Abstract
Background
Umbilical cord blood is used for the testing of various parameters in newborns. However, data on its applicability for hemostasis assays is insufficient.
Objective
To evaluate whether umbilical cord blood can be used for standard tests, thromboelastometry and thrombodynamics for preterm and term newborns.
Methods
187 newborns were included in the study. Blood was taken from the umbilical cord and by venipuncture of the newborn. Clotting times, fibrinogen, D-dimer, thromboelastometry and thrombodynamics were measured.
Results
Clotting times and fibrinogen indicated a hypocoagulable shift, while thromboelastometry and thrombodynamics showed a hypercoagulable shift in hemostasis in umbilical cord blood compared to newborn blood. D-dimer indicated an enhanced process of thrombus lysis in newborn blood compared to cord blood. Collecting blood into a tube with the addition of a contact pathway inhibitor did not significantly change the global assay parameters in either umbilical cord blood or newborn blood. In the thrombodynamics assay, spontaneous clotting was detected but suppressed by the addition of a tissue factor inhibitor.
Conclusions
Hemostasis in cord and newborn blood differs for both global and standard tests. Hypercoagulability in newborns registered with the global assay thrombodynamics is associated with the presence of tissue factor in the blood.
Impact statement
-
1.
We found a hypercoagulation shift in newborns compared with the adult references, possibly due to the presence of tissue factor in blood.
-
2.
Blood coagulation is enhanced in cord blood compared with blood sampled from the vein of a newborn according to thromboelastometry and thrombodynamics assays.
-
3.
Clotting times and fibrinogen concentrations in cord blood differ from these parameters in newborn blood.
-
4.
Studying of the (patho)physiological features of hemostasis in newborns should consider differences in cord blood and vein sampled blood.
Similar content being viewed by others
Introduction
Hemostasis in newborns differs from that in adults, depends on gestational age,1,2,3 and changes rapidly during the first weeks of life.3,4 Therefore, when studying hemostasis in newborns, it is necessary to focus on the correct reference ranges, accounting for gestational age and time passed after birth.
Classical hemostasis tests, such as measurements of the activated partial thromboplastin time (APTT), prothrombin time (PT) (as well as prothrombin and the international normalized ratio) and fibrinogen, have been thoroughly studied for newborns. Clotting times are longer in neonates than in adults.1 This is apparently due to reduced concentrations of clotting factors in the blood of newborns.1 According to data from various studies, the fibrinogen concentration in neonates does not change1,5,6 or is reduced7,8 compared to that in adults. Inhibitor concentrations are also reduced in neonates, but the sensitivity of clotting tests to clotting inhibitor deficiencies is poor due to the peculiarities of their design. Briefly, the activator concentrations used are so high that the time needed for slow-acting antithrombin9,10 to have a detectable impact on clotting before all fibrinogen is converted to fibrin is insufficient. The differences between the parameters of classical tests in newborns and adults become less pronounced with increasing gestational age1 and time after birth.11 Among the clotting and fibrinolysis markers, the D-dimer concentration is increased (probably as a result of trauma at birth) in neonates compared to adults.12,13,14 Studies have shown a decrease in D-dimer concentration with gestational age15 and a higher D-dimer concentration in newborns delivered by cesarian section than in those delivered vaginally.16,17
In some cases, global hemostasis tests are more informative for monitoring therapy and predicting coagulation disorders in newborns.18,19 According to global hemostasis tests, thrombin generation, thromboelastometry/thromboelastography and thrombodynamics, coagulation is enhanced in newborns than in adults.8,18,19 Additionally, in newborn blood, the thrombodynamics assay demonstrates the formation of spontaneous clots,8 which are formed due to the presence of clotting activators (activated factors, tissue factor (TF) or phospholipids) in the blood plasma.20 Thrombin generation test has shown that compared with term newborns, preterm newborns have increased coagulation,21 but thromboelastometry/ thromboelastography data are controversial.22,23,24
Another important point in the study of hemostasis in newborns is the widespread use of umbilical cord blood. The potential benefit of using umbilical cord blood is the ability to draw blood painlessly and obtain a larger volume of blood for analysis. Yet, there is still no clear consensus whether the cord blood can reflect the hemostasis state in newborns in the same manner as the newborns’ peripheral blood. The APTT, PT and fibrinogen concentrations in the peripheral blood of newborns and in umbilical cord blood are similar to those in the literature.25,26 Regarding the global assays thromboelastography and thrombin generation, in umbilical cord blood, coagulation is enhanced compared to that in peripheral blood.26
In this study, we investigated the feasibility of using cord blood in routine and global hemostasis tests for both healthy newborns and preterm newborns. In addition, we investigated the causes of spontaneous thrombus formation in the blood of newborns via a thrombodynamics assay.
