Abstract
Background
Increasing evidence indicates an association between statins and reduced prostate cancer-specific mortality (PCSM). However, significant bias may exist in these studies. One particularly challenging bias to assess is the healthy user effect, which may be quantified by screening patterns. We aimed to evaluate the association between statin use, screening, and PCSM in a dataset with detailed longitudinal information.
Methods
We used the Veterans Affairs Informatics and Computing Infrastructure to assemble a cohort of patients diagnosed with prostate cancer (PC) between 2000 and 2015. We collected patient-level demographic, comorbidity, and tumor data. We also assessed markers of preventive care utilization including cholesterol and prostate specific antigen (PSA) screening rates. Patients were considered prediagnosis statin users if they had at least one prescription one or more years prior to PC diagnosis. We evaluated PCSM using hierarchical Fine-Gray regression models and all-cause mortality (ACM) using a cox regression model.
Results
The final cohort contained 68,432 men including 40,772 (59.6%) prediagnosis statin users and 27,660 (40.4%) nonusers. Prediagnosis statin users had higher screening rates than nonusers for cholesterol (90 vs. 69%, p < 0.001) and PSA (76 vs. 67%, p < 0.001). In the model which excluded screening, prediagnosis statin users had improved PCSM (SHR 0.90, 95% CI 0.84–0.97; p = 0.004) and ACM (HR 0.96, 95% CI 0.93–0.99; p = 0.02). However, after including cholesterol and PSA screening rates, prediagnosis statin users and nonusers showed no differences in PCSM (SHR 0.98, 95% CI 0.91–1.06; p = 0.59) or ACM (HR 1.02, 95% CI 0.98–1.05; p = 0.25).
Conclusion
We found that statin users tend to have more screening than nonusers. When we considered screening utilization, we observed no relationship between statin use before a prostate cancer diagnosis and prostate cancer mortality.
Similar content being viewed by others
Background
Statin use has increasingly gained interest for its potential role to decrease mortality and improve prostate cancer-specific outcomes. Recent large-scale observational studies have demonstrated a protective effect of statin use on prostate cancer-specific mortality (PCSM) [1,2,3]. Many biological mechanisms have been proposed, including the inhibition of HMG-CoA reductase and thus the inhibition of mevalonate and cholesterol synthesis pathways is important for androgen deprivation and tumor growth [4, 5].
While these studies have revealed promising associations between statin use and prostate cancer (PC) prognosis, observational studies have historically been hindered by biases, including surveillance and healthy user effects [6,7,8]. Previous studies have long suspected the potential significant influence of unmeasured effects like increased health seeking behavior and screening linked to statin use [9,10,11]. However, there is a scarcity of empirical data on the healthy user effect in the context of statin use and PCSM.
Routine cholesterol testing and PSA screening tests may serve as surrogates of preventive care utilization and reveal the bias associated with statin use. These surrogates have not been regularly tested in observational studies likely because such granular information is often unavailable. However, the Veterans Affairs Informatics and Computing Infrastructure (VINCI) provides the opportunity to collect this data, gathered prospectively as part of routine care, and analyze the secondary effects of statin-related utilization of preventive care.
We sought evidence of the healthy user bias in a diverse population of patients with PC taking statins. We hypothesized that the association between statin use and reduced PCSM is confounded by healthy user effects. When we include markers of preventive care utilization, like PSA screening and cholesterol testing, we hypothesized no association between statin use and PCSM.
Methods
Data source
This study was conducted using VINCI, an electronic platform providing access to patient-level electronic health record information and administrative data for all veterans within the Veteran Affairs (VA) healthcare system. VINCI incorporates tumor registry data gathered at individual VA medical centers according to the protocols issued from the American College of Surgeons. We linked VINCI with the National Death Index (NDI) to obtain cause-specific mortality information (ICD-10 code C61 for PC) and with the American Community Survey to obtain zip-code level income and education. This study was approved by the local VA Institutional Review Board.
