostate cancer: A prospective phase II trial testing the oligometastasis hypothesis. Eur Urol. 2021;80:374–82.
Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36:446–53.
Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): Five-year results of a randomized phase II trial. Am Soc Clin Oncol. 2020;38:6_suppl, 10–10.
Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6:650–9.
Deek MP, Van der Eecken K, Sutera P, Deek RA, Fonteyne V, Mendes AA, et al. Long-term outcomes and genetic predictors of response to metastasis-directed therapy versus observation in oligometastatic prostate cancer: analysis of STOMP and ORIOLE trials. J Clin Oncol. 2022;40:3377–82.
Narayan V, Gladney W, Plesa G, Vapiwala N, Carpenter E, Maude SL, et al. A phase I clinical trial of PSMA-directed/TGFβ-insensitive CAR-T cells in metastatic castration-resistant prostate cancer. Am Soc Clin Oncol. 2019;37:7_suppl, TPS347.
Hofman MS, Sandhu S, Eu P, Price J, Akhurst T, Iravani A, et al. Lutetium-177 PSMA (LuPSMA) theranostics phase II trial: Efficacy, safety and QoL in patients with castrate-resistant prostate cancer treated with LuPSMA. Ann Oncol. 2017;28:v270.
Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804.
Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. TheraP: 177Lu-PSMA-617 (LuPSMA) versus cabazitaxel in metastatic castration-resistant prostate cancer (mCRPC) progressing after docetaxel—Overall survival after median follow-up of 3 years (ANZUP 1603). Am Soc Clin Oncol. 2022;40:16_suppl:5000.
Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl J Med. 2021;385:1091–103.
Advanced Accelerator Applications. PLUVICTO. U.S. Food and Drug Administration.
Weineisen M, Schottelius M, Simecek J, Baum RP, Yildiz A, Beykan S, et al. 68Ga-and 177Lu-labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J Nucl Med. 2015;56:1169–76.
Golan S, Frumer M, Zohar Y, Rosenbaum E, Yakimov M, Kedar D, et al. Neoadjuvant 177Lu-PSMA-I&T radionuclide treatment in patients with high-risk prostate cancer before radical prostatectomy: A single-arm Phase 1 trial. Eur Urol Oncol. 2022;7:S2588-9311(22)00165-1.
Dhiantravan N, Violet J, Eapen R, Alghazo O, Scalzo M, Jackson P, et al. Clinical trial protocol for LuTectomy: A Single-arm study of the dosimetry, safety, and potential benefit of 177Lu-PSMA-617 prior to prostatectomy. Eur Urol Focus. 2021;7:234–7.
Violet J, Jackson P, Ferdinandus J, Sandhu S, Akhurst T, Iravani A, et al. Dosimetry of (177)Lu-PSMA-617 in metastatic castration-resistant prostate cancer: Correlations between pretherapeutic imaging and whole-body tumor dosimetry with treatment outcomes. J Nucl Med. 2019;60:517–23.
Buteau JP, Martin AJ, Emmett L, Iravani A, Sandhu S, Joshua AM, et al. PSMA and FDG-PET as predictive and prognostic biomarkers in patients given [177Lu] Lu-PSMA-617 versus cabazitaxel for metastatic castration-resistant prostate cancer (TheraP): A biomarker analysis from a randomised, open-label, phase 2 trial. Lancet Oncol. 2022;23:1389–97.
Kuo P, Hesterman J, Rahbar K, Kendi AT, Wei XX, Fang B, et al. [68Ga] Ga-PSMA-11 PET baseline imaging as a prognostic tool for clinical outcomes to [177Lu] Lu-PSMA-617 in patients with mCRPC: A VISION substudy. Am Soc Clin Oncol. 2022;40:6_suppl, 5002.
Calais J, Czernin J, Thin P, Gartmann J, Nguyen K, Armstrong WR, et al. Safety of PSMA-targeted molecular radioligand therapy with 177Lu-PSMA-617: Results from the Prospective Multicenter Phase 2 Trial RESIST-PC (NCT03042312). J Nucl Med. 2021;62:1447–56.
Gafita A, Calais J, Grogan TR, Hadaschik B, Wang H, Weber M, et al. Nomograms to predict outcomes after (177)Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: An international, multicentre, retrospective study. Lancet Oncol. 2021;22:1115–25.
Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375:443–53.
Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–28.
Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N. Engl J Med. 2015;373:1697–708.
Velho PI, Qazi F, Hassan S, Carducci MA, Denmeade SR, Markowski MC, et al. Efficacy of radium-223 in bone-metastatic castration-resistant prostate cancer with and without homologous repair gene defects. Eur Urol. 2019;76:170–6.
Sonpavde G, Pond GR, Berry WR, de Wit R, Armstrong AJ, Eisenberger MA, et al. Serum alkaline phosphatase changes predict survival independent of PSA changes in men with castration-resistant prostate cancer and bone metastasis receiving chemotherapy. Urologic Oncol.: Seminars Original Investig. 2012;30:607–13.
van der Doelen MJ, Velho PI, Slootbeek PH, Naga SP, Bormann M, van Helvert S, et al. Impact of DNA damage repair defects on response to radium-223 and overall survival in metastatic castration-resistant prostate cancer. Eur J Cancer. 2020;136:16–24.