Materials and methods
Study population and specimen collection
The study was conducted in accordance with the Declaration of Helsinki. The study was approved by the ethics committee of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after academician Kulakov V.I. (Protocol code: 10, date of approval: 23 November 2017). The inclusion criteria were the presence of indications for cesarean section before the onset of labor and patient consent for inclusion in the study. The non-inclusion criteria were emergency cesarean section, critical health conditions of the mother or newborn. The exclusion criterion was the absence of at least one hemostasis test result.
Cord blood was drawn from the umbilical vein during the cesarean section birth immediately after separation of the placenta from the uterine wall. Delayed cord clamping was performed only for preterm infants with a gestational age of 36 weeks or less and when possible. In total, this procedure was performed on 14 newborns (50%). Peripheral blood was drawn from the intact peripheral vein (the median vein of the elbow, the medial vein of the forearm, or the dorsal venous plexus) during the first 2 h after birth. Demographic and clinical information was collected from each infant’s medical records.
Blood was drawn into standard 106 mM sodium citrate buffer tubes (S-Monovette, Sarstedt, Germany). Blood was sampled once both from the umbilical cord and from the newborns. Blood (4.5 ml) was taken from the umbilical cord. Blood (1.3 ml) was taken from all newborns, except 12 newborns, from which a total of 2.6 ml of blood was taken. In 12 patients, we collected an additional 1.3 ml of blood into a tube containing 50 µg/ml corn-trypsin inhibitor (CTI, Reagent I from Thrombodynamics Kit, HemaCore, LLC, Russia) mixed with sodium citrate to assess the effect of contact activation during blood sampling on the test results. These 12 newborns were randomly selected, excluding only neonates whose gestational age was less than 33 weeks and whose weight was less than 2000 g.
Three hundred microliters of whole blood were used for thromboelastometry. The remaining whole blood was processed by centrifugation at 1600 × g for 15 min to obtain platelet-poor plasma. Platelet-poor plasma was repeatedly processed by centrifugation at 10,000 × g for 5 min to obtain 120 µl of platelet-free plasma, which was used for the thrombodynamics assay. The remaining platelet-free plasma was frozen and stored at −80 °C for further investigation of the source of spontaneous clots in the thrombodynamics assay. The time interval between collection and analysis was less than 2 h.
Laboratory assays
Standard coagulation tests, including APTT, prothrombin, fibrinogen and the D-dimer concentration were performed using an ACL TOP 700 system and HemosIL® reagents (Instrumentation Laboratory, Lexington, MA).
Rotation thromboelastometry was performed on whole blood with a ROTEM® thromboelastometer (Pentapharm, Germany). A Non-Activated Thromboelastometry (NATEM) assay was performed, the following standard parameters were calculated as described in ref. 27, and further data analysis was conducted: clotting time (CT), clot formation time (CFT), angle (α) and maximum clot firmness (MCF).
A thrombodynamics assay was performed with a Thrombodynamics Analyzer and Thrombodynamics Kit (HemaCore, LLC, Russia) as described previously.28 This method is based on registering spatial fibrin clot growth after the activation of clotting in a thin layer of plasma after contact with an immobilized TF-bearing surface. The process of clot growth was registered by serial photos during the test. In some cases, spontaneous clotting (clot formation in the cuvette space not associated with the main clot growth) occurred and was described with a spontaneous clotting plot.8 The following standard parameters were calculated as described in ref. 29: lag time (Tlag), initial rate of clot growth (Vi), rate of clot growth (V), clot density (D) and spontaneous clotting time (Tsp).
Adult reference ranges for standard assays and thrombodynamics assays were obtained from the manufacturers. Adult reference ranges for thromboelastometry were obtained from the Clinical Diagnostic Laboratory of National Medical Research Center for Obstetrics, Gynecology and Perinatology.
Inhibition of spontaneous clotting in the thrombodynamics assay
In total, 100 nM of inactivated factor VII (FVIIai, which was prepared as described previously30 and used as a TF inhibitor), or 100 nM of nitrophorin 2 (a direct inhibitor of activated FIXa, NP2, kindly provided by Dr. JF Andersen) or vehicle was added to blood plasma that was recalcified with 20 mM calcium acetate (Reagent II from the Thrombodynamics Kit) and observed for 60 min in a Thrombodynamics Analyzer (the TF-bearing surface was not inserted). The effect of the inhibitors was evaluated by the time of spontaneous clots appearance (T10%), which was determined as the time the average light scattering intensity of the plasma volume distant from the activating surface reached 10% of the maximum light scattering measured after 60 min in the area where the plasma had completely clotted. The absence of spontaneous clotting is indicated by T10% = 60 min.
Statistical analysis
The median (min–max) or median (25–75%) was used to estimate the results. The Mann‒Whitney U test (nonrelated samples), Wilcoxon signed-rank test (related samples) for continuous data or Fisher’s exact test for categorical data were used for statistical analysis. To estimate the strength of the correlations, the Spearman correlation coefficient was calculated. The results were considered significant at p < 0.05. Statistical analysis was performed using Origin Pro 2018 (OriginLab Corp., Northampton, MA) software.
