Abstract
Cerebrovascular reactivity defines the ability of the cerebral vasculature to regulate its resistance in response to both local and systemic factors to ensure an adequate cerebral blood flow to meet the metabolic demands of the brain. The increasing adoption of near-infrared spectroscopy (NIRS) for non-invasive monitoring of cerebral oxygenation and perfusion allowed investigation of the mechanisms underlying cerebrovascular reactivity in the neonatal population, confirming important associations with pathological conditions including the development of brain injury and adverse neurodevelopmental outcomes. However, the current literature on neonatal cerebrovascular reactivity is mainly still based on small, observational studies and is characterised by methodological heterogeneity; this has hindered the routine application of NIRS-based monitoring of cerebrovascular reactivity to identify infants most at risk of brain injury. This review aims (1) to provide an updated review on neonatal cerebrovascular reactivity, assessed using NIRS; (2) to identify critical points that need to be addressed with targeted research; and (3) to propose feasibility trials in order to fill the current knowledge gaps and to possibly develop a preventive or curative approach for preterm brain injury.
Impact
-
NIRS monitoring has been largely applied in neonatal research to assess cerebrovascular reactivity in response to blood pressure, PaCO2 and other biochemical or metabolic factors, providing novel insights into the pathophysiological mechanisms underlying cerebral blood flow regulation. Despite these insights, the current literature shows important pitfalls that would benefit to be addressed in a series of targeted trials, proposed in the present review, in order to translate the assessment of cerebrovascular reactivity into routine monitoring in neonatal clinical practice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The ability of the cerebral vasculature to regulate its resistance in response to both local and systemic factors is defined as cerebrovascular reactivity (CR) and is aimed at maintaining adequate cerebral blood flow (CBF) to meet cerebral metabolic demand. Neonatal CR was first studied using radioactive tracer methods and Doppler sonography. The application of non-invasive and operator-independent near-infrared spectroscopy (NIRS) technique for cerebral regional tissue oxygen saturation (rStO2) monitoring in neonatal settings has shed further light on CR in several physiological and pathological conditions. Nevertheless, the methodological heterogeneity, along with the observational nature and the small sample sizes of current reports, hinders a routine application of CR monitoring for neonatal neuroprotection. On these premises, we aim to provide an updated review of NIRS-based evidence on neonatal CR and to identify critical issues and gaps that should be addressed with targeted trials to implement CR monitoring in neonatal intensive care.
Physiological mechanisms of cerebrovascular reactivity
Pressure-flow autoregulation
The modulation of vascular tone in relation to intraluminal pressure is a leading mechanism of CBF regulation, mediated by the mechanoreceptor properties of smooth muscle cells lining cerebral arteries. In response to increased intraluminal pressure, membrane depolarisation and calcium-dependent vasoconstriction occur, while the opposite happens at low intraluminal pressure, resulting in cerebral vasodilation.1 The classic depiction of cerebral pressure-flow autoregulation is a sigmoidal curve (Fig. 1), with stable CBF over a range of cerebral perfusion pressure (CPP), of which a main determinant in neonates is arterial blood pressure (ABP). When ABP values fall below the lower limit or rise above the upper limit of autoregulatory capacity, pressure-passive circulation occurs, with potential ischaemic or haemorrhagic complications.2 Recent evidence from adults, however, has revised this classical view by showing a much shorter plateau which still has a gentle slope, indicating some degree of pressure-passive CBF.3,4 Notably, ‘cerebral autoregulation’ is often used to refer to ‘cerebrovascular reactivity’. However, although the pressure-flow autoregulation of CBF is frequently involved in the interplay between CR mechanisms, the two terms are not synonymous, and their interchangeable use may contribute to the heterogeneity seen in current literature.
The main determinant of cerebral perfusion pressure (CPP, shown in the x-axis) in the neonatal brain is arterial blood pressure. The black line indicates pressure-flow regulation under physiological homoeostatic conditions (i.e., normal pH, PaCO2, PaO2, normoglycaemia, basal autonomic status). The red line illustrates changes in CBF and in its relationship with CPP under hypercapnia and other conditions determining cerebral vasodilation, which may reduce the vasodilatory reserve and shorten the autoregulatory plateau. The blue line illustrates the changes in CBF and in its relationship with CPP under hypocapnia or associated conditions, which may widen the autoregulatory plateau. The dashed black line indicates the effect of sympathetic activation on the pressure-flow regulation curve. The dotted vertical lines indicate the upper (ULA) and lower (LLA) limits of the autoregulatory plateau in different conditions.
Biochemical factors
Partial arterial pressure of oxygen (PaO2) and carbon dioxide (PaCO2) are potent chemo-modulators of cerebral vasculature, independent of intravascular pressure. While hypoxia and hypercapnia exert a vasodilatory effect on CBF, hyperoxia and hypocapnia lead to cerebral vasoconstriction. Vascular reactivity to PaO2 and PaCO2 is mediated by H+/K+ homoeostasis, secondary to changes in perivascular pH.5,6,7 CR to PaO2, PaCO2 and ABP can functionally interact (Fig. 1). For example, if the vasodilator pathway has been activated during hypercapnia, the slope of the autoregulatory plateau increases, predisposing to larger CBF fluctuations even when ABP/CPP are within the normal range.8
Metabolic factors
Cerebral metabolic demands can influence cerebral perfusion, as supported by the evidence of CBF changes in relation to glucose availability. In preterm newborns, hypoglycaemia is associated with a significant compensatory increase in CBF;9 following the restoration of normoglycaemia, CBF gradually decreases.10 A recent systematic review11 has also reported a similar negative correlation between blood glucose levels and either cerebral rStO2 or haemoglobin concentration,12,13,14 used as a proxy for CBF, in term and preterm neonates.
Sympathetic nervous system
The cerebral vasculature has abundant adrenergic receptors and is under precise autonomic control.15 Sympathetic activation shifts the autoregulatory plateau towards higher CPP, thereby protecting the brain against hyperperfusion (see Fig. 1).16 The sympathetic system appears to play a greater role in CBF regulation in the perinatal period than later in life.17 The relative immaturity of nitric oxide (NO)-induced vasodilatory mechanisms during early development, the greater sensitivity to exogenous norepinephrine and the higher sympathetic nerve density in neonatal compared to adult pial arteries may contribute to this finding.18
Functional activation
The CBF response to neuronal activation is referred to as neurovascular coupling. This response is mediated by the neurovascular unit (Fig. 2), which represents an interactive network of cerebral vessels, vascular cells (pericytes, smooth muscle and endothelial cells), glia (astrocytes and microglia) and perivascular neurons. Upon neuronal activation, vasoactive substances such as prostaglandins, NO and adenosine are released from both neurons and astrocytes, leading to the modulation of vascular smooth muscle.19 Studies using functional NIRS have provided insight into the neurovascular coupling response in newborns. In the adult brain, the classic ‘positive’ response is characterised by an increase in oxygenated haemoglobin (O2Hb) and a decrease in deoxygenated haemoglobin (HHb). In neonates, however, variable cerebral haemodynamic patterns have been reported in response to neuronal activation, including a ‘negative’ response with local O2Hb reduction, which may indicate that the oxygen consumption triggered by neuronal activity transiently outpaces the concomitant CBF increase.20,21,22,23,24 This variability may be due to developmental changes in the capacity of cerebral vasculature to produce functional hyperaemia in the low-density capillary bed of the neonatal brain.25,26
CO carbon monoxide, SMC smooth muscle cell, EC endothelial cell, Ach acetylcholine, PGs prostaglandins, EETs epoxyeicosatrienoic acids, VIP vasoactive intestinal polypeptide, CGRP calcitonin gene-related peptide, NA noradrenaline, DA dopamine, NPY neuropeptide Y, EDHF endothelium-derived hyperpolarizing factor, ET endothelin, GABA γ-aminobutyric acid, ROS reactive oxygen species. Reproduced with permission from Brew et al.38.