Study population
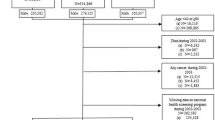
We identified 80,863 stage I–IV PC patients without prior malignancies diagnosed between 2000 and 2015. Patients were required to have at least 2 years of prediagnosis medical care at the VA to be included in the cohort. We sequentially eliminated 527 patients with unknown stage disease and 3005 patients with an unknown PSA value at the time of PC diagnosis. We then eliminated patients with one or more of the following variables missing: 5687 with missing Gleason score; 2219 patients with missing zip-code associated income data; 1910 patients with missing zip-code associated high school diploma rate; and 6 patients with missing treatment information to total 11,003 unique patients eliminated. We limited the cohort to patients with known race, eliminating 1428 patients with missing race. This resulted in a final cohort of 68,432 patients. All patients were followed until death or last follow-up with a VA provider with latest possible follow-up on December 31, 2017.
Exposure assessment
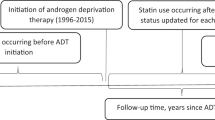
Information on statin use was obtained from the VA pharmacy data. In the primary analysis, we defined prediagnosis statin use as having at least one prescription filled one or more years before a PC diagnosis. In a sensitivity analysis, to account for regular statin users, we increased the minimum number of statin prescriptions one or more years before a PC diagnosis from one to five—those with less than five statin prescriptions were in the nonuser group. In the secondary analysis, we evaluated the influence of postdiagnosis statin use on PCSM. We defined postdiagnosis statin use as having at least one prescription filled within 1 year after a PC diagnosis.
Covariates
Demographic and comorbidities
We extracted the following patient-level variables: age, year of diagnosis, marital status, employment status, service-connected disability rating, race, alcohol history, tobacco history, body mass index (BMI), and zip-code income and education. We determined Charlson comorbidity index score from comorbid conditions patients had in the year prior to diagnosis using previously described methods [12,13,14]. We also obtained information on any use of aspirin, other non-steroidal anti-inflammatory drugs (NSAID), and 5-alpha reductase inhibitors (5-ARIs) in the 1 year prior to PC diagnosis from VA pharmacy data.
Disease and treatment characteristics
We collected PC staging information such as Gleason score, PSA, and clinical T/N/M stage. We collected treatment-related information such as radiation therapy, surgery, and androgen deprivation therapy (ADT).
Preventive care utilization
We defined the PSA screening rate as a ratio of the number of years in which a patient had at least one PSA measured divided by the number of years that the patient was in the VA system prior to PC diagnosis, with a maximum designation of 5 years. To avoid including PSA values associated with PC diagnosis, we only considered PSA lab tests conducted one or more years before the PC diagnosis date. For example, an annualized PSA screening rate of 75% for a patient with a 4-year history in the VA prior to a PC diagnosis signifies that he had at least one PSA lab value 3 of the 4 years. We defined the cholesterol screening rate similarly using low density lipoprotein (LDL) as the lab value.
Model building: base and expanded models
In the primary analysis, we used two models to evaluate the impact of prediagnosis statins on PCSM. The base model was designed to emulate models from previous studies by incorporating data routinely collected in cancer registries [1, 3, 15,16,17,18]. Specifically, the base model included demographic, comorbidity, and treatment-related information (as described previously in the “Covariates”). The expanded model added information acquired through the linked electronic health record, not routinely available in most registries. This included utilization of preventive care, specifically annualized PSA screening, and cholesterol screening. On sensitivity analysis, we also separated the preventive care measures in the expanded models to only include either annualized PSA screening or cholesterol screening. Tumor characteristics, including T/N/M staging variables, Gleason score, PSA at diagnosis, and risk group were not included as covariates in our primary analyses because they are on the causal pathway between exposure (prediagnosis use of statins) and outcome (PCSM) [19].
In the secondary analysis, we used the base and expanded models to evaluate the effect of postdiagnosis statins on PCSM. In the postdiagnosis statin setting, we included tumor characteristics as covariates in these models because they are not on the causal pathway between exposure (postdiagnosis use of statins) and outcomes (PCSM).
Primary and secondary outcomes
The primary outcome of our study was PCSM. The secondary outcome was all-cause mortality. For analysis of survival, we used data from the NDI to determine date and cause of death. Patients alive at the date of last follow-up were censored on that date. Survival was measured from the date of diagnosis to the date of censoring, non-PC death, or PC death.