Results
Study population characteristics
In total, 187 newborns (159 term and 28 preterm) born to 184 mothers were enrolled in the study. All newborns were delivered by cesarean section due to causes not related to maternal or fetal coagulation (Table 1). The newborns were divided into three groups (Table 1). The first group (n = 101) included apparently healthy newborns with a gestational age of 37 weeks or more; the following minor comorbidities were observed in some newborns: transient tachypnea of newborns, large weight for gestational age, ankyloglossia, congenital malformations of the fingers, and minor hemorrhage under the skin. The second group (n = 58) included newborns with a gestational age of 37 weeks or more with complications (Table 1). The third group (n = 28) included preterm newborns.
Congenital benign neoplasms were recorded in 5 newborns: hepatic hemangiomatosis, lymphangioma of the head and neck, liver cyst, ovarian cyst, and multicystic dysplastic kidney. Anemia was recorded in 24 newborns: 18 newborns with congenital anemia (minimum hematocrit value in the first 3 days of life of 135 (78–149) g/l, median (min–max)), 4 newborns with anemia of prematurity (minimum hematocrit value in the first two weeks of life of 89 (65–117) g/l), 1 newborn with anemia that developed on the 6th day of life (minimum hematocrit of 133 g/l) and 1 newborn with mild anemia (minimum hematocrit of 87 g/l on the 42nd day of life, after drainage of the neck lymphangioma cyst). Five newborns required red blood cell transfusions. Hemorrhagic disorders, including intraventricular (n = 7), pulmonary (n = 2), gastrointestinal (n = 4), adrenal (n = 1), subcutaneous (n = 3), hemorrhagic stroke (n = 1) disorders, and simultaneous gastric, pulmonary and subcutaneous bleeding (n = 1), were recorded in 19 newborns. We included one newborn with minor subcutaneous hemorrhage in the group of healthy newborns. No major thrombotic complications were observed before discharge.
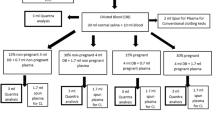
In some cases, blood collection from the umbilical cord or from the newborn was not possible, or a clot was found in the blood sample (Fig. 1).
Comparison of newborn coagulation assay parameters and the adult reference range
As the presence of the disease or gestational age could affect both hemostasis and the differences between umbilical cord blood and newborn blood, in addition to comparing coagulation parameters in the whole cohort of newborns, we separately analyzed 1) healthy full-term newborns, 2) full-term newborns with complications, and 3) preterm newborns.
First, we compared the parameters of hemostasis tests in newborns with the reference values for adults. We observed a prolongation of APTT and decreases in prothrombin and fibrinogen concentrations in both umbilical cord and newborn blood compared to the adult reference range (Fig. 2a–c). The neonatal D-dimer concentration in both the umbilical cord and newborn blood was higher than the adult reference range (Fig. 2d).
Standard assays: a – APTT (the number of umbilical cord-newborn pairs, n = 117), b – prothrombin (n = 118), c – fibrinogen (n = 117), d – D-dimer (n = 130). Thromboelastometry (NATEM test): e – CT (n = 137), f – CFT (n = 137), g – α (n = 137), h – MCF (n = 137). Thrombodynamics: i –Tlag (n = 134), j – V (n = 94), k – Tsp (n = 99), l – percentage of samples with spontaneous clots (n = 134). The box plots indicate the following parameters: the mean value (the dot inside the box), the median (the horizontal line inside the box), and the 25th and 75th percentiles (the bottom and top of the box, respectively). The shaded area indicates the reference range. For p values, the paired sample Wilcoxon signed rank test was used; for p* values, the F test was used.
Relative to the adult reference ranges, the thromboelastometry parameters CT, CFT and α were shifted to hypocoagulation in umbilical cord blood (Fig. 2e–g). The same parameters in the blood of newborns were within the reference range. The MCF was within the reference range for both cord blood and newborn blood (Fig. 2h).
Tlag and D in the thrombodynamics assay were within the adult reference range for both cord blood and newborn blood (Fig. 2i, Table 2). V and Vi were significantly higher than the reference range for both cord blood and newborn blood (Fig. 2j). The spontaneous clotting time was shorter than the reference range for both cord blood and newborn blood (Fig. 2k).
