Abstract
Background
Metabolic syndrome and its pharmacologic treatment can potentially influence the progression of prostate cancer in men receiving androgen deprivation therapy (ADT). We aimed to evaluate the association between metabolic syndrome and its pharmacologic treatment with time to castration-resistant prostate cancer (CRPC).
Methods
We identified 409 men with metastatic castration-sensitive prostate cancer receiving first line ADT from 1996 to 2014 at our institution. Information concerning metabolic syndrome, statin use, aspirin use, and metformin use at initiation of ADT was collected from medical records. Time to CRPC was defined as the duration between initiating ADT and diagnosis of CRPC based on the Prostate Cancer Working Group 3 definition. Flexible parametric survival models were used to calculate hazard ratios (HR, and 95% confidence intervals, CI) of the association between metabolic conditions and time from ADT initiation to CRPC.
Results
During a median follow-up of 59 months, 87% (N = 356) men progressed to CRPC. Median time to CRPC was 19 months. Fifty-six percent of men met the definition of metabolic syndrome. Controlling for demographic and prostate cancer-specific variables, metabolic syndrome was associated with shorter time to CRPC (HR 1.41, 95% CI 1.09–1.81). Importantly, in men with metabolic syndrome, statin use was associated with a slower progression to CRPC (HR 0.70, 95% CI 0.49–0.98).
Conclusions
Our study suggests that metabolic syndrome is a risk factor for earlier progression from castration-sensitive to castration-resistant prostate cancer and raises the possibility that treatment, such as statin use, may slow the time to progression.
Similar content being viewed by others
Introduction
Prostate cancer is currently diagnosed in 248,000 men in the United States each year [1]. While current controversies focus on overdiagnosis and treatment [2], metastatic prostate cancer remains a growing problem [3, 4]. Although newer treatments for metastatic prostate cancer increase life expectancy [5,6,7], almost all castration-sensitive prostate cancer (CSPC) patients treated with androgen deprivation therapy (ADT) progress to castration-resistant prostate cancer (CPRC) and death. Multiple mechanisms help to explain the development of CRPC including intracrine androgen synthesis, androgen receptor (AR) amplification, AR mutations, AR splice variants, and bypass pathways [8]. The risk of progression to CRPC is likely a complex interaction of genetic, environmental, and social factors.
Metabolic syndrome is also a major public health problem. It is related to an increased risk of cardiovascular disease, it is also associated with cancer development and progression, including prostate cancer. In a systemic review, metabolic syndrome was associated with a higher incidence of advanced and lethal prostate cancer [9]. An underpowered study of 82 men demonstrated that metabolic syndrome is potentially a risk factor for shorter time to progression to CRPC following treatment with ADT [10]. Furthermore, some of the individual components of metabolic syndrome (specifically obesity, insulin resistance, and dyslipidemia) have been identified as risk factors for the development of aggressive prostate cancer [11].
Pharmacologic treatments for metabolic syndrome and its sequelae, including statins [12, 13], metformin [14, 15], and aspirin [16], have been inversely associated with lethal prostate cancer. Interestingly, hypertension treatment has not been strongly associated with a protective effect [17, 18]. Although there is still a lack of large-scale, randomized trials, pharmacologic interventions for metabolic syndrome hold promise as a potential safe and effective secondary prevention approach for CRPC and prostate cancer death [19, 20]. Since no previous study has investigated the influence of the combination of metabolic syndrome and its pharmacologic treatment on progression of metastatic CSPC, we sought to determine the impact of metabolic syndrome and its pharmacologic treatment on the development of CRPC.