Statistical analysis
We tested for differences in covariates between exposure groups using Chi-Square and Wilcoxon rank-sum tests when appropriate. To assess the relationship between our preventive measures, we evaluated the association between screening rates of PSA and cholesterol with the Spearman test. We modeled PCSM using competing events of cancer versus non-cancer death with a Fine-Gray regression and reported subdistribution hazard ratios (SHR) with 95% confidence intervals (CI) [20]. We selected all variables significant at the 0.05 level in univariable analysis for multivariable analysis. Overall survival analysis was performed with a Cox Proportional Hazards model, again using a univariable screen for variable selection in the multivariable analysis.
We used R version 3.5.1 for analyses and figure design, using packages “tidyverse”, “cmprsk”, and “survival” for data manipulation and figure design [5], Fine-Gray regression [6], and Cox proportional hazards analysis [7], respectively. All p values were two-sided.
Results
Baseline characteristics
The final cohort contained 68,432 men including 40,772 (59.6%) prediagnosis statin users and 27,660 (40.4%) nonusers. On average, prediagnosis statin users had their first statin prescription 6.1 years before their PC diagnosis and had an average of 18.2 statin prescriptions in the exposure period. The median follow-up time was 5.87 years, and a total of 10,431 (15.2%) men were followed for at least 10 years. Prediagnosis statin users were older that nonusers (mean age 67.0 vs. 64.9, p < 0.001) and had a higher BMI (mean BMI 29.1 vs. 27.6, p < 0.001). They were more likely to have a Charlson score greater than or equal to 2 (14.7 vs. 10.5%, p < 0.001) and be married (55.1 vs. 46.3%, p < 0.001). Prediagnosis statin users were also less likely to be current smokers (27.5 vs. 35.1%, p < 0.001) and have an alcohol history (49.0 vs. 54.2%, p < 0.001).
Preventive care utilization
Prediagnosis statin users were more likely to have regular PSA screening (mean annualized rate 75 vs. 66%, p < 0.001). They were also more likely to have regular cholesterol screening (mean annualized rate 89 vs. 68%, p < 0.001). Prediagnosis statin users were more likely to have screening rates >80% for both PSA (61.4 vs. 44.4%, p < 0.001) and cholesterol (84.1 vs. 48.4%, p < 0.001) (Fig. 1). Overall, there was a statistically significant relationship between PSA and cholesterol screening rates (Spearman correlation coefficient of 0.46, p < 0.001) (Fig. 2).
Disease characteristics
Prediagnosis statin users had a lower median PSA at diagnosis (6.10 ng/mL vs. 6.60, p < 0.001). They were less likely to have T3 or T4 disease (2.5 vs. 3.0%, p < 0.001), high risk disease (23.7 vs. 25.2%, p < 0.001) and metastatic disease (2.9 vs. 3.3%, p = 0.002) (Table 1).
Survival analyses
In the cohort of 68,432 men, there were 11,576 deaths including 3515 from PC (1915 statin users and 1600 nonusers). The 10-year cumulative incidence of death from PC was 5.2% for prediagnosis statin users and 6.9% for nonusers (p = 0.005). The 10-year cumulative incidence of death from causes other than PC was 18.2% for prediagnosis statin users and 20.6% for nonusers (p < 0.001). Overall survival at 10 years was 76.6% and 72.5% (p < 0.001) for prediagnosis statin users and nonusers, respectively.
In the base model, prediagnosis statin use was associated with a decreased risk of both PCSM (SHR 0.90, 95% CI 0.84–0.97; p = 0.004) and ACM (HR 0.96, 95% CI 0.93–0.99; p = 0.02). However, after expanding the base model to include PSA and cholesterol screening rates, prediagnosis statin users and nonusers had no differences in PCSM (SHR 0.98, 95% CI 0.91–1.06; p = 0.59) and ACM (HR 1.02, 95% CI 0.98–1.05; p = 0.25). We explored in the expanded model individual interactions between prediagnosis statin use and race, treatment modality, ADT use, and PSA screening. Each stratification was nonsignificant: race (e.g., African-American, non-Hispanic White, other; p = 0.90), treatment modality (e.g., radiation, surgery, radiation/surgery; p = 0.43), ADT (e.g., yes and no; p = 0.18), and PSA screening rate (e.g., ≥80 and <80%; p = 0.72).