Comparison of coagulation assays in newborn and cord blood
We compared the hemostasis tests for all umbilical cord-newborn pairs included in the study, as well as separately for the groups. APTT was significantly longer in umbilical cord blood than in newborn blood in the whole cohort of patients (Fig. 2a), as well as in the groups of term newborns (groups 1 and 2); no significant changes were recorded for preterm newborns (group 3) (Table 2). There was a correlation between APTT in umbilical cord blood and that in newborn blood (RSpearman = 0.701, p < 0.001). Compared with that in newborn blood, prothrombin levels in umbilical cord blood were significantly lower in the whole population (Fig. 2b) and in groups 1 and 2. No significant changes were recorded in group 3 (Table 2). There was a correlation between prothrombin in umbilical cord blood and that in infant blood (RSpearman = 0.738, p < 0.001). The fibrinogen concentration was significantly lower in the umbilical cord blood than in the blood of newborns in the whole population (Fig. 2c) and in the group of healthy term newborns but not in groups 2 and 3 (Table 2). There was a correlation between the fibrinogen concentration in umbilical cord blood and that in newborn blood (RSpearman = 0.653, p < 0.001). The concentration of D-dimer was significantly higher in the peripheral blood than in the cord blood in the whole cohort of patients (Fig. 2d) as well as in each group (Table 2). No correlation was found between the D-dimer value in umbilical cord blood and that in infant blood.
All thromboelastometry parameters reflected a hypercoagulable shift in umbilical cord blood compared to newborn blood for the whole patient cohort (Fig. 2e–h) and for individual groups (Table 2). No correlation was found between thromboelastometry parameters in umbilical cord blood and those in newborn blood.
The thrombodynamics parameter Tlag did not differ significantly between umbilical cord blood and infant blood for the whole cohort (Fig. 2i); only slight shortening of Tlag in cord blood was observed in group 2 (Table 2). V and Vi were significantly higher, indicating hypercoagulable changes, in umbilical cord blood than in newborn blood for the whole cohort (Fig. 3j) and groups 1 and 2 (Table 2). D did not differ significantly in umbilical cord blood or newborn blood among the groups (Table 2). Tsp in cord blood was significantly shorter than that in newborn blood in the whole cohort (Fig. 2k) and in groups 1 and 3 (Table 2). The percentage of samples with spontaneous clots was also significantly higher in cord blood (Fig. 2l). With the exception of parameter D, which is associated with the fibrinogen concentration,31 there was no correlation regarding thrombodynamics parameters between umbilical cord blood and newborn blood (RSpearman = 0.641, p < 0.001).
Thromboelastometry (NATEM test): a – CT (the number of umbilical cord-newborn pairs n = 11), b – CFT (n = 11), c – α (n = 11), d – MCF (n = 11). Thrombodynamics: e –Tlag (n = 12), f – V (n = 10), g – Tsp (n = 9), h – percentage of samples with spontaneous clots (n = 12). The box plots indicate the following parameters: the mean value (the dot inside the box), the median (the horizontal line inside the box), and the 25th and 75th percentiles (the bottom and top of the box, respectively). The shaded area indicates the reference range. For p values, the paired sample Wilcoxon signed rank test was used; for p* values, the F test was used.
Next, we compared the hemostasis parameters of healthy term newborns with the same parameters with term newborns with various clinical complications (Table 1). There were no differences in any of the hemostasis tests in either cord blood or newborn blood (Table 2).
We also compared the parameters of hemostasis tests in healthy term newborns with the same parameters as those of preterm newborns. Compared with those of healthy term newborns, we detected the following differences in the blood of preterm newborns: prolonged APTT, decreased prothrombin, and increased Vi in the thrombodynamics assay (Table 2). In umbilical cord blood, the D-dimer concentration was increased, the CT in the thromboelastometry assay was decreased, and V in the thrombodynamics assay was increased in preterm newborns compared with healthy term newborns (Table 2). The other parameters did not differ significantly.
Sensitivity of coagulation tests to infection and hemorrhage
In total, 14 newborns had an infection (11 had congenital pneumonia, and 3 had infection specific to the perinatal period). This group had a significantly lower gestational age than the other group (median (25–75%): 36 (32–38) vs. 38 (37–39) weeks, p = 0.028) and newborn weight (2510 (1660–3428) vs. 3236 (2890–3480) g, p = 0.025). We compared hemostasis parameters in newborns with infection with those in all other newborns and revealed a prolonged APTT (56 (46–61) vs. 51 (44–57) sec, p = 0.011) and an increase in the Vi in the thrombodynamics assay (72 (70–75) vs. 69 (65–72) µm/min, p = 0.007) in the newborn blood. Additionally, the D-dimer concentration increased (1264 (999–3505) vs. 1430 (772–4649) ng/ml, p = 0.036), and Vi and V in the thrombodynamics assay were increased (82 (76–90) vs. 71 (69–76) µm/min, p = 0.004; 81 (72–92) vs. 53 (43–62) µm/min, p < 0.001) in the umbilical cord blood. It is likely that these differences are predominantly related to gestational age since these parameters were also different in groups 1 and 3. Thus, comparison of APTT between preterm newborns with infection (n = 6) and those without infection (n = 9) revealed no significant effect of infection (63 (55–70) vs. 57 (57–60) sec, p = 0.345).
