Abstract
Background
Multiparametric magnetic resonance imaging (MP-MRI) and MRI/ultrasound (US) fusion-guided biopsy are becoming more widely used techniques for prostate cancer (PCa) diagnosis and management. However, their widespread adoption and use, where available, are limited by cost and added time. These limitations could be minimized if a biparametric MRI (BP-MRI) focusing on T2-weighted and diffusion-weighted imaging is performed. Herein we report the cancer detection rate of BP-MRI compared with full MP-MRI.
Methods
Biopsy-naive and prior negative biopsy patients with clinical suspicion for PCa underwent MP-MRI with an imaging protocol incorporating narrow field-of-view T2-weighted, diffusion-weighted, and DCE pelvic MRI. Then patients underwent MRI/US fusion-guided biopsy of target lesions between November 2013 and October 2017. The pathology results were compared to the positivity of the DCE sequence compared to the BP-MRI findings alone.
Results
There were 648 targeted lesions biopsied in 344 patients. We defined biparametric screen filter positivity as both T2-weighted and diffusion-weighted imaging positivity for the same lesion. The majority of target lesions (552/648, 85%) were screen filter positive. For those that were screen filter negative, a minority (14/96, 15%) had DCE-positive findings. Of these, 2/3 (67%) cancer-positive cases were seen on T2-weighted imaging. For those 82 that were screen filter negative and DCE negative, the DCE phase would not have added imaging suspicion. Only 3/82 (3.7%) were cancer positive; 2 with low risk, grade group 1 cancer and 1 with intraductal carcinoma, all identified on targeted T2-weighted MRI positivity.
Conclusions
BP-MRI for the evaluation of PCa and for guiding MRI/US fusion-targeted biopsy has the advantages of reducing cost, time, and contrast exposure of MP-MRI by eliminating the DCE phase. These benefits are realized without forfeiting valuable diagnostic information, as shown by similar cancer detection rates of BP-MRI and MP-MRI in this study, particularly for clinically significant cases of PCa.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Prostate cancer (PCa) is the most common solid organ malignancy in American men and the leading cause of cancer mortality in this population, with 29,430 deaths projected in 2018 [1]. PCa is traditionally most often diagnosed on systematic transrectal ultrasound (TRUS)-guided extended-sextant prostate biopsy performed owing to suspicion from elevated prostate-specific antigen (PSA) or abnormal findings on digital rectal exam (DRE). Multiparametric magnetic resonance imaging (MP-MRI) and MRI/ultrasound (US) fusion-targeted biopsy (TBx) are relatively new techniques for detection of PCa that allow for targeted, image-guided sampling of lesions suspicious for cancer and reduction of sampling error, as well as evaluation of regional structures such as seminal vesicles, bones, and lymph nodes for PCa involvement. Studies have shown that TBx enables superior detection of clinically significant cases of PCa when compared to systematic biopsy alone [2, 3].
However, the use of MP-MRI to guide TBx is limited by several factors, including cost, time of image acquisition and interpretation, and use of intravenous, gadolinium contrast. MP-MRI of the prostate is comprised of anatomical T2-weighted imaging (T2W) in addition to functional imaging techniques, commonly diffusion-weighted imaging (DWI) and dynamic contrast enhancement (DCE) sequences. This typically requires 30–45 min of MRI gantry time, placement of intravenous access, and contrast administration [4, 5]. The use of contrast contributes additional cost and time, as well as the potential risk of gadolinium-based contrast administration complications. It has been proposed that these clinical risks and burdens could be reduced by eliminating the DCE sequence altogether. Instead, performing a more abbreviated biparametric-MRI (BP-MRI) would require less time (approximately 15–30 min less per case), cost less, and obviate the need for contrast administration and associated risks [4, 6]. As such, this diagnostic modality could significantly improve patient access while limiting many of the financial and time constraints institutions face with traditional MP-MRI [7].