NIRS-based assessment of cerebrovascular reactivity: surrogate signals and neonatal applications
The assessment of CBF fluctuations in response to physiological changes or stimuli is fundamental to evaluate the integrity of CR. This assessment evaluates the relationship between the input and the output (i.e., CBF) signals,27 and can be classified into static, which includes quantitative methods performing point measurements of CBF (e.g.,133Xe clearance), or dynamic.28,29 While in adults, transcranial Doppler sonography (TCD) can be used for dynamic CR monitoring, the small size of neonatal vessels hinders an accurate measurement of the vessel diameter, which is necessary to calculate blood flow within a specific artery.30 The small vessel size also causes frequent loss of signal, limiting the application of neonatal TCD to static CBF assessments.31,32,33
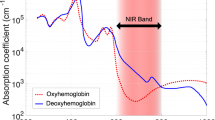
NIRS exploits the relative transparency of near-infrared light (700–950 nm) in biological tissue and the oxygen-dependent absorption of haemoglobin at different wavelengths to measure rStO2, derived from the changes in concentration of O2Hb and HHb within the vascular beds (Fig. 3). Fluctuations in cerebral rStO2 can reflect changes in CBF if other determinants of cerebral metabolism and oxygen delivery (i.e., arterial oxygen saturation, haemoglobin concentration, arterial–venous volume ratio, fractional inspired oxygen, tissue oxygen diffusivity) remain relatively constant.34 NIRS monitoring can be performed non-invasively and continuously for relatively prolonged periods; hence, cerebral rStO2 has been used as a surrogate for dynamic CBF monitoring, mainly for slow CBF changes. The changes in total cerebral haemoglobin concentration (∆tHb) or tissue haemoglobin index (THI), as a sum of ∆O2Hb and ∆HHb or directly measured using the isosbestic 805 nm wavelength, have been described as surrogate measurements of cerebral blood volume.12,35,36,37
a Absorption spectra for oxygenated haemoglobin (O2Hb), deoxygenated haemoglobin (HHb), and water. b Example of photon trajectory through biological tissue. S source, D detector, ρ source-detector distance. The shaded part highlights the area crossed by the photon. Adapted with permission from Martini et al.172.
The interaction between CBF and CPP is mediated by cerebrovascular resistance as follows, using an analogy of Ohm’s law:
Cerebrovascular resistance is determined by the vascular tone of the arterial smooth muscle cells. During brain development, the muscularis layer of the extra-striatal arterioles is initially limited to the pial vessels and superficial penetrators; consequently, in preterm infants, cerebral vasoreactivity occurs predominantly in the superficial and peripheral parenchyma of the brain.38
CPP is dependent on ABP and intracranial pressure. As intracranial pressure is assumed stable in neonates because of the open cranial sutures, the slow waves of ABP can presumably be used as a surrogate for low-frequency CPP changes. With high-frequency CPP changes and oscillations (>0.20 Hz), the autoregulatory processes become less able to stabilise CBF in the face of changing CPP.39 Therefore, these fast CPP oscillations are passed along unimpeded into CBF oscillations. In contrast, slower frequency oscillations (<0.20 Hz, but most effectively <0.05 Hz) can be counteracted by the cerebral arterioles and are dampened.40,41 Hence, continuous measurements of slow rhythmic oscillations in ABP and CBF have been used to assess the integrity of pressure-flow autoregulation36,42,43,44,45,46 and, in different neonatal cohorts, have also identified individual optimal ABP (ABPopt) ranges within which autoregulatory mechanisms are most effective, defined by the lowest values of the related CR coefficient indicating functional reactivity.47,48,49,50,51,52 Continuous ABP monitoring requires an indwelling arterial catheter53 and may not always be feasible. To date, evidence on the use of non-invasive continuous monitoring for blood pressure (e.g., beat-to-beat finger arterial devices) to assess neonatal pressure-flow autoregulation is limited,54,55 and the reliability of non-invasive blood pressure monitoring against invasive methods needs further improvements.56,57 Using blood pressure data measured non-invasively by arm cuffs at 15 min after birth, functional pressure-flow autoregulation was demonstrated in term neonates during the immediate postnatal transition, but not in preterm infants at this early phase.58
Being a direct determinant of cardiac output, heart rate (HR) can be considered a surrogate of systemic blood flow. Since continuous HR monitoring is non-invasive and universally available, HR can represent an alternative input signal to assess CR in response to systemic blood flow changes. A moving correlation coefficient between cerebral rStO2 and HR (TOHRx) has been proposed for CR monitoring in preterm infants, with rising values indicating loss of CR.47,48,59,60,61 Low or negative TOHRx values have been used to define ABPopt ranges during the first 24 h after birth.48 The recent neonatal application of non-invasive bedside devices for continuous cardiac output monitoring may facilitate the investigation of CR to systemic blood flow; current data, however, are limited. Two reports have found no association between cardiac output and cerebral rStO2 in term infants immediately after delivery62 or at different sleep positions.63
PaCO2 can also be used as an input to evaluate CBF-CO2 reactivity.8,64 End-tidal (etCO2) or transcutaneous CO2 (tCO2) monitoring allow continuous, non-invasive estimation of PaCO2. The relationship between tCO2 and THI in neonates has been evaluated by Dietz et al.,65 who documented a trend towards increased CO2 reactivity in healthy term neonates between days 1 and 4, whereas Aly et al.66 found that lower gestational age (GA), mechanical ventilation and increased PaCO2 were associated with stronger CO2 reactivity in the first week of life in preterm infants, with possible implications on the risk of brain injury. EtCO2 fluctuations have also been associated with concomitant changes in cerebral rStO2 and electroencephalographic brain activity.67 Other studies using punctual PaCO2 from blood gas analysis yielded variable results: in preterm infants, Kaiser et al. observed a progressive loss of pressure-flow autoregulation for PaCO2 ≥45 mmHg during the first week of life,8 whereas Hoffman et al. failed to demonstrate an overall association between PaCO2 and pressure-flow autoregulation during the transitional period.68 During the immediate postnatal period (i.e., 15 min after birth), Wolfsberger et al. observed that the vasodilative effect of PaCO2 on cerebral rStO2 was less pronounced in preterm compared to term neonates.69
Electroencephalographic techniques have been used to investigate CBF responses to neurophysiological changes. In preterm neonates, Roche-Labarbe et al. described that spontaneous bursts of electroencephalographic activity were coupled to a haemodynamic response characterised by an O2Hb decrease, followed by an increase and then a return to baseline.70 Tataranno et al. documented a decreased cerebral rStO2 and increased tissue oxygen extraction in extremely preterm infants with increasing intensity of spontaneous brain activity.71 Seizures are paroxysmic bursts of abnormal electrical brain activity. Available neonatal data during different types of seizures consistently report a reduction of cerebral rStO2,72,73,74 followed by a variable rStO2 increase.73,74 The observed rStO2 reduction was associated with an O2Hb decrease and an HHb increase mimicking a ‘negative functional response’ pattern,73 consistent with the elevated oxygen consumption associated with seizures which exceeds cerebral oxygen delivery.
Notably, CR assessment is only reliable if considerable variability in the input signal is present; if there is no variation, dependency cannot be determined. Some researchers have proposed adding more weight to epochs with high variability to correct for this factor.75,76,77 Furthermore, when compared to outer input signals such as those derived by electroencephalographic or electrocardiographic techniques, some NIRS instruments may have a lower sampling rate; this may affect the calculation accuracy of some CR metrics such as those for neurovascular coupling, especially for long-term monitoring periods.19,25,78
Proposed mathematical methodologies for CR assessment
Recent overviews, which also provide specific methodological details of the studies included, have confirmed the feasibility of examining CR in the neonatal population.79,80 To date, however, there is no clinical gold standard methodology for CR assessment; this is reflected in the numerous mathematical methods used for these purposes. These methodologies have mostly been applied to the investigation of pressure-flow autoregulation. Signal processing techniques for assessing neurovascular coupling have recently been reviewed by Hendrikx et al.19 and will not be addressed in this review.
Invasively measured ABP and cerebral rStO2 as surrogates for CPP and CBF, respectively, are included in different mathematical models to address pressure-flow autoregulation. The lack of standardisation on different levels in the quantification of pressure-flow autoregulation (Table 1) hinders the reproducibility of studies that have related the analyses with clinical outcomes.