There were a total of 19 patients with hemorrhage. This group had a significantly lower gestational age (33 (32–38) vs. 38 (38–39) weeks, p < 0.001) and newborn weight (2020 (1240–3270) vs. 3248 (2910–3495) g, p < 0.001) than the other patients. We compared the hemostasis parameters of the newborns with hemorrhages with those of all other newborns and found a prolonged APTT in the newborn blood (57 (44–65) vs. 48 (41–55) sec, p = 0.034); there were no significant differences in the other parameters. When we compared the APTT of the preterm newborns with hemorrhage (n = 11) with that of the newborns without hemorrhage (n = 17), we found that hemorrhage did not have a significant effect (61 (57–68) vs. 57 (55–60) sec, p = 0.480).
Effect of blood sampling in citrate tubes with CTI
Since we detected pronounced hypercoagulable changes in umbilical cord blood compared to newborn blood using global assays and considering their high sensitivity to the preanalytical stage, we decided to use citrate tubes with CTI to avoid the detected effects. Twelve newborns were included in this protocol.
We used the NATEM test in thromboelastometry; the activation occurred after recalcification of a whole blood sample from the walls of the cuvette, that is, by the contact pathway. Therefore, the expected effect of CTI should be a significant inhibition of coagulation. For example, in adult blood samples, CFT was prolonged by 2.6 ± 1.3 times from 210 ± 71 s to 477 ± 135 s (n = 7). CFT in cord blood was prolonged by 1.2 ± 0.5 times from 240 ± 156 s to 271 ± 176 s (n = 12, p = 0.037) when CTI was added to cord blood (Fig. 3b). MCF paradoxically but slightly increased in blood samples with CTI (Fig. 3a–d). No other significant changes were found. The absence of a CTI effect indicates that activation in both umbilical cord and newborn blood samples may be due to coagulation-activating components present directly in the blood.
There were no significant changes in thrombodynamics parameters with the addition of CTI to either umbilical cord blood or newborn blood (Fig. 3e–h).
We detected no difference between umbilical cord blood and infant blood in either global assay when examining the effects of CTI; this may be due to the small sample size.
Causes of neonatal hypercoagulation detected by laboratory tests
To determine the cause of hypercoagulation detected by thrombodynamics, we added an inhibitor of FIXa (NP2) or an inhibitor of TF (FVIIai) to 12 samples of neonatal plasma (either cord or peripheral), recalcified the plasma, and observed spontaneous clotting within the bulk of the plasma via a thrombodynamics analyzer (Fig. 4a).
a – Characteristic photos of spontaneous clots. The plasma samples from cord blood without (b) (the number of umbilical cord-newborn pairs, n = 8) and with (c) (n = 12) the addition of CTI to the blood collection tube with no inhibitor vs. samples with 100 nM of TF inhibitor inactivated FVIIa (FVIIai) or with 100 nM of the FIXa inhibitor nitrophorin 2 (NP2) and the plasma samples from newborn blood without (d) (n = 10) and with (e) (n = 10) the addition of CTI to the blood collection tube with no inhibitor vs. samples with 100 nM of FVIIai are presented. The box plots indicate the following parameters: the mean value (the dot inside the box), the median (the horizontal line inside the box), and the 25th and 75th percentiles (the bottom and top of the box, respectively). The shaded area indicates the reference range. For p values, the paired sample Wilcoxon signed rank test was used.
Addition of the FIXa inhibitor NP2 slightly prolonged T10% in cord blood both in blood samples collected into citrate tubes (Fig. 4b) and blood samples collected into citrate tubes with CTI (Fig. 4c). The addition of the TF inhibitor FVIIai significantly prolonged T10% in both cord blood and newborn blood regardless of the addition of CTI to the blood collection tube (Fig. 4b, c). The effect of FVIIai was much more pronounced than the effect of NP2. Similarly, FVIIai reduced the spontaneous clotting of newborn blood (Fig. 4d, e).
Discussion
A comparative study of umbilical cord blood and newborn blood using standard APTT and prothrombin tests revealed no significant differences between healthy term newborns and umbilical cord blood.25,26 Our results showed that APTT and prothrombin were significantly shifted toward hypocoagulation in umbilical cord blood compared to the blood of newborns. Most likely, these differences were associated with the preanalytical stage, namely, blood collection, since this is the most difficult process to standardize.32,33 In addition, in our study, the detected shift was small (the difference in the median values was 9% for APTT and 6% for prothrombin) which is comparable to the analytical reproducibility of these methods.34 Since there was a correlation between umbilical cord blood and newborn blood in terms of APTT and prothrombin, we suggest that both newborn blood and umbilical cord blood can be used to determine APTT and prothrombin.
Our data on the prolongation of APTT and the decrease in prothrombin in premature newborns compared to those in term newborns are in good agreement with the literature.24 We also recorded prolongation of APTT in groups of newborns with infection and with hemorrhage, but we suggest that this is associated with a decrease in gestational age in these newborns rather than with the pathophysiological mechanisms of thrombosis and hemostasis.