While these are compelling reasons to eliminate DCE in this setting, the potential of reducing diagnostic accuracy must be considered. Therefore, BP-MRI must first be shown not to overlook clinically significant PCa that a DCE phase would detect. It has been previously reported that BP-MRI in men with elevated PSA has a similar diagnostic accuracy compared to full MP-MRI [4]. Herein we aimed to determine the cancer detection rate (CDR) of BP-MRI compared to traditional MP-MRI in men who were either biopsy naive or had one or more prior negative prostate biopsies to further investigate the necessity of DCE for reliable detection of clinically significant PCa.
Methods
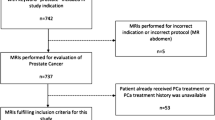
An Institutional Review Board-approved, HIPAA (Health Insurance Portability and Accountability Act)-compliant retrospective review of prostate MP-MRI and MRI/US fusion-TBx records from November 2013 to October 2017 was performed. Patients meeting our inclusion criteria were those who underwent MP-MRI for clinical suspicion of PCa based on elevated serum PSA level and/or abnormal DRE without prior PCa diagnosis. This included both biopsy naive men as well as those with a prior negative biopsy. Patients with suspicious lesions on MP-MRI underwent TBx. All patients also underwent concurrent 12-core extended-sextant biopsy at the time of TBx if they had not undergone a separate standard template biopsy within a 12-month period. For patients with more than one MRI/US fusion-TBx, only the initial MP-MRI and TBx session data were included in this study. Our inclusion criteria are summarized in Fig. 1.
Our standard prostate MP-MRI protocol consisted of a baseline non-contrast T1 phase, triplanar T2W imaging, DCE imaging, and DWI with multiple b-values, including a high b-value sequence from which an apparent diffusion coefficient map was derived [8]. All images were reviewed by a body radiologist with subspecialization in genitourinary MRI on an Intellispace Portal (Philips Medical systems, Eindhoven, The Netherlands) Picture Archiving Communications System. These were secondarily reviewed in a multidisciplinary prostate imaging conference consisting of body radiologists, genitourinary pathologists, and urologic oncologists. In this setting, each suspicious lesion was discussed and assigned a PI-RADS v.2 suspicion score, with denotation of imaging parameter positivity and three-dimensional segmentation of the prostate volume and regions of interest for TBx guidance in the DynaCad post-image processing software platform (Philips/InVivo Corp, Gainesville, FL, USA) as previously described [9].
Next, the results were interpreted according to BP-MRI screen filter and DCE positivity. BP-MRI screen filter positivity was defined as a foci having both T2W and DWI positivity. The zonal and anatomical location of BP-MRI screen filter-negative lesions that were positive on traditional MP-MRI were recorded.
Results
A total of 344 men met the inclusion criteria for this study that afforded 648 target lesions for analysis. Our study population comprised a median age of 65 years (SD 7.69, interquartile range 60–70), 204 (59%) of which were white, 65 (19%) black, 2 (1%) Hispanic/Latino, 2 (1%) Asian, and 71 (21%) where this demographic was not available (Table 1). The study population had a median PSA of 7.61 ng/mL (SD 9.96, interquartile range 5.29–10.7). Ninety-five patients (28%) were biopsy naive, while 249 (72%) had a history of one or more prior negative prostate biopsy sessions. Of those who had prior negative biopsy sessions before undergoing MRI and TBx, 145 men had 1 prior biopsy session, and 104 had ≥2 (range 1–9, SD 1.14).
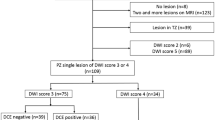
The imaging characteristics and subsequent surgical pathology of the 648 targeted lesions are recorded in Fig. 2. The majority of target lesions (552/648, 85%) were BP-MRI screen filter positive. Among these lesions, 201 (36%) were DCE positive.