To study cerebral autoregulation, a synchronised capture of the physiological signals from multiple monitoring systems into a single aggregated file is required. Several available data capture platforms compatible with current NIRS monitors have been recently summarised by Vesoulis et al.81 A major step in pre-processing of the acquired data is the removal of artefacts; although this essential component is often performed manually, ideally a reliable automated approach would speed up this stage, avoid any biases, and enable real-time analysis. As two key assumptions for the use of rStO2 as a surrogate for CBF are stable SpO2 and cerebral metabolic rate (CMRO2), correction for SpO2 and CMRO2 would ideally also be necessary. The total cerebral haemoglobin concentration has been proposed by Grubb et al. as a surrogate for CBF independent of the CMRO2.82
Pressure-flow autoregulation analysis, making use of the spontaneous oscillations in cerebral oxygenation and ABP, has been explored both in the time domain and frequency domain by correlation and coherence analysis, respectively. These approaches assume that the physiological parameters are considered stationary signals.
Time-domain methods
The time-based method of Pearson correlation (r) is the most straightforward approach. The correlation coefficient between rStO2 and ABP is used as a cut-off value to assess the presence or absence of pressure-flow autoregulation (the closer to 0, the higher the autoregulatory capacity); significant positive correlation is then considered to represent pressure-passive cerebral circulation in that epoch.83,84,85,86,87,88 A question remains whether the cerebrovascular transit time (i.e., the time needed for cerebral rStO2 to fully respond to a CBF change) is considered. In this regard, the moving correlation coefficient between cerebral rStO2 and ABP, which has been validated in hypotensive piglets and has been shown to correlate with TCD measurements of pressure-flow autoregulation in adult patients,44 has been largely used in neonatal clinical observational studies.85,89,90,91,92 The percentage of epochs with impaired pressure-flow autoregulation (defined as r above a predefined threshold), the strength of correlation, but also the amount of r variability during measurement can be assessed.93 These principles have been applied to other NIRS-based parameters to study pressure-flow autoregulation. For example, the moving correlation between THI and ABP has been used to determine individualised ABPopt.49,50,52,94,95 Similarly, the Pearson correlation between THI and tCO2 has been used to investigate CR to CO2 in the neonatal population.65,66
Frequency-domain methods
Coherence analysis has been applied to explore the relationship between NIRS measurements and ABP changes in the frequency domain.96 Notably, according to the Nyquist theorem, the sampling frequency of the collected data needs to be at least twice the highest frequency of interest in the signal. Similar to time-domain methods, a predefined threshold is used to define impaired pressure-flow autoregulation (the closer to 0, the higher the autoregulatory capacity). To correct for different epoch lengths and sample frequencies, Monte-Carlo simulations are performed to identify significant coherence in the given data segments;75,97 adaptations to the basic model of coherence are also made.76,98 Coherence describes the impairment of pressure-flow autoregulation quantitatively. The degree of dependence between two variables (e.g., ABP and rStO2) is measured (no units), and by performing transfer function analysis, the gain and phase are calculated. The gain estimates the degree of this impairment by describing the magnitude of change in the output signal (e.g., rStO2) in relation to a unity change in the input signal (e.g., ABP) at a given frequency. However, causality, i.e., what signal induces changes in the other signal, cannot be established by these methods. Wong et al.45 first used coherence and gain to assess rStO2-derived pressure-flow autoregulation in sick infants. This method was later validated by Hahn et al. in piglets.99 Transfer function with logarithmic transformation of the gain coefficient to provide the amplitude of the dampening response is described by Vesoulis et al.100 The phase describes the time delay between coherent oscillations of the two signals and can be seen as an additional factor in pressure-flow autoregulation analyses. Pressure-flow autoregulation can be studied in different frequency bands, ranging from ultra-low to high-frequency bands. Low-frequency bands correspond to slow oscillations in ABP and rStO2. Slow and prolonged periods of hypotension or hypertension of higher magnitudes are considered to be more injurious than fast and transient ABP changes of small magnitudes (‘high-pass filter’ principle of pressure-flow autoregulation).101
Time-frequency methods
Since biological signals tend to be non-linear and non-stationary, alternative methods based on linear equations, such as wavelet cross-correlation, have also been described.102 This approach incorporates a time element to frequency analysis. It makes no assumption about the stationarity of input signals, providing a framework for the analysis of non-stationary effects in cerebral haemodynamics. Another time-frequency method, defined as bivariate auto-regressive coherence (BiAR-COH), has been proposed by Riera et al.98 The main difference between the BiAR-COH and standard coherence methods is that the former demands both temporal and frequency dependence, whereas coherence only evaluates frequency dependence. This key feature discriminates changes in the two signals that are closely related in time from those that are not time-related and, therefore, have no mutual dependence. The same authors introduced the partial directed coherence (PDC) method to address the condition of directionality,76 so that the system is forced to consider only those events in which changes in CPP induce changes in CBF. Therefore, this approach not only analyses pressure-flow autoregulation, but also infers causality.76
Impaired cerebrovascular reactivity and neonatal outcomes
Impaired CR is a marker of disease severity and adverse outcome, both in term85,103,104,105,106,107,108,109,110 and preterm infants.60,76,83,84,91,98,111,112,113 The patient populations most often studied are neonates with hypoxic-ischaemic encephalopathy (HIE) and preterm infants during the postnatal transition, which will be discussed hereafter; specific study details on these populations are available in other reviews.80,114,115 Insights on other neonatal conditions are also available in recent targeted reviews.2,116
There is longstanding evidence of the association between perinatal hypoxia-ischaemia and altered CR, resulting in hypoxia-ischaemia-reperfusion injury.117,118,119 Cerebral hypoxia-ischaemia induces compensatory overproduction of NO,120 leading to persistent cerebral vasodilation that may disrupt the vessels’ autoregulatory capacities (the so-called vasoparalysis).115 Upon reperfusion, the disrupted CR results in a significant increase in CBF with no change in CMRO2.121 In animal models of hypoxia-ischaemia, this cerebral hyperaemia has been associated with histopathological evidence of brain damage.106,115 Consistent evidence of post-asphyxial cerebral hyperaemia has been obtained from human neonates with HIE using NIRS.103,115,122,123 This CBF increase positively correlates with the severity of the ischaemic hit, and is accompanied by an impaired CR to acute ABP and CO2 changes.119 The burden of cerebral hyperaemia and of CR impairment was greater in infants who later developed brain injury on MRI or showed poorer neurodevelopmental outcomes.85,103,104,105,106,107,108,110,124 Using wavelet coherence between NIRS and electroencephalographic signals, Das et al.109 recently showed that HIE infants with MRI brain abnormalities had poorer neurovascular coupling during the first 24 h compared to those with normal neuroimaging. Notably, the cerebral rStO2- electroencephalographic coherence during this early period was superior to the Sarnat score in predicting abnormal brain MRI.
Given the altered CR following perinatal asphyxia, defining ABPopt ranges of pressure-flow autoregulation is particularly important. Prolonged ABP deviations below ABPopt during the hypothermic treatment have been associated with increased MRI abnormalities in both the deep grey matter51,52 and white matter.50 Infants with more prolonged deviations below ABPopt also had greater motor and cognitive impairments at 21–32 months.49 Based on this evidence, monitoring pressure-flow autoregulation to establish and target ABPopt ranges with a tailored haemodynamic management (e.g., adjusting pharmacological cardiovascular support) is potentially an adjunctive neuroprotective strategy in neonatal HIE.
In the preterm population, the cardiovascular changes occurring during the first 72 h after birth, which define the so-called transitional period, are often associated with a significant haemodynamic instability together with CBF fluctuations that may cause disruption of the germinal matrix endothelium, increased intravascular pressure and125 result in intraventricular haemorrhage (IVH).126 Accordingly, specific NIRS patterns of cerebral haemodynamics have been reported in infants developing this complication, characterised by reduced cerebral rStO2 during the first 24 h60,91,127,128 followed by a transient increase,60,127 which suggest the hypoperfusion-hyperaemia alternance.