Castro E, Mejorada RL, Saez M, De Giorgi U, Aragón I, Laorden NR, et al. Impact of germline mutations in homologous recombination (HR) genes on the response to Radium-223 for metastatic castration-resistant prostate cancer (mCRPC). Ann Oncol. 2019;30:v343–v4.
Crumbaker M, Emmett L, Horvath LG, Joshua AM. Exceptional response to 177Lutetium prostate-specific membrane antigen in prostate cancer harboring DNA repair defects. JCO Precis Oncol. 2019;3:1–5.
Privé BM, Slootbeek PH, Laarhuis BI, Naga SP, van Der Doelen MJ, van Kalmthout LW, et al. Impact of DNA damage repair defects on response to PSMA radioligand therapy in metastatic castration-resistant prostate cancer. Prostate cancer Prostatic Dis. 2022;25:71–78.
Sutera P, Deek MP, Van der Eecken K, Wyatt AW, Kishan AU, Molitoris JK, et al. Genomic biomarkers to guide precision radiotherapy in prostate cancer. Prostate. 2022;82:S73–S85.
Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33.
Dosimetry for optimized, personalized radiopharmaceutical therapy. Seminars in Radiation Oncology, 2021.
Dawson LA, Kavanagh BD, Paulino AC, Das SK, Miften M, Li XA, et al. Radiation-associated kidney injury. Int J Radiat Oncol* Biol* Phys. 2010;76:S108–S115.
Emami B, Lyman J, Brown A, Cola L, Goitein M, Munzenrider J, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol* Biol* Phys. 1991;21:109–22.
Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42:5–19.
Bergsma H, Konijnenberg MW, van der Zwan WA, Kam BL, Teunissen JJ, Kooij PP, et al. Nephrotoxicity after PRRT with 177Lu-DOTA-octreotate. Eur J Nucl Med Mol Imaging. 2016;43:1802–11.
Schäfer H, Mayr S, Büttner-Herold M, Knorr K, Steinhelfer L, Böger CA, et al. Extensive 177Lu-PSMA Radioligand Therapy Can Lead to Radiation Nephropathy with a Renal Thrombotic Microangiopathy–like Picture. Eur Urol. 2022;7:S0302-2838(22)02401-0.
Radiopharmaceuticals for bone metastases. Seminars in Radiation Oncology, 2021.
Bayer HealthCare Pharmaceuticals. Xofigo. U.S. Food and Drug Administration.
Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23.
Spratt DE, Osborne JR, Zumsteg ZS, Rebeiz K, Leeman J, Rivera A, et al. Radium-223 outcomes after multiple lines of metastatic castration-resistant prostate cancer therapy in clinical practice: implication of pre-treatment spinal epidural disease. Prostate Cancer Prostatic Dis. 2016;19:271–6.
Smith M, Parker C, Saad F, Miller K, Tombal B, Ng QS, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:408–19.
Gillessen S, Choudhury A, Rodriguez-Vida A, Nole F, Gallardo Diaz E, Roumeguere TA, et al. Decreased fracture rate by mandating bone protecting agents in the EORTC 1333/PEACEIII trial combining Ra223 with enzalutamide versus enzalutamide alone: An updated safety analysis. Wolters Kluwer Health, 2021.
Morris MJ, Corey E, Guise TA, Gulley JL, Kevin Kelly W, Quinn DI, et al. Radium-223 mechanism of action: implications for use in treatment combinations. Nat Rev Urol. 2019;16:745–56.
Den RB, George D, Pieczonka C, McNamara M. Ra-223 treatment for bone metastases in castrate-resistant prostate cancer: Practical management issues for patient selection. Am J Clin Oncol. 2019;42:399–406.
Satapathy S, Sood A, Das CK, Mittal BR. Evolving role of 225Ac-PSMA radioligand therapy in metastatic castration-resistant prostate cancer—A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2021;24:880–90.
Hammer S, Hagemann UB, Zitzmann-Kolbe S, Larsen A, Ellingsen C, Geraudie S, et al. Preclinical efficacy of a Psma-targeted thorium-227 conjugate (Psma-Ttc), a targeted alpha therapy for prostate cancerpreclinical efficacy of Psma-Ttc in prostate cancer. Clin Cancer Res. 2020;26:1985–96.
Author information
Authors and Affiliations
Contributions
AYJ – study design, draft, manuscript preparation and revision; APK – study design, manuscript revision; QL – manuscript revision; ESA – conceptualization, supervision, study design, manuscript revision. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
AYJ was a paid consultant for Myovant. A.P.K has served as an unpaid consultant for Novartis and has received research support (to institution) from Merck, Bayer, Novartis and POINT. ESA has served as a paid consultant for Janssen, Astellas, Sanofi, Bayer, Bristol Myers Squibb, Amgen, Constellation, Blue Earth, Exact Sciences, Invitae, Curium, Pfizer, Merck, AstraZeneca, Clovis, and Eli Lilly; and has received research support (to his institution) from Janssen, Johnson & Johnson, Sanofi, Bristol Myers Squibb, Pfizer, AstraZeneca, Novartis, Curium, Constellation, Celgene, Merck, Bayer, Clovis, and Orion.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jia, A.Y., Kiess, A.P., Li, Q. et al. Radiotheranostics in advanced prostate cancer: Current and future directions. Prostate Cancer Prostatic Dis 27, 11–21 (2024). https://doi.org/10.1038/s41391-023-00670-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-023-00670-6
- Springer Nature Limited