In the expanded model, PSA screening was associated with decreased risks of both PCSM (SHR 0.52, 95% CI 0.46–0.59; p < 0.001) and ACM (HR 0.70, 95% CI 0.65–0.74; p < 0.001). Furthermore, cholesterol screening was also associated with decreased risks of both PCSM (SHR 0.83, 95% CI 0.72–0.96; p = 0.01) and ACM (HR 0.84, 95% CI 0.78–0.90; p < 0.001) (Table 2)
In the secondary analysis, postdiagnosis statin use was not associated with improved PCSM in either the base model (SHR 0.91, 95% CI 0.76–1.11, p = 0.36) or the expanded model (SHR 0.92, 95% CI 0.76–1.11, p = 0.37) (Supplementary Table 1).
Sensitivity analysis
The results were very similar to the base case when changing the criteria of statin exposure before PC diagnosis to at least five statin prescriptions (Supplementary Table 2). Furthermore, prediagnosis statin use was not associated with improved PCSM when the expanded model only included as a preventive care measure PSA screening (SHR 0.98, 95% CI 0.95–1.02; p = 0.36) or only cholesterol screening (SHR 0.98, 95% CI 0.99–1.06, p = 0.20).
Discussion
In this large population-based study, we found that the protective effect of statin use before a PC diagnosis on mortality was confounded by the healthy user effect. Like many others, we found that statins were associated with improved PC mortality when we only considered covariates routinely included in published studies [1, 3, 15,16,17,18]. However, after including markers of preventive care utilization not included in most observational analyses, the protective effect of statins on PC mortality was no longer observed. Furthermore, we found that statin use after a PC diagnosis was not associated with improved PC mortality in any models Table 3.
To our knowledge, many cancer registries and other large datasets do not include sufficient detail to permit estimation of preventive care utilization like annualized PSA screening rates. We could uncover this confounder because the VINCI dataset collects longitudinal data from the electronic medical record and contains most lab values, including PSAs and LDLs. Our results highlight the importance of collecting significant confounders including surrogates for health care utilization in large registries for more trustworthy comparative effectiveness research.
Similar to previous studies, we observed that statin users are much more likely to have their PSAs and cholesterol annually checked in the years preceding a PC diagnosis [7, 21, 22]. One study showed that in a Pennsylvania cohort of 20,783 new statin users, patients who regularly filled statin prescriptions were more likely than patients who filled only one prescription to receive PSA tests (HR 1.57, 95% CI 1.17–2.19), fecal occult blood tests (HR 1.31, 95% CI 1.12–1.53), screening mammograms (1.22, 95% CI 1.09–1.38), influenza vaccinations (HR 1.21, 95% CI 1.12–1.31), and pneumococcal vaccinations (HR 1.46, 95% CI 1.17–1.83) [21]. In another study of 141,086 new statin users, those who regularly filled statin prescriptions were also more likely to be screened with multiple tests. They were also less likely to have motor vehicle accidents (HR 0.75, 95% CI 0.72–0.79) and workplace accidents (HR 0.75; 95% CI 0.74–0.81). Furthermore, they were less likely to develop diseases unrelated to a biological effect of a statin (HR 0.87, 95% CI 0.86–0.89), including dental problems (HR 0.76, 95% CI 0.72–0.81) and drug dependency (HR 0.73, 95% CI 0.65–0.83) [22]. In our study, we observed statin users were less likely to be current smokers and have an alcohol history. These results raise the possibility that patients adherent to statins may also be more likely to make other healthy decisions that affect mortality.