Materials and methods
Study population and data collection
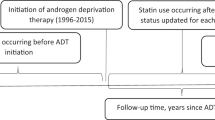
We identified 431 men with metastatic CSPC evaluated from 1996 to 2014 at either Brigham and Women’s Hospital or Dana-Farber Cancer Institute. Clinicopathological data were captured from an institutional clinical database as described previously [21] and from electronic medical records. The inclusion criteria were prostate cancer patients receiving first-line ADT for metastatic disease with longitudinal follow-up. Patients were excluded if they had insufficient follow-up data on prostate-specific antigen (PSA) level after ADT administration (N = 2), or if they received primary chemo-hormone therapy (N = 15), or if they received adjuvant ADT due to pathological lymph node positive after radical prostatectomy (N = 5), which left 409 patients for analysis. We collected pre-ADT information on metabolic syndrome, statin use, aspirin use, and metformin use within 6 months prior to administration of ADT. The study was approved by the institutional IRB (Protocol No: 2018P002714) with all participants providing written informed consent. There were no external funding sources (institutional resources only) and there are no conflicts of interest.
Exposure assessment
We used the modified Adult Treatment Panel (ATP) III criteria to define the presence or absence of metabolic syndrome [22]. A subject was categorized as having metabolic syndrome [22] if three or more of the following five criteria were met before start of ADT: blood pressure ≥130/85 mmHg or pharmacologic treatment, fasting triglyceride level ≥150 mg/dl or pharmacologic treatment, fasting HDL cholesterol level < 40 mg/dl or pharmacologic treatment, fasting blood sugar ≥100 mg/dl or pharmacologic treatment, and BMI ≥ 30 kg/m2. We used BMI ≥ 30 kg/m2 as an indicator of obesity as a surrogate for waist circumference >40 inches, because waist circumference was not available. This substitution has been validated previously [10, 23, 24]. Patients were defined as statin/aspirin/metformin users if they were using statin/aspirin/metformin before start of ADT.
Definition of CRPC
The primary outcome was CRPC using the Prostate Cancer Clinical Trials Working Group 3 (PCWG3) criteria: in a patient with testosterone <50 ng/dl as (1) new metastatic disease, or (2) an increase in two consecutive PSA levels obtained at least one week apart with a minimum PSA greater than 1.0 ng/ml, or (3) initiation of secondary hormone treatment for increasing PSA [25].
Covariates
The potential covariates included: age at ADT initiation, year of ADT initiation, self-reported race, smoking, biopsy Gleason score at diagnosis, primary treatment, clinical stage at ADT initiation, PSA at ADT initiation, and volume of disease at ADT initiation. The volume of disease was assessed by whole-body scintigraphy and MRI or CT staging scans and based on the CHAARTED trial definition [5]. Synchronous metastasis was defined as metastases within ≤3 months of initial diagnosis of prostate cancer, and metachronous metastasis was defined as metastasis diagnosed >3 months after initial diagnosis [26].
Statistical analysis
All patients were followed from the date of initiation of ADT until CRPC, death, loss to follow-up, or end of follow-up (July 31, 2019), whichever event came first. Proportional-hazards flexible parametric survival analysis [27] was used to estimate hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for the association between metabolic syndrome and time to CRPC, both with and without adjustment of potentially relevant epidemiologic (age at ADT initiation, year of ADT initiation, self-reported race, and smoking) and prostate cancer-specific factors (Gleason score, primary treatment, clinical stage at ADT initiation, and PSA at ADT initiation). HRs were also estimated for joint categories of metabolic syndrome and pharmacologic treatment, and for the individual components of metabolic syndrome. In addition to estimating proportional hazards, we fitted flexible parametric survival models [27] including time-dependent effects of metabolic syndrome. We further calculated the cumulative incidence of CRPC by metabolic syndrome status, taking the competing risk of death into account and using regression standardization to control for confounding. All flexible parametric models were fitted with five degrees of freedom for the baseline hazard and three degrees of freedom for time-varying effects [27]. Time since ADT initiation was the underlying time-scale for all analyses. Significance tests for all comparisons were two-sided and p values <0.05 were considered statistically significant. Missing variables were treated as separate categories, or if numbers were too small for a separate category (statin/aspirin/metformin use), coded as zeros. For metabolic syndrome, we performed a worst-case analysis in which all men with missing information were a) assumed to have and b) not to have metabolic syndrome. All analyses were carried out using STATA 15.0 (StataCorp, Texas, USA) and R version 3.6.2 (R Foundation for Statistical Computing, Wien, Austria).