Numerous NIRS studies have shown impaired CR in preterm infants developing IVH.60,61,76,83,84,91,98,111,112,129 Severe IVH development has been associated with a significantly higher time burden of a pressure-passive circulation on day 2, which was also the median age at IVH detection.84 An independent association between high-magnitude cerebral pressure-flow passivity in the low-frequency range and IVH development has also been found.112 With regard to ABPopt, infants with greater deviations below or above ABPopt ranges had higher IVH rates compared to those whose ABP laid close to optimal ranges during the transition.47,48 Significantly higher TOHRx values were also reported in infants who developed IVH compared to those who did not.60,61 In contrast, one study observed lower amplitudes of cross-correlation, semblance and gain between cerebral rStO2 and HR, measured by wavelet analysis, in a small number of preterm infants developing IVH/pulmonary haemorrhage compared to those who did not, possibly reflecting the extreme haemodynamic instability associated with haemorrhages.130
The link between CR indices and systemic blood flow, as well as their relationship to IVH has also been reported. Low superior vena cava (SVC) flow has been pathophysiologically linked to intracranial bleeding in preterm neonates.125,131 Low SVC flow infants also had higher BiAR-COH and PDC, indicating impaired pressure-flow autoregulation, and were more prone to developing severe IVH.76,98 A negative correlation between COx and left ventricular output at 24 h of life in a cohort of neonates that developed IVH has also been reported.91 These findings align with the report of lower left ventricular output and cerebral rStO2 within the first day of life in infants who later developed IVH,127 and may reflect CR impairment associated with fluctuations of systemic blood flow before or around the time of bleeding. The association between other factors influencing CR, such as PaCO2 and IVH,132 warrants further targeted investigations.
Dopamine and impaired pressure-flow autoregulation: causality or association?
Circulatory failure and the need for cardiovascular support using inotropic/vasopressor medications have been associated with impaired cerebral pressure-flow autoregulation. Due to its alpha-, beta-adrenergic and dopaminergic activity, dopamine has long been used as a vasopressor-inotrope to support circulation and maintain adequate perfusion of critical organs, such as the brain.133 Dopamine’s impact on cerebral pressure-flow autoregulation, secondary to its effects on vascular tone or inotropism, remains inconclusive. Several NIRS studies reported that, in hypotensive preterm infants treated with dopamine, both CBF and ABP increase together,134,135,136,137 indicating that cerebral pressure-passive circulation or a small positive slope of the autoregulatory plateau92,134 may persist over a range of ABP. Moreover, time periods with impaired pressure-flow autoregulation in hypotensive infants were reported to increase with dopamine treatment in a dose-dependent fashion.138,139,140 However, the methodology and design of these studies could not address whether dopamine directly impaired the autoregulatory capacity or was merely an indicator of illness. In contrast, when dopamine effects were investigated in newborn piglets with induced hypotension, an improvement of cerebral pressure-flow autoregulation at low ABP, proportional to dopamine dose, was observed.141 Recent data from the HIP trial, where hypotensive preterm neonates were randomly assigned to either dopamine or placebo infusion,142 reported a significantly impaired pressure-flow autoregulatory capacity in hypotensive compared to normotensive infants, but not in relation to dopamine treatment.111 This, however, was not an adequately powered study; therefore, no firm conclusions can be drawn. Although currently available evidence suggests that autoregulatory capacity may be impaired by hypotension and its underlying causes rather than by dopamine treatment, larger targeted studies are needed to validate these findings and to define the complex relationship existing between these factors. In addition, data on the impact of other inotropic and vasoactive medications on pressure-flow autoregulation in preterm infants remain very limited and scarce.
Discussion
Current evidence on neonatal CR is based on many observational studies which, over the years, have pointed towards physiological associations between altered CR and an increased risk of brain injury. To move forward towards a preventive and therapeutic approach in neonatal CR research, multiple aspects need to be considered.
First, most of the available neonatal literature is derived from single-centred studies, based on small and heterogeneous cohorts which are under-powered and potentially involve biases and confounders (e.g., different types of brain injury, lack of PaCO2 data, use of different monitoring windows etc.), hindering comparison between studies to quantify the independent impact of CR impairment.
Second, an agreement on the multiple methods proposed for CR estimation is needed. The heterogeneity in the monitoring devices, recording methods, pre-processing steps and mathematical models applied for CR assessment hampers the identification of possible gold standard methodology that shows the best sensitivity and specificity for outcome prediction. The comprehensive integration of different multimodality monitoring signals, as well as the adoption of the Findable, Accessible, Interoperable, and Reusable approach143 for an open database with high-resolution data on cerebral rStO2, ABP, HR and standardised outcomes would facilitate the comparison and evaluation of their performances in predicting outcome.
Third, if an acceptable and reliable methodology for CR assessment is agreed upon, continuous real-time monitoring of CR may allow a personalised approach to neonatal intensive care, aimed at optimising CR and reducing neonatal brain injury. Continuous CR assessment combining rStO2, ABP and tCO2 monitoring should ideally be measured in at-risk neonates. However, such a comprehensive and multi-modal monitoring may be technically challenging (e.g., skin frailty, signal noise, availability of intra-arterial catheter), especially in extremely preterm neonates. In this regard, the validation of CR measurements using less invasive parameters (e.g., HR) as input signals for outcome prediction would facilitate CR monitoring and application in neonatal settings. Further improvements in the design of NIRS probes to suit the fragile skin of extremely preterm infants may also support long-term CR monitoring.
Some of the above aspects have been tackled by adult neurointensive care research groups, especially following traumatic brain injury (TBI), sepsis and stroke.144,145,146 In this setting, a Delphi consensus stating that CR status is uniquely dependent on an individual patient at any specific time, excluding the use of universal and absolute thresholds for CPP, was formalised, and a research agenda was proposed to establish and validate CR assessment methods against outcome, together with prospective safety, feasibility and efficacy studies to investigate the application of CR-guided clinical management.147 Of note, a study in adults with TBI, investigating the feasibility of automated assessment of optimal CPP based on individualised CR monitoring, is currently recruiting.148,149,150
A consensus approach similar to the abovementioned one may help to establish a future research agenda including collaborative clinical NIRS-based trials, targeted to address the current questions on neonatal CR listed in Table 2. These feasibility trials may represent the first step towards a randomised controlled trial based on continuous real-time CR monitoring, using dedicated software and aimed to assess whether a proposed treatment strategy (i.e., actively maintaining ABP within an optimal CR range) may improve neurological and neurodevelopmental outcomes. The goals are to identify infants with CR impairment predictive of brain injury, followed by interventional trials to prevent or correct the CR impairment for neonatal neuroprotection. Indeed, the SafeBoosC trials have pioneered the multi-centred approach in assessing the benefit of clinical interventions to optimise cerebral rStO2 in preterm neonates.151,152,153,154,155 In this regard, the formation of an international, multicentre working group to coordinate clinical trials and collated data management would support achieving these research targets.
References
Frösen, J. & Joutel, A. Smooth muscle cells of intracranial vessels: from development to disease. Cardiovasc. Res. 114, 501–512 (2018).
Kooi, E. M. W. & Richter, A. E. Cerebral autoregulation in sick infants: current insights. Clin. Perinatol. 47, 449–467 (2020).
Tan, C. O. Defining the characteristic relationship between arterial pressure and cerebral flow. J. Appl. Physiol. (1985) 113, 1194–1200 (2012).
Willie, C. K., Tzeng, Y. C., Fisher, J. A. & Ainslie, P. N. Integrative regulation of human brain blood flow. J. Physiol. 592, 841–859 (2014).
Pierce, W. J. & Harder, D. R. In Neurophysiological Basis of Cerebral Blood Flow Control an Introduction (eds Mraovitch, S. & Sercombe, R.) 153–158 (John Libbey, 1996).
Lindauer, U., Vogt, J., Schuh-Hofer, S., Dreier, J. P. & Dirnagl, U. Cerebrovascular vasodilation to extraluminal acidosis occurs via combined activation of ATP-sensitive and Ca2+-activated potassium channels. J. Cereb. Blood Flow. Metab. 23, 1227–1238 (2003).
Vutskits, L. Cerebral blood flow in the neonate. Paediatr. Anaesth. 24, 22–29 (2014).
Kaiser, J. R., Gauss, C. H. & Williams, D. K. The effects of hypercapnia on cerebral autoregulation in ventilated very low birth weight infants. Pediatr. Res. 58, 931–935 (2005).
Pryds, O., Greisen, G. & Friis-Hansen, B. Compensatory increase of CBF in preterm infants during hypoglycaemia. Acta Paediatr. Scand. 77, 632–637 (1988).
Skov, L. & Pryds, O. Capillary recruitment for preservation of cerebral glucose influx in hypoglycemic, preterm newborns: evidence for a glucose sensor? Pediatrics 90, 193–195 (1992).