Interestingly, we observed that PSA screening was associated with a substantial reduction in all-cause mortality (HR 0.70, 95% CI 0.65–0.74). No randomized evidence indicates that PSA screening reduces all-cause mortality [23,24,25]. This likely represents a significant healthy user bias in our study. Furthermore, we found that PSA screening was associated with a substantial reduction in PC mortality (SHR 0.59, 95% CI 0.50–0.69; p < 0.001). In our study, PSA screening was associated with a much larger reduction in PC mortality than reported at 13 years of follow-up in the European Randomized Study of Screening for PC (HR 0.79, 95% CI 0.69–0.91) [23]. This may also indicate a healthy user bias, which would be theoretically minimized through the virtues of randomization. Prediagnosis statin users, who were more likely to have annual PSA screening, had lower PC disease burden compared with nonusers. Given the nonrandomized, observational nature of our study, we do not claim there is a causal relationship between PSA screening and improved PC mortality. The effect of PSA screening on improved PC mortality may stem from earlier detection of disease, but it may also reflect a lead time bias [26, 27].
There are a large number of recent observational studies showing strong associations between statin use and improved PC outcomes [1, 3, 15,16,17,18]. These studies are supported by many biological studies showing inhibition of PC inflammation, cell proliferation, angiogenesis, invasion, and promotion of apoptosis [28]. A recent analysis involving nationwide Danish registries evaluated post-diagnostic statin use in 31,790 PC patients and reported a 17% decrease in mortality [3]. In another study involving 11,772 patients with PC from the United Kingdom, statin use before PC diagnosis decreased the risks of both PCSM (HR 0.55, 95% CI 0.41–0.74), and ACM (HR 0.66, 95% CI 0.53–0.81) [29]. Another group showed statin use before a PC diagnosis resulted in a 19% reduction in mortality in a subset of 27,752 patients. In this same study, statin use before a cancer diagnosis in 26 other cancer types also showed 13–17% reductions in cancer-specific mortality [1]. None of the aforementioned studies included differences in PSA testing or any other surrogates of preventive care or health care utilization between statin users and nonusers in their survival analyses. Though Danish and UK patients undergo less PSA testing than patients in western countries, PSA testing and other utilization of health care services may still be different between statin users and nonusers.
Prospective evidence exists suggesting that in the PSA screening era, statins are not associated with increased PC incidence. In a cohort study of 9457 men 55 years old or older at randomization to the placebo arm of the PC Prevention Trial, statin use during the trial was not associated with the risk of PC (HR 1.03, 95% CI 0.82–1.30) [30]. Importantly, as part of the protocol, patients in both arms of the trial were instructed to receive annual PSA screening and digital rectal examinations. This likely masked any potential statin-correlated healthy user effects present in a real-world setting and is an important corroboration of our findings. Some randomized evidence also indicates that statins are not associated with changes in tumor proliferation in PC patients. In perhaps the first study to test statins in PC patients in a randomized, double-blind, placebo-controlled setting, a total of 160 statin-naïve PC patients scheduled for radical prostatectomy were randomized to use atorvastatin or placebo daily from recruitment to surgery for a median of 27 days. Overall, atorvastatin did not significantly lower tumor proliferation index Ki-67 or PSA compared with placebo [31].
The major strengths of our study include the large study size with up to 16 years of follow-up for assessment of all-cause and PCSM with high-quality registry data. We also had the ability to ascertain screening rates because of VINCI’s unique longitudinal collection of lab data. There were also limitations to our study. We did not have information about patients’ diet or physical activity. Furthermore, statin use was ascertained by receipt of a prescription of a statin. Therefore, misclassification of exposure was possible if patients did not take their statins as instructed or received statins from non-VA providers. Furthermore, we used the NDI for cause-specific mortality, therefore introducing the possibility of misclassification for the cause of death. However, we believe there were no indications of differential bias as studies have shown that death certificates accurately reflect PCSM [32].
In summary, using a detailed longitudinal dataset, we observed that prediagnosis statin users utilize more preventive screening than nonusers. When considering rates of PSA and cholesterol screening, we observed that the association between prediagnosis statin use and the reduced risk of PCSM was nullified. Our study contributes compelling evidence that statin use is more likely an indicator of increased health care use than a cause for reduced PCSM. We recommend cautious interpretation of observational analyses attributing protective effects of statins and other similar medications on cancer-specific mortality.
References
Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367:1792–802. https://doi.org/10.1056/NEJMoa1201735.