We found a decrease in the fibrinogen concentration in cord blood compared to that in newborn blood, but its correlation with the fibrinogen concentration in newborn blood also makes it possible to use cord blood for analysis. Our data are consistent with that reported in the literature.26
Our study demonstrated that the D-dimer concentration is higher in newborns than in adults, which is consistent with reports in the literature.12,13,14 An increase in the D-dimer concentration reflected an increase in thrombus lysis. It is known that in newborns, compared to those in adults, the concentrations of factor XIII and prothrombin are decreased,35 which makes the clot weaker and more susceptible to lysis. In addition, the TAFI is lower in newborns than in adults,36 which leads to increased lysis in newborns. This indicates that lysis in newborns should be enhanced. Direct comparisons of the lysis process also show that the clot is weaker in newborns and that lysis is better in newborns than in adults.37 An increase in the D-dimer concentration with decreasing gestational age has also been noted.15 In our study, we did not find differences in newborn blood between the term and premature preterm groups; however, this difference was detected in umbilical cord blood. Additionally, we found a significant increase in the D-dimer concentration in the peripheral blood of newborns compared to that in the umbilical cord blood, which could be due to umbilical cord injury. We suppose that during the first days of life, the D-dimer concentration in the blood of a newborn increases dramatically due to injury, and the D-dimer concentration becomes nonindicative (i.e., does not reflect generalized prothrombotic processes). Conversely, D-dimer from cord blood essentially reflect the situation before birth and may indicate pathological processes that began in utero. We detected an increase in D-dimer in umbilical cord blood but not in newborns with congenital infections. This finding is consistent with the literature, which has shown that newborns with sepsis have higher D-dimer concentrations than do newborns without sepsis in the control group.38 Regarding the D-dimer concentration, there was no correlation between umbilical cord blood and newborn blood.
We assume that prolongation of APTT and increases in prothrombin, fibrinogen and D-dimer concentrations in newborn blood compared with those in cord blood are associated with the development of hemostatic and proinflammatory responses to umbilical cord injury, which progress within 2 h after birth, when blood is sampled from newborns.
Thromboelastometry, as in previous research,26 showed a shift toward hypercoagulation in umbilical cord blood compared to newborn blood, with a lack of correlation in parameters between the umbilical cord and newborn blood. Adding a factor XII inhibitor to the blood did not affect the test parameters, although we used the NATEM test, in which activation occurs through the contact pathway. This suggests that extrinsic clotting activators, such as TF or phospholipid microvesicles, are present in both newborn and cord blood.12,39,40 No differences in thromboelastometry parameters according to gestational age were found. Thromboelastometry showed no sensitivity to infectious or hemorrhagic disorders.
Thrombodynamics showed increased coagulation in newborn blood compared to that in adult blood and the presence of spontaneous clots, which are not observed in healthy adults.41 This is consistent with previous studies.8,42 The rate of clot growth was higher in umbilical cord blood, and spontaneous clots formed more intensely in the umbilical cord blood than in the newborn blood, while regarding thrombodynamics parameters, there was no correlation observed between the umbilical cord blood and newborn blood. In the study,42 there was no significant difference in the thrombodynamics parameters between the umbilical cord blood and newborn blood; however, the study used a small sample of patients (n = 12). No dependence on gestational age was found, as in our earlier work.8 The addition of a contact activation inhibitor during blood collection did not affect the test parameters, which indicates the insignificant contribution of the contact activation of coagulation during blood collection. Thrombodynamics were sensitive to infectious diseases: the initial rate of clot growth increased in the presence of infection in both umbilical cord blood and newborn blood. Thus, thrombodynamics appears to be a promising test for diagnosing inflammatory conditions in newborns.
The addition of factor IXa and TF inhibitors to plasma showed that the formation of spontaneous clots decreases when TF is inhibited, which indirectly confirms the assumption that significant concentrations of TF are present in both the newborn and cord blood, which is consistent with the literature.12,39,40 The reasons for this are not entirely clear, but it may be due to a physiological mechanism to protect the child from blood loss. Thus, from cell culture experiments, it is known that umbilical vein cells express more TF than saphenous vein cells43; the placenta is also capable of secreting microparticles with TF.44
We suggest that global assays indicate a procoagulant shift in cord blood compared with newborn blood caused by the higher concentration of TF in the umbilical cord due to physiological reasons or preanalytical difficulties.
Conclusions
We detected significant differences in APTT, prothrombin and fibrinogen concentrations between the umbilical cord blood and peripheral blood of a newborn, and there was a correlation between these two blood sources, which suggests that it is possible to use either umbilical cord blood or peripheral blood for studies using the reference range corresponding to the type of blood. On the first day of life, it seems reasonable to use umbilical cord blood to determine the D-dimer concentration since, in this case, sensitivity to infectious diseases is observed. In contrast, global hemostasis assays, thromboelastometry and thrombodynamics showed a hypercoagulable shift in cord blood without correlation with newborn blood; thus, only peripheral blood from newborns should be assessed in these studies.