Mattersberger, C., Schmölzer, G. M., Urlesberger, B. & Pichler, G. Blood glucose and lactate levels and cerebral oxygenation in preterm and term neonates-a systematic qualitative review of the literature. Front. Pediatr. 8, 361 (2020).
von Siebenthal, K. et al. Variability of cerebral hemoglobin concentration in very preterm infants during the first 6 h of life. Adv. Exp. Med. Biol. 566, 91–97 (2005).
Zhang, G., Cai, S. & Li, J. Hyperglycaemia is negatively associated with systemic and cerebral oxygen transport in neonates after the norwood procedure. Cardiol. Young. 22, 49–56 (2012).
Matterberger, C. et al. Blood glucose and cerebral tissue oxygenation immediately after birth – an observational study. J. Pediatr. 200, 19–23 (2018).
Goadsby, P. J. Autonomic nervous system control of the cerebral circulation. Handb. Clin. Neurol. 117, 193–201 (2013).
Pryds, O. Control of cerebral circulation in the high-risk neonate. Ann. Neurol. 30, 321–329 (1991).
Toda, N., Shimizu, I., Okamura, T. & Miyazaki, M. Age-dependent change in the response of isolated beagle cerebral arteries to vasoactive agents. J. Cardiovasc. Pharm. 8, 681–688 (1986).
Bevan, R. et al. Responsiveness of human infant cerebral arteries to sympathetic nerve stimulation and vasoactive agents. Pediatr. Res. 44, 730–739 (1998).
Hendrikx, D. et al. Measurement of neurovascular coupling in neonates. Front. Physiol. 10, 65 (2019).
Sakatani, K., Chen, S., Lichty, W., Zuo, H. & Wang, Y. P. Cerebral blood oxygenation changes induced by auditory stimulation in newborn infants measured by near infrared spectroscopy. Early Hum. Dev. 55, 229–236 (1999).
Kusaka, T. et al. Noninvasive optical imaging in the visual cortex in young infants. Hum. Brain Mapp. 22, 122–132 (2004).
Meek, J. H. et al. Regional hemodynamic responses to visual stimulation in awake infants. Pediatr. Res. 43, 840–843 (1998).
Karen, T. et al. Hemodynamic response to visual stimulation in newborn infants using functional near-infrared spectroscopy. Hum. Brain Mapp. 29, 453–460 (2008).
Zaramella, P. et al. Brain auditory activation measured by near-infrared spectroscopy (NIRS) in neonates. Pediatr. Res. 49, 213–219 (2001).
Kozberg, M. & Hillman, E. Neurovascular coupling and energy metabolism in the developing brain. Prog. Brain Res. 225, 213–242 (2016).
Inocencio, I. M. et al. Cerebral haemodynamic response to somatosensory stimulation in preterm lambs and 7-10-day old lambs born at term: direct synchrotron microangiography assessment. J. Cereb. Blood Flow. Metab. 42, 315–328 (2022).
Fantini, S., Sassaroli, A., Tgavalekos, K. T. & Kornbluth, J. Cerebral blood flow and autoregulation: current measurement techniques and prospects for noninvasive optical methods. Neurophotonics 3, 031411 (2016).
Weindling, A. M. & Kissack, C. M. Blood pressure and tissue oxygenation in the newborn baby at risk of brain damage. Biol. Neonate 79, 241–245 (2001).
Greisen, G. Autoregulation of cerebral blood flow in newborn babies. Early Hum. Dev. 81, 423–428 (2005).
Bellapart, J. & Fraser, J. F. Transcranial Doppler assessment of cerebral autoregulation. Ultrasound Med. Biol. 35, 883–893 (2009).
Boylan, G. B., Young, K., Panerai, R. B., Rennie, J. M. & Evans, D. H. Dynamic cerebral autoregulation in sick newborn infants. Pediatr. Res. 48, 12–17 (2000).
Boylan, G. B. et al. Cerebral blood flow velocity during neonatal seizures. Arch. Dis. Child Fetal Neonatal Ed. 80, F105–F110 (1999).
Panerai, R. B., Kelsall, A. W., Rennie, J. M. & Evans, D. H. Cerebral autoregulation dynamics in premature newborns. Stroke 26, 74–80 (1995).
Pellicer, A. & Bravo, M. C. Near-infrared spectroscopy: a methodology-focused review. Semin. Fetal Neonatal Med. 16, 42–49 (2011).
Wyatt, J. S. et al. Quantitation of cerebral blood volume in human infants by near-infrared spectroscopy. J. Appl. Physiol. (1985) 68, 1086–1091 (1990).
Lee, J. K. et al. Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke 40, 1820–1826 (2009).
Pryds, O., Greisen, G., Skov, L. L. & Friis-Hansen, B. Carbon dioxide-related changes in cerebral blood volume and cerebral blood flow in mechanically ventilated preterm neonates: comparison of near infrared spectrophotometry and 133Xenon clearance. Pediatr. Res. 27, 445–449 (1990).
Brew, N., Walker, D. & Wong, F. Y. Cerebral vascular regulation and brain injury in preterm infants. Am. J. Physiol. Regul. Integr. Comp. Physiol. 306, R773–R786 (2014).
Zhang, R., Zuckerman, J. H., Giller, C. A. & Levine, B. D. Transfer function analysis of dynamic cerebral autoregulation in humans. Am. J. Physiol. 274, H233–H241 (1998).
Claassen, J. A., Meel-van den Abeelen, A. S., Simpson, D. M. & Panerai, R. B., International Cerebral Autoregulation Research Network (CARNet). Transfer function analysis of dynamic cerebral autoregulation: a white paper from the International Cerebral Autoregulation Research Network. J. Cereb. Blood Flow. Metab. 36, 665–680 (2016).
Diehl, R. R., Linden, D., Lücke, D. & Berlit, P. Spontaneous blood pressure oscillations and cerebral autoregulation. Clin. Auton. Res. 8, 7–12 (1998).
Schat, T. E. et al. Assessing cerebrovascular autoregulation in infants with necrotizing enterocolitis using near-infrared spectroscopy. Pediatr. Res. 79, 76–80 (2016).
Kooi, E. M. W. et al. Cerebrovascular autoregulation in preterm infants during and after surgical ligation of the ductus arteriosus, a comparison between two surgical approaches. Front. Pediatr. 8, 334 (2020).
Brady, K. M. et al. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke 38, 2818–2825 (2007).
Wong, F. Y. et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics 121, e604–e611 (2008).
JS, S. et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr. Res. 61, 467–473 (2007).
da Costa, C. S. et al. Monitoring of cerebrovascular reactivity for determination of optimal blood pressure in preterm infants. J. Pediatr. 167, 86–91 (2015).
da Costa, C. S., Czosnyka, M., Smielewski, P. & Austin, T. Optimal mean arterial blood pressure in extremely preterm infants within the first 24 h of life. J. Pediatr. 203, 242–248 (2018).
Burton, V. J. et al. A pilot cohort study of cerebral autoregulation and 2-year neurodevelopmental outcomes in neonates with hypoxic-ischemic encephalopathy who received therapeutic hypothermia. BMC Neurol. 15, 209 (2015).
Lee, J. K. et al. Optimizing cerebral autoregulation may decrease neonatal regional hypoxic-ischemic brain injury. Dev. Neurosci. 39, 248–256 (2017).
Carrasco, M. et al. Cerebral autoregulation and conventional and diffusion tensor imaging magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy. Pediatr. Neurol. 82, 36–43 (2018).
Tekes, A. et al. Apparent diffusion coefficient scalars correlate with near-infrared spectroscopy markers of cerebrovascular autoregulation in neonates cooled for perinatal hypoxic-ischemic injury. AJNR Am. J. Neuroradiol. 36, 188–193 (2015).
Hermansen, M. C. & Hermansen, M. G. Intravascular catheter complications in the neonatal intensive care unit. Clin. Perinatol. 32, 141–156 (2005).
Fyfe, K. L. et al. Gestational age at birth affects maturation of baroreflex control. J. Pediatr. 166, 559–565 (2015).
Fyfe, K. L. et al. Preterm infants exhibit greater variability in cerebrovascular control than term infants. Sleep 38, 1411–1421 (2015).
Andriessen, P. et al. Feasibility of noninvasive continuous finger arterial blood pressure measurements in very young children, aged 0-4 years. Pediatr. Res. 63, 691–696 (2008).