Results
The clinical characteristics of the 409 included patients are summarized in Table 1 (by metabolic syndrome) and Supplementary Table 1 (by statin use). The median age at ADT initiation was 66 years. The majority of patients (85%) self-reported as White. More than half of the patients (54%) had a biopsy Gleason score >7 at cancer diagnosis; and 20%, 74%, 6% of patients had positive lymph node, bone metastasis, and visceral metastasis at ADT initiation, respectively. Forty-nine percent of patients had not received prior local therapy for prostate cancer with curative intent. The median PSA level at ADT initiation was 27.8 (interquartile range [IQR] 9.5–109.0) ng/ml. Metabolic syndrome was present in 56% of patients. In men without metabolic syndrome, 43% had no metabolic factor, 31% had one factor and 26% had two factors.
During a median follow-up time of 59 (IQR 36–105) months, 356 men with prostate cancer developed CRPC. Median time to CRPC in these men was 19 months (IQR 10–45). In multivariable models controlling for both demographic and prostate cancer-specific factors, metabolic syndrome (HR 1.41, 95% CI 1.09–1.81) was significantly associated with a shorter time to CRPC (Table 2). Of the individual components of metabolic syndrome, only an elevated fasting glucose was significantly associated with time to CRPC (Table 2). Results were similar in analysis adjusting for volume of disease instead of M stage (HR = 1.41, 95% CI = 1.09–1.83) (Supplementary Table 2). In a worst-case analysis examining missing information, the HR for metabolic syndrome ranged from 1.18 (95% CI 0.94–1.48) in a scenario in which men with missing information was assumed not to have metabolic syndrome, to 1.50 (95% CI 1.19–1.89) in a scenario in which all men with missing information were assumed to have metabolic syndrome (Supplementary Table 3).
In analyses examining the time-varying effect of metabolic syndrome, the association of time to CRPC was strongest in the second year after start of ADT (HRyear 2 2.06, 95% CI 1.34–3.16) (Supplementary Table 4). In year five, the rate of progression to CRPC remained elevated among men who had not had an early event (HRyear 5 1.54, 95% CI 1.00–2.39).
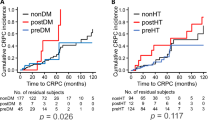
The cumulative incidence of CRPC in men with and without metabolic syndrome, both (a) unadjusted and (b) controlling for confounders is illustrated in Fig. 1. Five years after start of ADT, 82% (95% CI 76–88%) of men with metabolic syndrome had progressed to CRPC, compared with 66% (95% CI 58–74%) of men without.
We additionally examined joint categories of metabolic syndrome and pharmacologic treatments (Table 3). In multivariable models adjusting for demographic factors, men with metabolic syndrome and statin use had a slower progression to CRPC (HR 0.66, 95% CI 0.47–0.93) compared with men with metabolic syndrome but without statin use. The association remained significant in models with additional adjustment for Gleason score, primary treatment, clinical stage at ADT initiation, and PSA at ADT initiation (HR 0.70, 95% CI 0.49–0.98). In addition, men who used statins, but did not fulfill the criteria for metabolic syndrome, had the lowest rate of PSA progression (HR 0.32, 95% CI 0.15–0.65). Results were similar in a sensitivity analysis using only increased triglyceride level or decreased HDL cholesterol level as the reference group while adjusting for the other components of metabolic syndrome (Supplementary Table 5). The other medications were not significantly associated with time to PSA progression in the subgroup of patients with metabolic syndrome.