For the 96 (15%) target lesions that were BP-MRI screen filter negative, imaging characteristics, anatomical location, and final surgical pathology were reviewed. A small minority (14/96, 15%) exhibited DCE positivity: 4 (29%) in the central gland (CG) and 10 (71%) in the peripheral zone (PZ). Among these, 3 (21%) harbored cancer. While two lesions were more favorable risk PCa (Gleason 3 + 3 = 6, grade group 1 and low volume Gleason 3 + 4 = 7, grade group 2), one did contain 4 + 5 = 9 disease. All but one cancer-positive case was appreciated on the T2W phase (Fig. 2).
Among the 82/96 (85%) BP-MRI screen filter and DCE-negative lesions, 26 (32%) were located in the CG, 53 (65%) in the PZ, 1 (1%) located at the CG/PZ junction, and 1 (2%) in the seminal vesicles. As such, the DCE phase would not have provided any additional clinical benefit nor upgraded PI-RADS v.2 suspicion score [10]. In these screen-negative cases, where only T2W or DWI was positive, surgical pathology found PCa in 3/82 (4%) lesions. All three lesions were detected on T2W imaging in isolation. Two cases were low-risk disease (Gleason 3 + 3 = 6, grade group 1) and one case harbored variant intraductal carcinoma, which could not formally be graded. The comprehensive pathology findings of screen-negative TBx lesions are illustrated in Fig. 2.
Discussion
MP-MRI and TBx are modern, ever-expanding techniques for optimized detection of PCa compared to traditional TRUS-guided systematic or extended-sextant biopsy. These novel modalities are becoming widely available and integrated into daily clinical practice [11]. Nevertheless, the substantial costs and experience necessary for conducting high-fidelity imaging protocols and interpreting these imaging studies continues to pose a challenge for many providers [12]. More specifically, traditional MP-MRI protocols are costly and time-consuming, and controversy exists surrounding the use of contrast. These are modifiable factors that have limited access to certain patient populations due to regulations imparted by insurance providers and health-care payer systems [13, 14]
Currently, most prostate biopsies are prompted by an elevated serum PSA level or abnormal DRE. However, PSA screening and systematic biopsy alone often find clinically insignificant PCa, which has been shown to lead to overtreatment with limited benefit in cancer-specific or overall survival measures [15, 16]. With the widespread use of active surveillance for lower-risk PCa, prostate MRI leads to better risk stratification and increased confidence for safe cancer surveillance and is supported in current American Urological Association and the Society for Abdominal Radiology guidelines [17,18,19]. Additionally, differentiation of clinically significant cases of cancer in patients initially diagnosed with possible active surveillance-eligible low-risk disease has been quantified and calculated with MRI-based risk calculators [8]. Evidence supporting the role of MRI as a screening tool in biopsy-naive men had been limited to smaller series and relied largely on the negative predictive value of the diagnostic imaging study [9, 20]. However, recent investigation by an international, multicenter randomized trial demonstrated the benefit of MRI and MRI-directed biopsy over the standard TRUS-guided systematic biopsy approach in a population of biopsy-naive men [21]. As MRI finds more wide-spread adoption and implementation in the workflow of PCa detection, even prior to first biopsy, it is imperative to be cost-conscious to allow for wider public health reach of benefit.
One potential strategy for reducing the cost, study time, and contrast-associated risks is elimination of the DCE MRI phase, thus relying solely on BP-MRI that consists of just the T2W and DWI MRI sequences. This would require less time and less overall imaging and radiologic interpretation-associated expenses, as well as obviating the need for intravenous catheter insertion and contrast administration. However, up to this point, there is a paucity of evidence in support of omitting the DCE phase and only obtaining sequences in a BP-MRI study to safely detect PCa in this setting.
BP-MRI is a less-invasive imaging modality that has potential as a more rapid, affordable screening tool for PCa coupled with standard screening with PSA and DRE. A multidisciplinary group at the National Cancer Institute evaluated 143 biopsy-naive men who underwent full MP-MRI. They observed that using the BP-MRI sequences alone superiorly predicted the presence of PCa compared to PSA level or PSA density (PSAD) alone. When analyzed in conjunction with PSA and PSAD, integrating BP-MRI data significantly improved the sensitivity and specificity of clinically significant PCa detection [6]. These findings were further validated by the same center in a subsequent cohort of 59 biopsy-naive patients [22].