Ricci, Z. et al. Arterial pressure monitoring in pediatric patients undergoing cardiac surgery: an observational study comparing invasive and non-invasive measurements. Pediatr. Cardiol. 40, 1231–1237 (2019).
Baik, N. et al. Blood pressure during the immediate neonatal transition: is the mean arterial blood pressure relevant for the cerebral regional oxygenation? Neonatology 112, 97–102 (2017).
Mitra, S. et al. Heart rate passivity of cerebral tissue oxygenation is associated with predictors of poor outcome in preterm infants. Acta Paediatr. 103, e374–e382 (2014).
Cimatti, A. G. et al. Cerebral oxygenation and autoregulation in very preterm infants developing ivh during the transitional period: a pilot study. Front. Pediatr. 8, 381 (2020).
Martini, S. et al. Clinical determinants of cerebrovascular reactivity in very preterm infants during the transitional period. Pediatr. Res. 92, 135–141 (2022).
Baik-Schneditz, N. et al. Cardiac output and cerebral oxygenation in term neonates during neonatal transition. Children (Basel) 8, 439 (2021).
Wu, T. W., Lien, R. I., Seri, I. & Noori, S. Changes in cardiac output and cerebral oxygenation during prone and supine sleep positioning in healthy term infants. Arch. Dis. Child Fetal Neonatal Ed. 102, F483–F489 (2017).
Lampe, R., Botkin, N., Turova, V., Blumenstein, T. & Alves-Pinto, A. Mathematical modelling of cerebral blood circulation and cerebral autoregulation: towards preventing intracranial hemorrhages in preterm newborns. Comput Math. Methods Med. 2014, 965275 (2014).
Dietz, V. et al. CO2 reactivity of the cerebral hemoglobin concentration in healthy term newborns measured by near infrared spectrophotometry. Biol. Neonate 75, 85–90 (1999).
Aly, S. et al. Factors affecting cerebrovascular reactivity to CO2 in premature infants. J. Perinat. Med. 47, 979–985 (2019).
Dix, L. M. L. et al. Carbon dioxide fluctuations are associated with changes in cerebral oxygenation and electrical activity in infants born preterm. J. Pediatr. 187, 66–72.e61 (2017).
Hoffman, S. B., Lakhani, A. & Viscardi, R. M. The association between carbon dioxide, cerebral blood flow, and autoregulation in the premature infant. J. Perinatol. 41, 324–329 (2021).
Wolfsberger, C. H. et al. Impact of carbon dioxide on cerebral oxygenation and vital parameters in stable preterm and term infants immediately after birth. Neonatology 119, 10–17 (2022).
Roche-Labarbe, N., Wallois, F., Ponchel, E., Kongolo, G. & Grebe, R. Coupled oxygenation oscillation measured by NIRS and intermittent cerebral activation on EEG in premature infants. Neuroimage 36, 718–727 (2007).
Tataranno, M. L. et al. Early oxygen-utilization and brain activity in preterm infants. PLoS One 10, e0124623 (2015).
Silas, R., Sehgal, A., Walker, A. M. & Wong, F. Y. Cerebral oxygenation during subclinical seizures in neonatal hypoxic-ischaemic encephalopathy. Eur. J. Paediatr. Neurol. 16, 304–307 (2012).
Roche-Labarbe, N. et al. NIRS-measured oxy- and deoxyhemoglobin changes associated with EEG spike-and-wave discharges in children. Epilepsia 49, 1871–1880 (2008).
Martini, S., Paoletti, V., Faldella, G. & Corvaglia, L. Cerebral oxygenation patterns during electroclinical neonatal seizures. Neuropediatrics 50, 408–409 (2019).
Hahn, G. H., Christensen, K. B., Leung, T. S. & Greisen, G. Precision of coherence analysis to detect cerebral autoregulation by near-infrared spectroscopy in preterm infants. J. Biomed. Opt. 15, 037002 (2010).
Riera, J., Cabañas, F., Serrano, J. J., Madero, R. & Pellicer, A. New developments in cerebral blood flow autoregulation analysis in preterm infants: a mechanistic approach. Pediatr. Res. 79, 460–465 (2016).
Wong, F. Y., Silas, R., Hew, S., Samarasinghe, T. & Walker, A. M. Cerebral oxygenation is highly sensitive to blood pressure variability in sick preterm infants. PLoS One 7, e43165 (2012).
Inocencio, I. M. et al. The cerebral haemodynamic response to somatosensory stimulation in preterm newborn lambs is reduced with dopamine or dobutamine infusion. Exp. Neurol. 341, 113687 (2021).
Thewissen, L. et al. Measuring near-infrared spectroscopy derived cerebral autoregulation in neonates: from research tool toward bedside multimodal monitoring. Front. Pediatr. 6, 117 (2018).
Kooi, E. M. W. et al. Measuring cerebrovascular autoregulation in preterm infants using near-infrared spectroscopy: an overview of the literature. Expert Rev. Neurother. 17, 801–818 (2017).
Vesoulis, Z. A., Mintzer, J. P. & Chock, V. Y. Neonatal NIRS monitoring: recommendations for data capture and review of analytics. J. Perinatol. 41, 675–688 (2021).
Grubb, R. L., Raichle, M. E., Eichling, J. O. & Ter-Pogossian, M. M. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke 5, 630–639 (1974).
Alderliesten, T. et al. Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J. Pediatr. 162, 698–704.e692 (2013).
Hoffman, S. B., Cheng, Y. J., Magder, L. S., Shet, N. & Viscardi, R. M. Cerebral autoregulation in premature infants during the first 96 h of life and relationship to adverse outcomes. Arch. Dis. Child Fetal Neonatal Ed. 104, F473–F479 (2019).
Vesoulis, Z. A., Liao, S. M. & Mathur, A. M. Late failure of cerebral autoregulation in hypoxic-ischemic encephalopathy is associated with brain injury: a pilot study. Physiol. Meas. 39, 125004 (2018).
Lemmers, P. M., Toet, M., van Schelven, L. J. & van Bel, F. Cerebral oxygenation and cerebral oxygen extraction in the preterm infant: the impact of respiratory distress syndrome. Exp. Brain Res 173, 458–467 (2006).
Polavarapu, S. R., Fitzgerald, G. D., Contag, S. & Hoffman, S. B. Utility of prenatal doppler ultrasound to predict neonatal impaired cerebral autoregulation. J. Perinatol. 38, 474–481 (2018).
Richter, A. E., Scherjon, S. A., Dikkers, R., Bos, A. F. & Kooi, E. M. W. Antenatal magnesium sulfate and preeclampsia differentially affect neonatal cerebral oxygenation. Neonatology 117, 331–340 (2020).
MM, G. et al. Relationship between cerebrovascular dysautoregulation and arterial blood pressure in the premature infant. J. Perinatol. 31, 722–729 (2011).
Cohen, E. et al. Cerebrovascular autoregulation in preterm fetal growth restricted neonates. Arch. Dis. Child Fetal Neonatal Ed. 104, F467–F472 (2019).
Sortica da Costa, C. et al. Changes in hemodynamics, cerebral oxygenation and cerebrovascular reactivity during the early transitional circulation in preterm infants. Pediatr. Res. 86, 247–253 (2019).
Eriksen, V. R., Hahn, G. H. & Greisen, G. Dopamine therapy is associated with impaired cerebral autoregulation in preterm infants. Acta Paediatr. 103, 1221–1226 (2014).
Chock, V. Y. et al. Cerebral oxygenation and autoregulation in preterm infants (Early Nirs Study). J. Pediatr. 227, 94–100.e1 (2020).
Chavez-Valdez, R. et al. Sex-specific associations between cerebrovascular blood pressure autoregulation and cardiopulmonary injury in neonatal encephalopathy and therapeutic hypothermia. Pediatr. Res. 81, 759–766 (2017).
Howlett, J. A. et al. Cerebrovascular autoregulation and neurologic injury in neonatal hypoxic-ischemic encephalopathy. Pediatr. Res. 74, 525–535 (2013).
Eriksen, V. R., Hahn, G. H. & Greisen, G. Cerebral autoregulation in the preterm newborn using near-infrared spectroscopy: a comparison of time-domain and frequency-domain analyses. J. Biomed. Opt. 20, 037009 (2015).
Thewissen, L. et al. Cerebral autoregulation and activity after propofol for endotracheal intubation in preterm neonates. Pediatr. Res. 84, 719–725 (2018).