The cumulative incidence of CRPC among men with metabolic syndrome but without statin use, both (a) unadjusted and (b) controlling for confounders is present in Fig. 2. Five years after start of ADT, 91% (95% CI 83–98%) of men with metabolic syndrome but without statin use had progressed to CRPC. The corresponding percentage was 79% (95% CI 71–86%) in men with metabolic syndrome and statin use, and 71% (95% CI 63–79%) in men with neither. The median time to CRPC was 15 months in men with metabolic syndrome but without statin use, 23 months in men with metabolic syndrome and statin use, and 25 months in men with neither.
Discussion
In this cohort study of metastatic CSPC men initiating ADT, metabolic syndrome was associated with earlier progression to CRPC. In addition, statin use was significantly associated with a slower progression to CRPC in the subgroup of men with metabolic syndrome, suggesting that this drug might influence prostate cancer outcomes. Overall, the present study suggests that metabolic syndrome and its pharmacologic treatment may affect ADT response in men with metastatic CSPC.
Our research builds upon the results of a smaller study of 82 men [10], in whom metabolic syndrome was associated with a shorter time to CRPC (HR 2.55, 95% CI 1.37–4.77). This study population had a lower prevalence of advanced disease, and one-third of men did not have metastatic prostate cancer and therefore possible should have been followed without treatment or have benefited from potentially curative therapy such as radiotherapy plus ADT.
An additional strength of our study defining metabolic syndrome according to universally accepted ATP III definition, which is the best current predictor of the sequelae of metabolic syndrome including cardiovascular events and progression to type 2 diabetes [28,29,30]. Moreover, our study sample size and larger number of events over long-term follow-up provided greater power and ability to do subgroup analyses. Lastly, the information of pharmacologic treatment of metabolic syndrome allowed us to investigate whether the excess risk associated with metabolic syndrome could be offset by medications.
In line with previous reports [31,32,33,34,35,36], we did not observe associations between the individual components of metabolic syndrome (increased blood pressure, obesity, and abnormal cholesterol or triglyceride levels) and ADT response. The only component that demonstrated an association was fasting glucose ≥100 mg/dl. Several prior studies have reported that elevated fasting glucose level is associated with prostate cancer development and aggressiveness. Wright et al examined 1,734 men treated with radical prostatectomy or radiation therapy and demonstrated that a fasting glucose level ≥100 mg/dl was associated with a 50% increased risk of PSA progression compared with those with fasting glucose <100 mg/dl [37]. A second similar study of 5,126 cancer-free men, there was higher risk of fatal prostate cancer in those with hyperglycemia before diagnosis [38]. These studies demonstrate an association, but a large-scale long-term prospective study would be needed to demonstrate if better glucose control could improve ADT response in metastatic CSPC patients.
Consistent with the hypothesis that better control would translate to improve survival, we demonstrated a potential protective effect of statin. Our findings are consistent with statins as a protective factor for prostate cancer progression. Previous research has suggested that statins can reduce disease progression in prostate cancer patients receiving ADT [21, 39] and lower the incidence of prostate cancer mortality in men with PTEN-loss prostate cancer [12]. Pharmacologic intervention being associated with protection was not observed to the same extent in patients with metabolic syndrome taking aspirin or metformin. This is consistent with prior work demonstrating that drugs for metabolic diseases (such as anti-diabetes, anti-dyslipidemia, and anti-hypertension) do not reduce the risk of cancer-specific mortality in patients with ADT [29]. The possible reasons for these conflicting findings include different primary outcome (prostate cancer-specific mortality versus PSA progression), different subjects (all stage prostate cancer patients with primary ADT versus metastatic CSPC patients with ADT), and different categorizations (all subjects versus subjects with metabolic syndrome only). Potentially, it can provide clinicians with guidance for the treatment of men initiating ADT.
Prior work examining metformin has in some but not all studies demonstrate a benefit. Margel et al. retrospectively analyzed 3,837 diabetes mellitus men with prostate cancer and found the increasing duration of metformin use was associated with improvement in both all-cause and prostate cancer–specific mortality [40]. Studies have also demonstrated that metformin is not protective. A 6,537 men Finnish cohort demonstrated a higher risk of prostate cancer death among metformin users compared with nonusers [41]. While our study did not demonstrate a benefit, it was not designed to explore the role of metformin on ADT response in metastatic CSPC patients, given the limited to men with metabolic syndrome and only 25 men received metformin in our study. Randomized trials are underway to investigate metformin and prostate cancer including STAMPEDE which will determine if metformin decreases progression in men with metastatic prostate cancer [42].