Joentausta RM, Rannikko A, Murtola TJ. Prostate cancer survival among statin users after prostatectomy in a Finnish nationwide cohort. Prostate. 2019. https://doi.org/10.1002/pros.23768.
Larsen SB, Dehlendorff C, Skriver C, Dalton SO, Jespersen CG, Borre M, et al. Postdiagnosis statin use and mortality in danish patients with prostate cancer. J Clin Oncol. 2017;35:3290–7. https://doi.org/10.1200/JCO.2016.71.89814.
Boudreau DM, Yu O, Johnson J. Statin use and cancer risk: a comprehensive review. Expert Opin Drug Saf. 2010;9:603–21. https://doi.org/10.1517/14740331003662620.
Altwairgi AK. Statins are potential anticancerous agents (review). Oncol Rep. 2015;33:1019–39. https://doi.org/10.3892/or.2015.3741.
Chubak J, Boudreau DM, Wirtz HS, McKnight B, Weiss NS. Threats to validity of nonrandomized studies of postdiagnosis exposures on cancer recurrence and survival. J Natl Cancer Inst. 2013;105:1456–62. https://doi.org/10.1093/jnci/djt211.
Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med. 2011;26:546–50. https://doi.org/10.1007/s11606-010-1609-1.
Emilsson L, García-Albéniz X, Logan RW, Caniglia EC, Kalager M, Hernán MA. Examining bias in studies of statin treatment and survival in patients with cancer. JAMA Oncol. 2018;4:63. https://doi.org/10.1001/jamaoncol.2017.2752.
Park HS, Schoenfeld JD, Mailhot RB, Shive M, Hartman RI, Ogembo R, et al. Statins and prostate cancer recurrence following radical prostatectomy or radiotherapy: a systematic review and meta-analysis. Ann Oncol. 2013;24:1427–34. https://doi.org/10.1093/annonc/mdt077.
Scosyrev E, Tobis S, Donsky H, Wu G, Joseph J, Rashid H, et al. Statin use and the risk of biochemical recurrence of prostate cancer after definitive local therapy: a meta-analysis of eight cohort studies. BJU Int. 2013;111:E71–7. https://doi.org/10.1111/j.1464-410X.2012.11527.x.
Aminsharifi A, Howard LE, Amling CL, Aronson WJ, Cooperberg MR, Kane CJ, et al. Statins are associated with increased biochemical recurrence after radical prostatectomy in diabetic men but no association was seen in men also taking metformin: results from the SEARCH database. Clin Genitourin Cancer. 2019;17:e140–9. https://doi.org/10.1016/j.clgc.2018.09.020.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. http://www.ncbi.nlm.nih.gov/pubmed/3558716. Accessed 28 Jul 2019.
Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. http://www.ncbi.nlm.nih.gov/pubmed/16224307. Accessed 28 Jul 2019.
Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–67. http://www.ncbi.nlm.nih.gov/pubmed/11146273. Accessed 28 Jul 2019.
Anderson-Carter I, Posielski N, Liou J, Khemees T, Downs T, Abel J, et al. The impact of statins in combination with androgen deprivation therapyin patients with advanced prostate cancer: a large observational study. Urol Oncol. 2019;37:130–7. https://doi.org/10.1016/J.UROLONC.2018.11.017.
Mucci LA, Stampfer MJ. Mounting evidence for prediagnostic use of statins in reducing risk of lethal prostate cancer. J Clin Oncol. 2014;32:1–2. https://doi.org/10.1200/JCO.2013.53.2770.
Wu S-Y, Fang S-C, Shih H-J, Wen Y-C, Shao Y-HJ. Mortality associated with statins in men with advanced prostate cancer treated with androgen deprivation therapy. Eur J Cancer. 2019;112:109–17. https://doi.org/10.1016/j.ejca.2018.11.032.
Van Rompay MI, Solomon KR, Nickel JC, Ranganathan G, Kantoff PW, McKinlay JB. Prostate cancer incidence and mortality among men using statins and non-statin lipid-lowering medications. Eur J Cancer. 2019;112:118–26. https://doi.org/10.1016/j.ejca.2018.11.033.
Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–95. https://doi.org/10.1097/EDE.0b013e3181a819a1.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. https://doi.org/10.1080/01621459.1999.10474144.
Brookhart MA, Patrick AR, Dormuth C, Avorn J, Shrank W, Cadarette SM, et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. 2007;166:348–54. https://doi.org/10.1093/aje/kwm070.
Dormuth CR, Patrick AR, Shrank WH, Wright JM, Glynn RJ, Sutherland J, et al. Statin adherence and risk of accidents: a cautionary tale. Circulation. 2009;119:2051–7. https://doi.org/10.1161/CIRCULATIONAHA.108.824151.
Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Zappa M, Nelen V, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet (Lond, Engl). 2014;384:2027–35. https://doi.org/10.1016/S0140-6736(14)60525-0.
Tsodikov A, Gulati R, Heijnsdijk EAM, Pinsky PF, Moss SM, Qiu S, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med. 2017;167:449. https://doi.org/10.7326/M16-2586.
Crawford ED, Grubb R, Black A, Andriole GL, Chen MH, Izmirlian G, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. J Clin Oncol. 2011;29:355–61. https://doi.org/10.1200/JCO.2010.30.5979.
Telesca D, Etzioni R, Gulati R. Estimating lead time and overdiagnosis associated with PSA screening from prostate cancer incidence trends. Biometrics. 2008;64:10–9. https://doi.org/10.1111/j.1541-0420.2007.00825.x.
Draisma G, Boer R, Otto SJ, van der Cruijsen I, Damhuis R, Schröder FH, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European randomized study of screening for prostate cancer. J Natl Cancer Inst. 2003;95:868–78. https://doi.org/10.1093/jnci/95.12.868.
Alfaqih MA, Allott EH, Hamilton RJ, Freeman MR, Freedland SJ. The current evidence on statin use and prostate cancer prevention: are we there yet? Nat Rev Urol. 2017;14:107–19. https://doi.org/10.1038/nrurol.2016.199.
Yu O, Eberg M, Benayoun S, Aprikian A, Batist G, Suissa S, et al. Use of statins and the risk of death in patients with prostate cancer. J Clin Oncol. 2014;32:5–11. https://doi.org/10.1200/JCO.2013.49.4757.
Platz EA, Tangen CM, Goodman PJ, Till C, Parnes HL, Figg WD et al. Statin drug use is not associated with prostate cancer risk in men who are regularly screened. J Urol. 2014. https://doi.org/10.1016/j.juro.2014.01.095.
Murtola TJ, Syvälä H, Tolonen T, Helminen M, Riikonen J, Koskimäki J, et al. Atorvastatin versus placebo for prostate cancer before radical prostatectomy—a randomized, double-blind, placebo-controlled clinical trial. Eur Urol. 2018;74:697–701. https://doi.org/10.1016/J.EURURO.2018.06.037.
Penson DF, Albertsen PC, Nelson PS, Barry M, Stanford JL. Determining cause of death in prostate cancer: are death certificates valid? J Natl Cancer Inst. 2001;93:1822–3. https://doi.org/10.1093/jnci/93.23.1822.
Funding
The project described was partially supported by the National Institutes of Health, Grant TL1TR001443 (AK, PR, RRS, AKB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
PR discloses previous salary support from Peptide Logic, LLC. AKB discloses consulting fees from Boston Consulting Group. RRS discloses consulting fees from Boston Consulting Group. AK discloses previous consulting fees from and ownership stake in Sympto Health. JDM disclose consulting fees from Boston Consulting Group and ownership stake in Sympto Health.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kumar, A., Riviere, P., Luterstein, E. et al. Associations among statins, preventive care, and prostate cancer mortality. Prostate Cancer Prostatic Dis 23, 475–485 (2020). https://doi.org/10.1038/s41391-020-0207-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-020-0207-5
- Springer Nature Limited
This article is cited by
-
Prostate cancer cell proliferation is influenced by LDL-cholesterol availability and cholesteryl ester turnover
Cancer & Metabolism (2022)
-
Population-wide impacts of aspirin, statins, and metformin use on prostate cancer incidence and mortality
Scientific Reports (2021)