Riera, J. et al. New time-frequency method for cerebral autoregulation in newborns: predictive capacity for clinical outcomes. J. Pediatr. 165, 897–902.e891 (2014).
Hahn, G. H., Heiring, C., Pryds, O. & Greisen, G. Applicability of near-infrared spectroscopy to measure cerebral autoregulation noninvasively in neonates: a validation study in piglets. Pediatr. Res. 70, 166–170 (2011).
Vesoulis, Z. A., Liao, S. M., Trivedi, S. B., Ters, N. E. & Mathur, A. M. A novel method for assessing cerebral autoregulation in preterm infants using transfer function analysis. Pediatr. Res. 79, 453–459 (2016).
Panerai, R. B. Assessment of cerebral pressure autoregulation in humans–a review of measurement methods. Physiol. Meas. 19, 305–338 (1998).
Rowley, A. B. et al. Synchronization between arterial blood pressure and cerebral oxyhaemoglobin concentration investigated by wavelet cross-correlation. Physiol. Meas. 28, 161–173 (2007).
Arriaga-Redondo, M. et al. Lack of variability in cerebral oximetry tendency in infants with severe hypoxic-ischemic encephalopathy under hypothermia. Ther. Hypothermia Temp. Manag. 9, 243–250 (2019).
Jain, S. V. et al. Cerebral regional oxygen saturation trends in infants with hypoxic-ischemic encephalopathy. Early Hum. Dev. 113, 55–61 (2017).
Lemmers, P. M. et al. Cerebral oxygenation and brain activity after perinatal asphyxia: does hypothermia change their prognostic value? Pediatr. Res. 74, 180–185 (2013).
Nakamura, S. et al. Simultaneous measurement of cerebral hemoglobin oxygen saturation and blood volume in asphyxiated neonates by near-infrared time-resolved spectroscopy. Brain Dev. 37, 925–932 (2015).
Wintermark, P., Hansen, A., Warfield, S. K., Dukhovny, D. & Soul, J. S. Near-infrared spectroscopy versus magnetic resonance imaging to study brain perfusion in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neuroimage 85, 287–293 (2014).
Massaro, A. N. et al. Exploratory assessment of the relationship between hemoglobin volume phase index, magnetic resonance imaging, and functional outcome in neonates with hypoxic-ischemic encephalopathy. Neurocrit. Care 35, 121–129 (2021).
Das, Y. et al. Wavelet-based neurovascular coupling can predict brain abnormalities in neonatal encephalopathy. Neuroimage Clin. 32, 102856 (2021).
Massaro, A. N. et al. Impaired cerebral autoregulation and brain injury in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. J. Neurophysiol. 114, 818–824 (2015).
Thewissen, L. et al. Cerebral oxygen saturation and autoregulation during hypotension in extremely preterm infants. Pediatr. Res. 90, 373–380 (2021).
O’Leary, H. et al. Elevated cerebral pressure passivity is associated with prematurity-related intracranial hemorrhage. Pediatrics 124, 302–309 (2009).
Stammwitz, A., von Siebenthal, K., Bucher, H. U. & Wolf, M. Can the assessment of spontaneous oscillations by near infrared spectrophotometry predict neurological outcome of preterm infants? Adv. Exp. Med. Biol. 876, 521–531 (2016).
Mitra, S., Bale, G., Meek, J., Tachtsidis, I. & Robertson, N. J. Cerebral near infrared spectroscopy monitoring in term infants with hypoxic ischemic encephalopathy – a systematic review. Front Neurol. 11, 393 (2020).
Kleuskens, D. G. et al. Pathophysiology of cerebral hyperperfusion in term neonates with hypoxic-ischemic encephalopathy: a systematic review for future research. Front. Pediatr. 9, 631258 (2021).
Leon, R. L. et al. Cerebral blood flow monitoring in high-risk fetal and neonatal populations. Front. Pediatr. 9, 748345 (2021).
Sankaran, K. Hypoxic-ischemic encephalopathy: cerebrovascular carbon dioxide reactivity in neonates. Am. J. Perinatol. 1, 114–117 (1984).
Greisen, G. Effect of cerebral blood flow and cerebrovascular autoregulation on the distribution, type and extent of cerebral injury. Brain Pathol. 2, 223–228 (1992).
Pryds, O., Greisen, G., Lou, H. & Friis-Hansen, B. Vasoparalysis associated with brain damage in asphyxiated term infants. J. Pediatr. 117, 119–125 (1990).
Hama-Tomioka, K. et al. Roles of neuronal nitric oxide synthase, oxidative stress, and propofol in N-methyl-D-aspartate-induced dilatation of cerebral arterioles. Br. J. Anaesth. 108, 21–29 (2012).
Short, B. L., Walker, L. K. & Traystman, R. J. Impaired cerebral autoregulation in the newborn lamb during recovery from severe, prolonged hypoxia, combined with carotid artery and jugular vein ligation. Crit. Care Med. 22, 1262–1268 (1994).
Dehaes, M. et al. Cerebral oxygen metabolism in neonatal hypoxic ischemic encephalopathy during and after therapeutic hypothermia. J. Cereb. Blood Flow. Metab. 34, 87–94 (2014).
Ancora, G. et al. Early predictors of short-term neurodevelopmental outcome in asphyxiated cooled infants. A combined brain amplitude integrated electroencephalography and near infrared spectroscopy study. Brain Dev. 35, 26–31 (2013).
Tian, F., Tarumi, T., Liu, H., Zhang, R. & Chalak, L. Wavelet coherence analysis of dynamic cerebral autoregulation in neonatal hypoxic-ischemic encephalopathy. Neuroimage Clin. 11, 124–132 (2016).
Kluckow, M. & Evans, N. Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 82, F188–F194 (2000).
Volpe, J. J. Intraventricular hemorrhage in the premature infant–current concepts. Part II. Ann. Neurol. 25, 109–116 (1989).
Noori, S., McCoy, M., Anderson, M. P., Ramji, F. & Seri, I. Changes in cardiac function and cerebral blood flow in relation to peri/intraventricular hemorrhage in extremely preterm infants. J. Pediatr. 164, 264–270.e261–e263 (2014).
Meek, J. H., Tyszczuk, L., Elwell, C. E. & Wyatt, J. S. Low cerebral blood flow is a risk factor for severe intraventricular haemorrhage. Arch. Dis. Child Fetal Neonatal Ed. 81, F15–F18 (1999).
Caicedo, A. et al. Impaired cerebral autoregulation using near-infrared spectroscopy and its relation to clinical outcomes in premature infants. Adv. Exp. Med. Biol. 701, 233–239 (2011).
Beausoleil, T. P., Janaillac, M., Barrington, K. J., Lapointe, A. & Dehaes, M. Cerebral oxygen saturation and peripheral perfusion in the extremely premature infant with intraventricular and/or pulmonary haemorrhage early in life. Sci. Rep. 8, 6511 (2018).
Osborn, D. A., Evans, N. & Kluckow, M. Hemodynamic and antecedent risk factors of early and late periventricular/intraventricular hemorrhage in premature infants. Pediatrics 112, 33–39 (2003).
Pryds, O., Greisen, G., Lou, H. & Friis-Hansen, B. Heterogeneity of cerebral vasoreactivity in preterm infants supported by mechanical ventilation. J. Pediatr. 115, 638–645 (1989).
Noori, S. & Seri, I. Neonatal blood pressure support: the use of inotropes, lusitropes, and other vasopressor agents. Clin. Perinatol. 39, 221–238 (2012).
Munro, M. J., Walker, A. M. & Barfield, C. P. Hypotensive extremely low birth weight infants have reduced cerebral blood flow. Pediatrics 114, 1591–1596 (2004).
Seri, I., Rudas, G., Bors, Z., Kanyicska, B. & Tulassay, T. Effects of low-dose dopamine infusion on cardiovascular and renal functions, cerebral blood flow, and plasma catecholamine levels in sick preterm neonates. Pediatr. Res. 34, 742–749 (1993).
Jayasinghe, D., Gill, A. B. & Levene, M. I. CBF reactivity in hypotensive and normotensive preterm infants. Pediatr. Res. 54, 848–853 (2003).
Pellicer, A. et al. Cardiovascular support for low birth weight infants and cerebral hemodynamics: a randomized, blinded, clinical trial. Pediatrics 115, 1501–1512 (2005).