To our knowledge, this is the first study to demonstrate a possible influence of statins on ADT response in patients with metabolic syndrome. This is the largest study performed in this patient population to date, with detailed information on established prognostic factors including demographic factors and prostate cancer-specific factors. Despite these strengths, several limitations should be noted. First, this is a retrospective, observational study with the inherent incomplete adjustment for confounders as in all studies of this nature. This might be particularly relevant for the findings related to statin use and time to CRPC. As observed in a previous report [21], statin users tend to have less advanced prostate cancer than non-statin users. Others have found that statin use is a marker of good health and access to high-quality health care [43]. Although we adjusted for several potential confounders in the present analysis, we lacked information on other factors such as the use of antihypertentive drugs, performance status, income, education, health care use, other comorbid conditions, and their medications. Second, we analyzed prevalent use of statins and other drugs, which is possibly susceptible for bias. This is exemplified in a previous meta-analysis, in which the authors found that observational studies using prevalent statin users over-estimated the protective effect of statin use on all-cause mortality in comparison to randomized-controlled trials [43]. Future, preferably randomized controlled trials or observational studies with information on start and end-dates of statin use [44], should address these possible biases. Third, though metastatic CRPC is a uniformly lethal condition, because we lacked detailed information on cause of death, we could not examine prostate cancer-specific death.
In conclusion, our data suggest that metabolic syndrome is a risk factor for earlier development of CRPC. Men with metabolic syndrome may benefit from statins to delay CRPC development. Further research is needed to better understand the possible interplay between metabolic factors and statin use on clinical outcomes of prostate cancer.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30.
Welch HG, Albertsen PC. Reconsidering prostate cancer mortality - the future of PSA screening. N. Engl J Med. 2020;382:1557–63.
Siegel DA, O’Neil ME, Richards TB, Dowling NF, Weir HK. Prostate cancer incidence and survival, by stage and race/ethnicity - United States, 2001-2017. MMWR Morb Mortal Wkly Rep. 2020;69:1473–80.
Cook MB, Hurwitz LM, Geczik AM, Butler EN. An up-to-date assessment of US prostate cancer incidence rates by stage and race: a novel approach combining multiple imputation with age and delay adjustment. Eur Urol. 2021;79:33–41.
Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–46.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–60.
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121–31.
Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013;32:5501–11.
Hammarsten J, Damber JE, Haghsheno MA, Mellström D, Peeker R. A stage-dependent link between metabolic syndrome components and incident prostate cancer. Nat Rev Urol. 2018;15:321–33.
Flanagan J, Gray PK, Hahn N, Hayes J, Myers LJ, Carney-Doebbeling C, et al. Presence of the metabolic syndrome is associated with shorter time to castration-resistant prostate cancer. Ann Oncol. 2011;22:801–7.
Hammarsten J, Peeker R. Urological aspects of the metabolic syndrome. Nat Rev Urol. 2011;8:483–94.
Allott EH, Ebot EM, Stopsack KH, Gonzalez-Feliciano AG, Markt SC, Wilson KM, et al. Statin use is associated with lower risk of PTEN-null and lethal prostate cancer. Clin Cancer Res. 2020;26:1086–93.
Wu SY, Fang SC, Shih HJ, Wen YC, Shao YJ. Mortality associated with statins in men with advanced prostate cancer treated with androgen deprivation therapy. Eur J Cancer. 2019;112:109–17.
Richards KA, Liou JI, Cryns VL, Downs TM, Abel EJ, Jarrard DF. Metformin use is associated with improved survival for patients with advanced prostate cancer on androgen deprivation therapy. J Urol. 2018;200:1256–63.