Kuhl et al. reported similar findings in their evaluation of 542 men with an elevated serum PSA (≥3 ng/mL) who underwent MP-MRI with a separate BP-MRI interpretation [4]. In their study, complete MP-MRI with contrast enhancement only affected the diagnosis of one clinically significant case of PCa out of a total of 139 (<1%), which would not have been otherwise detected with BP-MRI. In the current study, performing the DCE phase of the MP-MRI would permit detection of three screen-negative lesions that were PCa positive on TBx. However, two of these lesions would have been identified and targeted based upon T2W. Kuhl et al. found that the diagnostic accuracy of BP-MRI for clinically significant PCa (89.1%, 483 of 542) was comparable, if not slightly better, than that of the full MP-MRI with DCE (87.2%, 473 of 542) for evaluation and suspicion scoring prior to biopsy. In their study, BP-MRI was achieved in <9 min while providing “diagnostic accuracy and CDRs that are equivalent to those of conventional full multiparametric contrast-enhanced MR imaging protocols,” which is a much longer diagnostic study requiring 30–45 min to perform [4].
It should be mentioned that radiologists were not blinded to the DCE phase of each case, and therefore it is possible that their interpretation of the T2W and DWI sequences and PI-RADS scoring may have been affected by their viewing of the DCE series. Furthermore, one would consider whether a prospective analysis with only BP-MRI studies as a screening in biopsy-naive men would render different CDRs for each suspicion score level compared to those we have previously reported for our full MP-MRI protocol of imaging [9]. In our patient series, we were not able to identify any specific patient-related features where the data from DCE MRI would augment the decision-making process and detection of PCa, consistent with the PI-RADS v.2 suspicion scoring system where DCE findings play only a minor role in determining the presence of clinically significant PCas, only pertinent in cases where otherwise equivocal lesions scored a PI-RADS 3 based on T2W and DWI features would benefit from DCE data [23, 24]. Additionally, the number of cases with a single MRI sequence prompting TBx and PCa detection was very limited and did not allow for a well-powered statistical analysis of the accuracy of each independent MRI parameter.
Depending on an institution’s policies for renal function and gadolinium contrast, adding the DCE phase to MP-MRI may increase patient preparation time for glomerular filtration rate (GFR) assessment or possibly even exclude them. Even if an institution does not perform GFR assessment with Group II agents, patients must be screened for prior contrast reactions and potentially premedicated, if indicated [25]. Furthermore, intravenous access must be obtained that in itself can be a time-consuming task. In total, this likely adds at least 20 min to the imaging study time, which has been estimated at approximately 45 min based on prior publications [6, 7]. Interpreting the DCE phase adds an average 5 min per MP-MRI for a subspecialty body imager. When taken together, the time savings of a BP-MRI when compared to MP-MRI has been estimated by two independent institutions to be approximately three-fold, potentially reducing prostate MRI volume/access by 50–67% when performing these studies during normal business hours [4, 7].
Additional benefits of reduced scan duration and table time include patient comfort and satisfaction. Anxiety and claustrophobia during prostate MRI can be significant barriers for some patients, and increased patient compliance during the scan reduces motion and artifacts, improving the study’s image quality. Improved patient experience leads to increased compliance both during the scan itself and for follow-up imaging, which is particularly important for patients undergoing active surveillance.