The data obtained in this work can be used when planning studies of the (patho)physiological features of hemostasis in newborns and organizing a screening study of hemostasis in newborns.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Monagle, P. et al. Developmental haemostasis. Thromb. Haemost. 95, 362–372 (2006).
Andrew, M. et al. Development of the human coagulation system in the full-term infant. Blood 70, 165–172 (1987).
Andrew, M. et al. Development of the human coagulation system in the healthy premature infant. Blood 72, 1651–1657 (1988).
Monagle, P. & Massicotte, P. Developmental haemostasis: Secondary haemostasis. Semin. Fetal Neonatal. Med. 16, 294–300 (2011).
Andrew, M. et al. Maturation of the hemostatic system during childhood. Blood 80, 1998–2005 (1992).
Schneider, D. M., von Tempelhoff, G. F., Herrle, B. & Heilmann, L. Maternal and cord blood hemostasis at delivery. J. Perinat. Med. 25, 55–61 (1997).
Bleyer, W. A., Hakami, N. & Shepard, T. H. The development of hemostasis in the human fetus and newborn infant. J. Pediatr. 79, 838–853 (1971).
Koltsova, E. M. et al. Impaired platelet activity and hypercoagulation in healthy term and moderately preterm newborns during the early neonatal period. Pediatr. Res. 85, 63–71 (2019).
Blajchman, M. A. An overview of the mechanism of action of antithrombin and its inherited deficiency states. Blood Coagul. Fibrinolysis 5, 5–12 (1994).
Mitsiakos, G. et al. Neonatal haemostatic parameters in correlation to gestational age and birth weight. Int. J. Lab. Hematol. 44, 952–958 (2022).
Lippi, G. et al. Routine coagulation tests in newborn and young infants. J. Thromb. Thrombolysis 24, 153–155 (2007).
Tay, S. P., Cheong, S. K. & Boo, N. Y. Circulating tissue factor, tissue factor pathway inhibitor and D-dimer in umbilical cord blood of normal term neonates and adult plasma. Blood Coagul. Fibrinolysis 14, 125–129 (2003).
Knöfler, R. et al. Molecular Markers of the Endothelium, the Coagulation and the Fibrinolytic Systems in Healthy Newborns. Semin Thromb. Hemost. 24, 453–461 (1998).
Khalilov, Z. İ., Ünsal, A. & Altuntaş, N. The D-dimer reference intervals in healty term newborns. Transfus. Apher. Sci. 61, 103493 (2022).
Hudson, I. R. et al. Increased concentrations of D-dimers in newborn infants. Arch. Dis. Child 65, 383–384 (1990).
Nako, Y., Tomomasa, T. & Morikawa, A. Plasma thrombomodulin level in newborn infants with and without perinatal asphyxia. Acta Paediatr. 86, 91–95 (1997).
Murtha, A. Umbilical venous d-dimer concentrations with and without labor. Obstet. Gynecol. 92, 184–186 (1998).
Katsaras, G. Ν et al. The use of thromboelastography (TEG) and rotational thromboelastometry (ROTEM) in neonates: a systematic review. Eur. J. Pediatr. 180, 3455–3470 (2021).
Murphy, C. A. et al. The role of the calibrated automated thrombogram in neonates: describing mechanisms of neonatal haemostasis and evaluating haemostatic drugs. Eur. J. Pediatr. 181, 23–33 (2022).
Lipets, E. et al. Circulating contact-pathway-activating microparticles together with factors IXa and XIa induce spontaneous clotting in plasma of hematology and cardiologic patients. PLoS One 9, e87692 (2014).
Neary, E. et al. Coagulation indices in very preterm infants from cord blood and postnatal samples. J. Throm Haemost. 13, 2021–2030 (2015).
Raffaeli, G. et al. Thromboelastographic profiles of healthy very low birthweight infants serially during their first month. Arch. Dis. Child Fetal Neonatal Ed. 105, 412–418 (2020).
Strauss, T. et al. Clot formation of neonates tested by thromboelastography correlates with gestational age. Thromb. Haemost. 103, 344–350 (2010).
Wiegele, M. et al. Establishing reference ranges of cord blood: point-of-care hemostatic function assessment in preterm and term neonates. Pediatr. Res 90, 452–458 (2021).
Nielsen, S. T. et al. Coagulation parameters in the newborn and infant – the Copenhagen Baby Heart and COMPARE studies. Clin. Chem. Lab. Med. 60, 261–270 (2021).
Raffaeli, G. et al. Is placental blood a reliable source for the evaluation of neonatal hemostasis at birth? Transfusion 60, 1069–1077 (2020).
Whiting, D. & DiNardo, J. A. TEG and ROTEM: Technology and clinical applications. Am. J. Hematol. 89, 228–232 (2014).
Koltsova, E. M. et al. Determination of fibrin clot growth and spatial thrombin propagation in the presence of different types of phospholipid surfaces. Platelets 32, 1031–1037 (2021).