He K, Hu H, Ye S, Wang H, Cui R, Yi L. The effect of metformin therapy on incidence and prognosis in prostate cancer: a systematic review and meta-analysis. Sci Rep. 2019;9:2218.
Downer MK, Allard CB, Preston MA, Wilson KM, Kenfield SA, Chan JM, et al. Aspirin use and lethal prostate cancer in the health professionals follow-up study. Eur Urol Oncol. 2019;2:126–34.
Siltari A, Murtola TJ, Talala K, Taari K, Tammela TLJ, Auvinen A. Antihypertensive drug use and prostate cancer-specific mortality in Finnish men. PloS one. 2020;15:e0234269.
Santala EE, Rannikko A, Murtola TJ. Antihypertensive drugs and prostate cancer survival after radical prostatectomy in Finland-A nationwide cohort study. Int J Cancer. 2019;144:440–7.
Penney KL, Stampfer MJ. The time is ripe for a randomized trial of metformin in clinically localized prostate cancer. J Clin Oncol. 2013;31:3054–5.
Hamilton RJ. Making sense of the statin-prostate cancer relationship: is it time for a randomized controlled trial? Eur Urol Focus. 2017;3:221–2.
Harshman LC, Wang X, Nakabayashi M, Xie W, Valenca L, Werner L, et al. Statin use at the time of initiation of androgen deprivation therapy and time to progression in patients with hormone-sensitive prostate cancer. JAMA Oncol. 2015;1:495–504.
Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–7.
Deedwania P, Barter P, Carmena R, Fruchart JC, Grundy SM, Haffner S, et al. Reduction of low-density lipoprotein cholesterol in patients with coronary heart disease and metabolic syndrome: analysis of the Treating to New Targets study. Lancet. 2006;368:919–28.
Huptas S, Geiss HC, Otto C, Parhofer KG. Effect of atorvastatin (10 mg/day) on glucose metabolism in patients with the metabolic syndrome. Am J Cardiol. 2006;98:66–9.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34:1402–18.
Aluwini SS, Mehra N, Lolkema MP, Oprea-Lager DE, Yakar D, Stoevelaar H, et al. Oligometastatic prostate cancer: results of a dutch multidisciplinary consensus meeting. Eur Urol Oncol. 2020;3:231–38.
Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata J. 2009;9:265–90.
Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM, San Antonio Heart Study. The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes Care. 2003;26:3153–9.
Saely CH, Koch L, Schmid F, Marte T, Aczel S, Langer P, et al. Adult Treatment Panel III 2001 but not International Diabetes Federation 2005 criteria of the metabolic syndrome predict clinical cardiovascular events in subjects who underwent coronary angiography. Diabetes Care. 2006;29:901–7.
Tong PC, Kong AP, So WY, Yang X, Ho CS, Ma RC, et al. The usefulness of the International Diabetes Federation and the National Cholesterol Education Program’s Adult Treatment Panel III definitions of the metabolic syndrome in predicting coronary heart disease in subjects with type 2 diabetes. Diabetes Care. 2007;30:1206–11.
Smith MR, Bae K, Efstathiou JA, Hanks GE, Pilepich MV, Sandler HM, et al. Diabetes and mortality in men with locally advanced prostate cancer: RTOG 92-02. J Clin Oncol. 2008;26:4333–9.
Bosco C, Wong C, Garmo H, Crawley D, Holmberg L, Hammar N, et al. Drugs for metabolic conditions and prostate cancer death in men on GnRH agonists. BJU Int. 2018;121:260–7.
Dambal S, Howard LE, Allott EH, Aronson WJ, Kane CJ, Amling CL, et al. Serum lipids prior to starting androgen deprivation therapy and risk of castration resistant prostate cancer and metastasis: results from the SEARCH database. J Urol. 2020;203:120–7.