Immediate hypersensitivity reactions to gadolinium contrast are very rare, occurring in approximately 0.079% of all administrations. Although most immediate hypersensitivity reactions are mild, the incidence of moderate and severe reactions together is 0.013% per MR contrast media dose and 0.021% per person, which is rare but not low enough to be disregarded [26]. Furthermore, residual gadolinium has been shown to be deposited in tissues, particularly in the brain, and the effects of this deposition are currently unknown [27]. While gadolinium retention has not been directly linked to adverse health effects in patients with normal kidney function, in 2017 the Food and Drug Administration (FDA) issued a new class warning to alert the public about the potential for it to remain in the body for months or years after receiving the gadolinium contrast agent. Resultantly, the FDA now mandates that patients receive proper counseling on this matter before receiving a gadolinium contrast agent [28]. Our study suggests that BP-MRI has equitable PCa detection compared to traditional MP-MRI, so using this curtailed MRI study would eliminate these possible gadolinium-associated risks while still maintaining the diagnostic benefits.
Conclusions
BP-MRI for the detection of PCa and for guiding MRI/US fusion-targeted prostate biopsy has the advantages of reducing cost, time, and contrast exposure compared to traditional MP-MRI by eliminating the DCE phase. Of those with biparametric screen-negative findings, DCE did not in isolation find any clinically significant PCa. This abbreviated imaging study does not significantly sacrifice diagnostic yield and may permit greater access to the diagnostic benefits of prostate MRI in this setting.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7.
Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MG. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol. 2015;68:438.
Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313:390.
Kuhl CK, Bruhn R, Krämer N, Nebelung S, Heidenreich A, Schrading S. Abbreviated biparametric prostate MR imaging in men with elevated prostate-specific antigen. Radiology. 2017;285:493–505.
Bjurlin MA, Meng X, Nobin JL, Wysock JS, Lepor H, Rosenkrantz AB, et al. Optimization of prostate biopsy: the role of magnetic resonance imaging targeted biopsy in detection, localization and risk assessment. J Urol. 2014;192:648–58.
Rais-Bahrami S, Siddiqui MM, Vourganti S, Turkbey B, Rastinehad AR, Stamatakis L, et al. Diagnostic value of biparametric magnetic resonance imaging (MRI) as an adjunct to prostate-specific antigen (PSA)-based detection of prostate cancer in men without prior biopsies. BJU Int. 2015;115:381–8.
Porter KK, King A, Galgano S, et al. Financial implications of biparametric prostate MRI. In preparation (2018).
Lai WS, Gordetsky JB, Thomas JV, Nix JW, Rais-Bahrami S, et al. Factors predicting prostate cancer upgrading on magnetic resonance imaging-targeted biopsy in an active surveillance population. Cancer. 2017;123:1941–8.
Yarlagadda VK, Lai WS, Gordetsky JB, Porter KK, Nix JW, Thomas JV, et al. MRI/US fusion-guided prostate biopsy allows for equivalent cancer detection with significantly fewer needle cores in biopsy-naive men. Diagn Interv Radiol. 2018 ;24:115–20.
Gaur S, Harmon S, Mehralivand S, Bednarova S, Calio BP, Sugano D, et al. Prospective comparison of PI-RADS version 2 and qualitative in-house categorization system in detection of prostate cancer. J Magn Reson Imaging. 2018;48:1326–35.
Muthigi A, Sidana A, George AK, Kongnyuy M, Maruf M, Valayil S, et al. Current beliefs and practice patterns among urologists regarding prostate magnetic resonance imaging and magnetic resonance-targeted biopsy. Urol Oncol. 2017;35:32.e1–7.
Vourganti S, Starkweather N, Wojtowycz A. MR/US fusion technology: what makes it tick? Curr Urol Rep. 2017;18:20.
Gorin MA, Walsh PC. Magnetic resonance imaging prior to first prostate biopsy-are we there yet? Eur Urol. 2018. https://doi.org/10.1016/j.eururo.2018.05.018
Faria R, Soares MO, Spackman E, Ahmed HU, Brown LC, Kaplan R, et al. Optimising the diagnosis of prostate cancer in the era of multiparametric magnetic resonance imaging: a cost-effectiveness analysis based on the Prostate MR Imaging Study (PROMIS). Eur Urol. 2018;73:23–30.
Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–13.
Glaser ZA, Gordetsky JB, Porter KK, Varambally S, Rais-Bahrami S. Prostate cancer imaging and biomarkers guiding safe selection of active surveillance. Front Oncol. 2017;7:256.