Balandina, A. N. et al. Thrombodynamics-A new global hemostasis assay for heparin monitoring in patients under the anticoagulant treatment. PLoS One 13, e0199900 (2018).
Lipets, E. N. et al. Use of Thrombodynamics for revealing the participation of platelet, erythrocyte, endothelial, and monocyte microparticles in coagulation activation and propagation. PLoS One 15, 1–24 (2020).
Ovanesov, M. V., Ananyeva, N. M., Panteleev, M. A., Ataullakhanov, F. I. & Saenko, E. L. Initiation and propagation of coagulation from tissue factor-bearing cell monolayers to plasma: initiator cells do not regulate spatial growth rate. J. Thromb. Haemost. 3, 321–331 (2005).
Gosselin, R. C. & Marlar, R. A. Preanalytical Variables in Coagulation Testing: Setting the Stage for Accurate Results. Semin Thromb. Hemost. 45, 433–448 (2019).
Dashkevich, N. M. et al. Effect of pre-analytical conditions on the thrombodynamics assay. Thromb. Res. 133, 472–476 (2014).
Yis, O. M., Bugdayci, G., Pehlivan, M. B., Yildiz, R. N. & Alisik, M. Analytical performance evaluation of Sysmex CS-2500 and Stago STA Compact. Blood Coagul. Fibrinolysis 31, 324–329 (2020).
Achey, M. A. et al. The Developing Balance of Thrombosis and Hemorrhage in Pediatric Surgery: Clinical Implications of Age-Related Changes in Hemostasis. Clin. Appl Thromb. Hemost. 26, 107602962092909 (2020).
Gursoy, T. et al. Thrombin activatable fibrinolysis inhibitor activity (TAFIa) levels in neonates with meconium-stained amniotic fluid. J. Matern Fetal Neonat. Med. 21, 123–128 (2008).
Nellenbach, K. A. et al. Comparison of Neonatal and Adult Fibrin Clot Properties between Porcine and Human Plasma. Anesthesiology 132, 1091–1101 (2020).
Al-Biltagi, M. et al. Plasma D-dimer level in early and late-onset neonatal sepsis. World J. Crit. Care Med 11, 139–148 (2022).
Schweintzger, S. et al. Microparticles in newborn cord blood: Slight elevation after normal delivery. Thromb. Res. 128, 62–67 (2011).
Korbal, P., Słomka, A., Sadowska-Krawczenko, I. & Żekanowska, E. Evaluation of tissue factor bearing microparticles in the cord blood of preterm and term newborns. Thromb. Res. 153, 95–96 (2017).
Sinauridze, E. I. et al. Thrombodynamics, a new global coagulation test: Measurement of heparin efficiency. Talanta 180, 282–291 (2018).
Haidl, H. et al. New insights into neonatal coagulation: normal clot formation despite lower intra-clot thrombin levels. Pediatr. Res. 86, 719–724 (2019).
Grabowski, E. F. et al. Comparison of Tissue Factor Pathway in Human Umbilical Vein and Adult Saphenous Vein Endothelial Cells: Implications for Newborn Hemostasis and for Laboratory Models of Endothelial Cell Function. Pediatr. Res. 46, 742–742 (1999).
Uszyński, M., Żekanowska, E., Uszyński, W., Kuczyński, J. & Żyliński, A. Microparticles (MPs), tissue factor (TF) and tissue factor inhibitor (TFPI) in cord blood plasma. A preliminary study and literature survey of procoagulant properties of MPs. Eur. J. Obstetr Gynecol. Reprod. Biol. 158, 37–41 (2011).
Funding
Russian Science Foundation, grant number 22-15-00164.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.N.B. and N.K.T.; methodology, E.M.K.; formal analysis, E.M.K. and A.N.B.; investigation, E.M.K., B.V.A., M.A.S., E.N.L, L.A.T., A.L.K., E.N.B.; data curation, B.V.A., M.A.S.; writing – original draft preparation, E.M.K., A.N.B.; writing – review and editing, N.K.T., E.N.B, M.A.S. and L.V.K.; visualization, A.N.B. and E.M.K.; supervision, F.I.A., V.V.Z., D.N.D and G.T.S; project administration, B.V.A.; funding acquisition, A.N.B. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
FIA is a former employee and cofounder of Hemacore, LLC. Hemacore LLC holds several patents and patent applications related to the diagnostic use of spatial clot growth and has developed the assay under the trade name Thrombodynamics®. Hemacore LLC did not have any additional role in the design of the study, collection and analysis of the data, or the decision to publish or prepare the manuscript. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. All other authors declare no competing financial interests.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after academician Kulakov V.I. (Protocol code: 10, date of approval: 23 November 2017).
Informed consent
Written informed consent was obtained from the legal representatives (parents) of the newborns to publish this paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arutunyan, B.V., Koltsova, E.M., Shpilyuk, M.A. et al. Comparison of standard and global hemostasis assays in cord and peripheral blood of newborns. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03475-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03475-y
- Springer Nature America, Inc.