Abstract
The receptor tyrosine kinase Ret, a key gain-of-function mutated oncoprotein in thyroid carcinomas, has recently been implicated in other cancer types. While Ret copy number gains and mutations have been reported at low frequencies in breast tumors, we and others have reported that Ret is overexpressed in about 40% of human tumors and this correlates with poor patient prognosis. Ret activation regulates numerous intracellular pathways related to proliferation and inflammation, but it is not known whether abnormal Ret expression is sufficient to induce mammary carcinomas. Using a novel doxycycline-inducible transgenic mouse model with the MMTV promoter controlling Ret expression, we show that overexpression of wild-type Ret in the mammary epithelium produces mammary tumors, displaying a morphology that recapitulates characteristics of human luminal breast tumors. Ret-evoked tumors are estrogen receptor positive and negative for progesterone receptor. Moreover, tumors rapidly regress after doxycycline withdrawal, indicating that Ret is the driving oncoprotein. Using next-generation sequencing, we examined the levels of transcripts in these tumors, confirming a luminal signature. Ret-evoked tumors have been passaged in mice and used to test novel therapeutic approaches. Importantly, we have determined that tumors are resistant to endocrine therapy, but respond successfully to treatment with a Ret kinase inhibitor. Our data provide the first compelling evidence for an oncogenic role of non-mutated Ret in the mammary gland and are an incentive for clinical development of Ret as a cancer biomarker and therapeutic target.
Similar content being viewed by others
Introduction
In breast cancer, the oncogenic potential of several receptor tyrosine kinases (RTK), of e.g., the ErbB and FGFR families, has been studied for many years [1]. The Rearranged during transfection (Ret) RTK is a recent player in this disease. The first oncogenic Ret mutations were rearrangements identified in thyroid cancer [2]. Although Ret fusion proteins [3, 4] and point mutations [5] appear to be infrequent in breast cancer, we and others have reported that Ret RNA and protein levels are high in around 40% of primary tumors [6,7,8] and we have shown that high Ret correlates with decreased overall survival [7]. Elevated Ret has been found in different breast cancer subtypes, including estrogen receptor positive (ER+) and Her2/ErbB2 overexpressing tumors [7]. A higher percentage of Ret-positive tumors was found in breast tumors of patients who failed endocrine therapy [9], suggesting a role for Ret in resistance. Using an ER+ orthotropic Ret-dependent model, we found that Ret inhibition reduced tumor outgrowth and lung metastatic potential [7]. These studies provided the incentive to further explore the role of Ret in breast cancer and test its potential as a therapeutic target [10].
Here we present our studies on Ret using a bitransgenic mouse strain with a mammary gland-specific promoter controlling Ret expression in a doxycycline-inducible system [11, 12]. We show that chronic expression of wild-type (WT) Ret leads to the development of ER+ mammary tumors, with features of the human luminal subtype.
Results and discussion
To test whether WT Ret is tumorigenic, we created a novel transgenic mouse (Fig. 1a, top) using the doxycycline regulatory system [11]. Tissue-specific transgene expression was determined by measuring luciferase activity and only animals on doxycycline were positive (Fig. 1a, middle). Western blot (WB) analysis for Ret was performed on mammary gland lysates from Ret/MTB, MTB/− and Ret/− adult female mice following 1 week of doxycycline treatment. Ret was induced in Ret/MTB, but not in uninduced Ret/MTB-transgenics, or doxycycline treated Ret/− or MTB/− controls (Fig. 1a, bottom). No abnormalities were found in 1 week-induced Ret/MTB mammary glands (Supplementary Figure S1a).
Transgenic mouse model with MMTV‐driven human wild-type Ret expression under the control of a doxycycline‐inducible system. a We generated the pTetO-Ret expression vector by cloning the human Ret51 (hRet51, NM_020975) from MCF7 cells into pTAMILA-PS [11]. This vector also contains an IRES-firefly luciferase expression cassette that serves as a surrogate reporter. Founder lines were generated in FVB/N mice. Transgenic mice carrying the TetO-Ret construct (Ret/−) were mated with mouse mammary tumor virus-reverse tetracycline transactivator (MMTV-rtTA) mice (MTB/−) [11] to obtain bitransgenics, designated as Ret/MTB. Transgenic and control mice were administered a control diet (Kliba Nafag #3302.PM.V20, Irradiation > 25 Gy) or one enriched with 200 mg/kg doxycycline in the chow (Sigma by Chow Manufactures). Mice were housed under hygienic conditions according to the Swiss guidelines governing animal experimentation. Ret/MTB adult female mice were monitored for luciferase activity after 1 week of doxycycline or control food (nf). Lysates from mammary tissue and MCF7 cells as a positive control were analyzed by WB. b Mammary glands were analyzed by WM, H&E- and IHC for pS10H3. Scale bars, 50 µm; 1.25 cm. Quantification of the indicated gland areas and pS10H3-staining are shown. p values by Mann–Whitney test. WB shows Ret levels, phospho-Ret and Ret-mediated signaling after 8 weeks of induction. c IHC for ER, CK8/18, CK14 and Ret were carried out on paraffin sections of mammary glands. Scale bars, 50 µm. The antibodies used, the protocols and the quantification, are described in the Supplementary Data. d Mice were monitored every 1–2 weeks for tumor formation. Luciferase activity in Ret/MTB females (A–F) monitored after 5 months of doxycycline-induction (D–F), tumors developed in 2 mice (E,F). WB shows Ret and phospho-Ret in independent tumors. *unspecific band in the pY1062Ret blot. e Quantification of mRNA levels of Ret transgene was performed by qRT-PCR. RNA was extracted using TRI Reagent (MRC), then 2 µg was reversed transcribed with Ready-to-go You-Prime First-Strand Beads (GE Healthcare) and 1–4 µl of cDNA was used for the PCR reactions. qRT-PCR was carried out using specific primers (Supplementary Table S1) with the StepOne Real-Time PCR System (Applied Biosystems) instrument and software. For each primer set, a calibration curve was done. Expression values were normalized to GAPDH values and represented as absolute values. Columns, means of the values ± s.e.m. from independent females. R range, T threshold