Hirata Y, Shiota M, Kobayashi T, Kashiwagi E, Takeuchi A, Inokuchi J, et al. Prognostic significance of diabetes mellitus and dyslipidemia in men receiving androgen-deprivation therapy for metastatic prostate cancer. Prostate Int. 2019;7:166–70.
Hu MB, Yang T, Hu JM, Zhu WH, Jiang HW, Ding Q. Prognostic factors in Chinese patients with prostate cancer receiving primary androgen deprivation therapy: validation of Japan Cancer of the Prostate Risk Assessment (J-CAPRA) score and impacts of pre-existing obesity and diabetes mellitus. Int J Clin Oncol. 2018;23:591–8.
Shiota M, Takeuchi A, Sugimoto M, Kashiwagi E, Dejima T, Kiyoshima K, et al. Prognostic impact of serum testosterone and body mass index before androgen-deprivation therapy in metastatic prostate cancer. Anticancer Res. 2015;35:6925–32.
Wright JL, Plymate SR, Porter MP, Gore JL, Lin DW, Hu E, et al. Hyperglycemia and prostate cancer recurrence in men treated for localized prostate cancer. Prostate Cancer Prostatic Dis. 2013;16:204–8.
Marrone MT, Selvin E, Barber JR, Platz EA, Joshu CE. Hyperglycemia, classified with multiple biomarkers simultaneously in men without diabetes, and risk of fatal prostate cancer. Cancer Prev Res (Philos). 2019;12:103–12.
Hamilton RJ, Ding K, Crook JM, O’Callaghan CJ, Higano CS, Dearnaley DP, et al. The association between statin use and outcomes in patients initiating androgen deprivation therapy. Eur Urol. 2021;79:446–52.
Margel D, Urbach DR, Lipscombe LL, Bell CM, Kulkarni G, Austin PC, et al. Metformin use and all-cause and prostate cancer-specific mortality among men with diabetes. J Clin Oncol. 2013;31:3069–75.
Vihervuori VJ, Talala K, Taari K, Lahtela J, Tammela TLJ, Auvinen A, et al. Antidiabetic drugs and prostate cancer prognosis in a finnish population-based cohort. Cancer Epidemiol Biomark Prev. 2021;30:982–9.
Gillessen S, Gilson C, James N, Adler A, Sydes MR, Clarke N, STAMPEDE Trial Management Group. Repurposing metformin as therapy for prostate cancer within the STAMPEDE trial platform. Eur Urol. 2016;70:906–8.
Danaei G, Tavakkoli M, Hernán MA. Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta-analysis of statins. Am J Epidemiol. 2012;175:250–62.
Emilsson L, García-Albéniz X, Logan RW, Caniglia EC, Kalager M, Hernán MA. Examining bias in studies of statin treatment and survival in patients with cancer. JAMA Oncol. 2018;4:63–70.
Acknowledgements
We thank Dr. Sweeney for critical review of the manuscript, Junaid Nabi and Grace Shaw for administrative assistance.
Funding
JHG was supported by a scholarship from Kaohsiung Municipal Siaogang Hospital. ASK is supported by the DiNovi Family Foundation. AP is supported by William Casey and the Swedish Society for Medical Research. LAM and KLP are supported by the Prostate Cancer Foundation.
Author information
Authors and Affiliations
Contributions
Conception and design JHG, KLP, ASK, LAM; acquisition of data MP, JHG; analysis and interpretation of data All authors; drafting of the manuscript All authors; critical revision of the manuscript for important intellectual content All authors; statistical analysis JHG, AP; obtaining funding ASK; administrative, technical, or material support ASK; supervision LAM, ASK. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki. The study was approved by the institutional IRB (Protocol No: 2018P002714). All participants provided written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Geng, JH., Plym, A., Penney, K.L. et al. Metabolic syndrome and its pharmacologic treatment are associated with the time to castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 25, 320–326 (2022). https://doi.org/10.1038/s41391-022-00494-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-022-00494-w
- Springer Nature Limited
This article is cited by
-
The effects of diet on prostate cancer outcomes
Nature Reviews Urology (2022)