Alderliesten, T. et al. Hypotension in preterm neonates: low blood pressure alone does not affect neurodevelopmental outcome. J. Pediatr. 164, 986–991 (2014).
Chock, V. Y., Ramamoorthy, C. & Van Meurs, K. P. Cerebral autoregulation in neonates with a hemodynamically significant patent ductus arteriosus. J. Pediatr. 160, 936–942 (2012).
Solanki, N. S. & Hoffman, S. B. Association between dopamine and cerebral autoregulation in preterm neonates. Pediatr. Res. 88, 618–622 (2020).
Eriksen, V. R., Rasmussen, M. B., Hahn, G. H. & Greisen, G. Dopamine therapy does not affect cerebral autoregulation during hypotension in newborn piglets. PLoS One 12, e0170738 (2017).
Dempsey, E. M. et al. Hypotension in preterm infants (HIP) randomised trial. Arch. Dis. Child Fetal Neonatal Ed. 106, 398–403 (2021).
Fair Principles – Go Fair; https://www.go-fair.org/fair-principles (accessed February 9, 2023).
Beishon, L. C. & Minhas, J. S. Cerebral autoregulation and neurovascular coupling in acute and chronic stroke. Front. Neurol. 12, 720770 (2021).
Rosenblatt, K. et al. Cerebral autoregulation-guided optimal blood pressure in sepsis-associated encephalopathy: a case series. J. Intensive Care Med. 35, 1453–1464 (2020).
Zeiler, F. A. et al. Critical thresholds of intracranial pressure-derived continuous cerebrovascular reactivity indices for outcome prediction in noncraniectomized patients with traumatic brain injury. J. Neurotrauma 35, 1107–1115 (2018).
Depreitere, B. et al. Cerebrovascular autoregulation monitoring in the management of adult severe traumatic brain injury: a Delphi consensus of clinicians. Neurocrit Care 34, 731–738 (2021).
Beqiri, E. et al. Feasibility of individualised severe traumatic brain injury management using an automated assessment of optimal cerebral perfusion pressure: The Cogitate Phase II Study Protocol. BMJ Open 9, e030727 (2019).
Tas, J. et al. Targeting autoregulation-guided cerebral perfusion pressure after traumatic brain injury (Cogitate): a feasibility randomized controlled clinical trial. J. Neurotrauma 38, 2790–2800 (2021).
Tas, J. et al. An update on the cogitate phase II study: feasibility and safety of targeting an optimal cerebral perfusion pressure as a patient-tailored therapy in severe traumatic brain injury. Acta Neurochir. Suppl. 131, 143–147 (2021).
Plomgaard, A. M. et al. Early cerebral hypoxia in extremely preterm infants and neurodevelopmental impairment at 2 year of age: a post hoc analysis of the Safeboosc II Trial. PLoS One 17, e0262640 (2022).
Hansen, M. L. et al. Cerebral near-infrared spectroscopy monitoring versus treatment as usual for extremely preterm infants: a protocol for the Safeboosc Randomised Clinical Phase III Trial. Trials 20, 811 (2019).
Plomgaard, A. M. et al. No neurodevelopmental benefit of cerebral oximetry in the first randomised trial (Safeboosc II) in preterm infants during the first days of life. Acta Paediatr. 108, 275–281 (2019).
Plomgaard, A. M. et al. Early biomarkers of brain injury and cerebral hypo- and hyperoxia in the Safeboosc II Trial. PLoS One 12, e0173440 (2017).
Riera, J. et al. The Safeboosc Phase II Clinical Trial: an analysis of the interventions related with the oximeter readings. Arch. Dis. Child Fetal Neonatal Ed. 101, F333–F338 (2016).
TE, S. et al. Assessing cerebrovascular autoregulation in infants with necrotizing enterocolitis using near-infrared spectroscopy. Pediatr. Res. 79, 76–80 (2016).
Tsuji, M. et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics 106, 625–632 (2000).
Scholkmann, F., Spichtig, S., Muehlemann, T. & Wolf, M. How to detect and reduce movement artifacts in near-infrared imaging using moving standard deviation and spline interpolation. Physiol. Meas. 31, 649–662 (2010).
Ayaz, H., Izzetoglu, M., Shewokis, P. A. & Onaral, B. Sliding-window motion artifact rejection for functional near-infrared spectroscopy. Annu. Int Conf. IEEE Eng. Med. Biol. Soc. 2010, 6567–6570 (2010).
Zhang, Y. et al. Spectral analysis of systemic and cerebral cardiovascular variabilities in preterm infants: relationship with Clinical Risk Index for Babies (CRIB). Physiol. Meas. 32, 1913–1928 (2011).
Hahn, G. H. et al. Cerebral autoregulation in the first day after preterm birth: no evidence of association with systemic inflammation. Pediatr. Res. 71, 253–260 (2012).
De Smet, D., Vanderhaegen, J., Naulaers, G. & Van Huffel, S. New measurements for assessment of impaired cerebral autoregulation using near-infrared spectroscopy. Adv. Exp. Med. Biol. 645, 273–278 (2009).
Votava-Smith, J. K. et al. Impaired cerebral autoregulation in preoperative newborn infants with congenital heart disease. J. Thorac. Cardiovasc Surg. 154, 1038–1044 (2017).
Papademetriou, M. D., Tachtsidis, I., Elliot, M. J., Hoskote, A. & Elwell, C. E. Multichannel near infrared spectroscopy indicates regional variations in cerebral autoregulation in infants supported on extracorporeal membrane oxygenation. J. Biomed. Opt. 17, 067008 (2012).
Caicedo, A. et al. Differences in the cerebral hemodynamics regulation mechanisms of premature infants with intra-ventricular hemorrhage assessed by means of phase rectified signal averaging. Annu. Int Conf. IEEE Eng. Med. Biol. Soc. 2014, 4208–4211 (2014).
Giovannella, M. et al. Validation of diffuse correlation spectroscopy against. J. Cereb. Blood Flow. Metab. 40, 2055–2065 (2020).
Roche-Labarbe, N. et al. Noninvasive optical measures of CBV, StO(2), CBF Index, and rCMRO(2) in human premature neonates’ brains in the first six weeks of life. Hum. Brain Mapp. 31, 341–352 (2010).
Durduran, T. et al. Optical measurement of cerebral hemodynamics and oxygen metabolism in neonates with congenital heart defects. J. Biomed. Opt. 15, 037004 (2010).
Kovacsova, Z. et al. Investigation of confounding factors in measuring tissue saturation with NIRS spatially resolved spectroscopy. Adv. Exp. Med. Biol. 1072, 307–312 (2018).
Bale, G. et al. Near-infrared spectroscopy measured cerebral blood flow from spontaneous oxygenation changes in neonatal brain injury. Adv. Exp. Med. Biol. 1232, 3–9 (2020).
Kleiser, S. et al. In vivo precision assessment of a near-infrared spectroscopy-based tissue oximeter (Oxyprem V1.3) in neonates considering systemic hemodynamic fluctuations. J. Biomed. Opt. 23, 1–10 (2018).
Martini, S. & Corvaglia, L. Splanchnic NIRS monitoring in neonatal care: rationale, current applications and future perspectives. J. Perinatol. 38, 431–443 (2018).
Funding
Financial support of publication costs by the European Society for Paediatric Research (ESPR) is gratefully acknowledged.
Author information
Authors and Affiliations
Consortia
Contributions
All members of the European Special Interest Group “Near InfraRed Spectroscopy” are listed below. All these members have substantially contributed to the conception and revision of the manuscript and approved the final version to be published. S.M. and L.T. wrote the first manuscript draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martini, S., Thewissen, L., Austin, T. et al. Near-infrared spectroscopy monitoring of neonatal cerebrovascular reactivity: where are we now?. Pediatr Res (2023). https://doi.org/10.1038/s41390-023-02574-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-023-02574-6
- Springer Nature America, Inc.
This article is cited by
-
Cardiovascular and cerebrovascular effects of caffeine maintenance in preterm infants during the transitional period
Pediatric Research (2024)
-
Blood pressure and cerebral oxygenation with physiologically-based cord clamping: sub-study of the BabyDUCC trial
Pediatric Research (2024)
-
Neuromonitoring in neonatal intensive care units—an important need towards individualized neuroprotective care
European Journal of Pediatrics (2024)