Loeb S, Byrne N, Makarov DV, Lepor H, Walter D. Use of conservative management for low-risk prostate cancer in the Veterans Affairs Integrated Health Care System from 2005–15. JAMA. 2018. https://doi.org/10.1001/jama.2018.5616
Gordetsky JB, Saylor B, Bae S, Nix JW, Rais-Bahrami S. Prostate cancer management choices in patients undergoing multiparametric magnetic resonance imaging/ultrasound fusion biopsy compared to systematic biopsy. Urol Oncol. 2018;36:241.e7–13.
Rosenkrantz AB, Verma S, Choyke P, Eberhardt SC, Eggener SE, Gaitonde K, et al. Prostate magnetic resonance imaging and magnetic resonance imaging targeted biopsy in patients with a prior negative biopsy: a Consensus Statement by AUA and SAR. J Urol. 2016;196:1613–8.
Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–22.
Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378:1767–77.
Fascelli M, Rais-Bahrami S, Sankineni S, Brown AM, George AK, Ho R, et al. Combined biparametric prostate magnetic resonance imaging and prostate-specific antigen in the detection of prostate cancer: a validation study in a biopsy-naïve patient population. Urology. 2016;88:125–34.
Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS prostate imaging - reporting and data system: 2015, Version 2. Eur Urol. 2016;69:16–40.
Sheridan AD, Nath SK, Syed JS, Aneja S, Sprenkle PC, Weinreb JC, et al. Risk of clinically significant prostate cancer associated with prostate imaging reporting and data system category 3 (equivocal) lesions identified on multiparametric prostate MRI. AJR Am J Roentgenol. 2017;210:347–57.
American College of Radiology. Manual on contrast media, version 10.3. American College of Radiology; 2017.
Jung JW, Kang HR, Kim MH, Lee W, Min KU, Han MH, et al. Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology. 2012;264:414–22.
Malayeri AA, Brooks KM, Bryant LH, Evers R, Kumar P, Reich DS, et al. National Institutes of Health perspective on reports of gadolinium deposition in the brain. J Am Coll Radiol. 2016;13:237–41.
FDA in brief: FDA requires new class warning and additional research on retention in the body of gadolinium from gadolinium-based contrast agents used in magnetic resonance imaging. 2017. https://www.fda.gov/NewsEvents/Newsroom/FDAInBrief/ucm589604.htm
Acknowledgements
This work was funded in part by Junior Faculty Development Grant (ACS-IRG 001-53) and by Developmental funds from the UAB Comprehensive Cancer Center Support Grant (NCI P30 CA 013148) to Soroush Rais-Bahrami.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JWN and SR-B serve as consultants to Philips/InVivo Corp. The other authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sherrer, R.L., Glaser, Z.A., Gordetsky, J.B. et al. Comparison of biparametric MRI to full multiparametric MRI for detection of clinically significant prostate cancer. Prostate Cancer Prostatic Dis 22, 331–336 (2019). https://doi.org/10.1038/s41391-018-0107-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-018-0107-0
- Springer Nature Limited
This article is cited by
-
Biparametric versus multiparametric MRI for the detection of clinically significant prostate cancer in a diverse, multiethnic population
Abdominal Radiology (2024)
-
A nomogram based on biparametric magnetic resonance imaging for detection of clinically significant prostate cancer in biopsy-naïve patients
Cancer Imaging (2023)
-
The role of preoperative prostatic shape in the recovery of urinary continence after robotic radical prostatectomy: a single cohort analysis
Prostate Cancer and Prostatic Diseases (2023)
-
Parametric maps of spatial two-tissue compartment model for prostate dynamic contrast enhanced MRI - comparison with the standard tofts model in the diagnosis of prostate cancer
Physical and Engineering Sciences in Medicine (2023)
-
Radiomics vs radiologist in prostate cancer. Results from a systematic review
World Journal of Urology (2023)