Next, we analyzed mammary glands from Ret/MTB adult females chronically treated 8 weeks with doxycycline. Morphological examination of whole mounts (WM) revealed hyperplastic changes (Fig. 1b, top) and WB confirmed Ret expression and activation (Fig. 1b, lower). Ret-positive glands were larger than controls, with excess side branching and secondary branch extensions, resulting in an increase in areas covered by ducts and surrounding fat (Fig. 1b, top right). Control mammary glands from doxycycline-treated MTB/− and Ret/− mice, and Ret/MTB animals maintained in its absence for > 1 year, were normal (not shown). IHC with anti-phospho (p)-S10-histone 3 (pS10H3) revealed that ducts of induced glands have an increase in pS10H3-positive cells, compared with controls (Fig. 1b, middle), indicating higher Ret-driven proliferation. Prior to hyperplasia, no significant changes were seen (Supplementary Figure S1b). An analysis of markers for specific cell compartments showed that Ret-induced hyperplasia is caused by the expansion of luminal ER+ and CK8/18+ cells, and not basal CK14+ cells (Fig. 1c).
Chronic expression of Ret in mammary epithelium induces solid tumors with characteristics of human luminal breast cancer
Chronic Ret induction resulted in tumor development in Ret/MTB females. Tumors expressed the transgene, shown by bioluminescence and Ret protein; phosphorylated Ret and Erk, both indicators of activity were also detected (Fig. 1d). Since the Ret ligands neurturin (Nrtn) and artemin (Artn), and the Ret co-receptor GFRα1 are expressed in transgenic glands and tumors, these likely contribute to Ret activation (Supplementary Table S1–2 and Supplementary Figure S1c).
Transgene levels were quantified by qRT-PCR for human Ret. Ret mRNA was present in doxycycline-induced hyperplasia and tumors, but not in uninduced tissue (Fig. 1e). Ret/MTB-tumors express higher levels of Ret compared to hyperplastic glands, suggesting that not all cells in hyperplasia express the transgene. To examine this, we performed Ret IHC. Ret/MTB tumors show homogeneously high Ret levels, while Ret expression in the surrounding hyperplastic tissue is heterogeneous (Supplementary Figure S1d), as was previously shown using this system [13]. These results suggest that chronic Ret expression is sufficient to form tumors.
Focal tumors arose stochastically in Ret/MTB females, averaging 1-2 per mouse. In virgin females, tumor incidence was 37.0% (n = 27) following 6.5 ± 1.6 months of induction. Glands of chronically doxycycline-treated MTB/−, Ret/− (n = 18), and untreated Ret/MTB-mice (n = 6), developed no tumors for > 1 year (Fig. 2a). We also compared tumor onset in nulliparous (V) and multiparous (M) Ret/MTB mice. In parous mice, tumors occurred with similar latency (6.7 ± 3.2 months), but with a higher incidence reaching 76.8% (n = 13), compared to virgins (Fig. 2a). Since the MMTV promotor contains binding sites for steroid hormone receptors that are activated during pregnancy, we consider it likely that increased transcriptional activity of MMTV-rTTA leads to increased Ret expression, which contributes to higher tumor incidence in multiparous females. In addition, the hormonal environment could impact on mammary epithelial cell fate and ensuing tumor development in the transgenics. In human breast tumors, higher levels of Ret are seen compared to normal human tissue [8].. The mechanism underlying this observation is not fully understood. Interestingly, it has been reported that the transcription factor TFAP2C drives Ret expression and controls ER expression as well as other luminal signature target genes [14]. Estrogen stimulation increases Ret expression in breast tumor cell lines [6, 15]; however, cells persistently treated with anti-estrogenic drugs also show increased Ret levels [7]. Thus, it is tempting to speculate that higher Ret levels in tumors might be related to a balance between the action of specific transcription factor and the lifetime hormonal milieu.
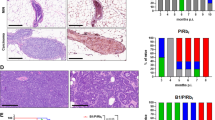
Histological features of Ret/MTB-evoked carcinomas. a Kaplan–Meier plots show tumor-free survival curves of control and Ret/MTB mice. M: multiparous, V: nulliparous, p value by Log-Rank test. b Left column, H&E-staining of 3 independent primary tumors (A–C). Right columns, IHC on primary tumors with the indicated antibodies. Scale bars, 50 µm. The MMTV-Neu tumor section is a positive control (ErbB2-Control). c Results of clustering the global gene expression of 6 Ret/MTB-tumors in the breast cancer subtypes. RNA from fragments of independent primary tumors was obtained using RNeasy Mini Kit (Qiagen). After quality control, mRNA samples were subject to NGS using Ilumina HiSeq 2500 Deep-sequencing (GSE83897). To predict tumor intrinsic subtypes, hierarchical clustering of Ret/MTB-tumors based on global RNA-seq profiles was performed using the PAM50 gene model and the correlation scores are indicated. d GSEA was carried out using publicly available sets. GSEA shows the correlation between indicators of proliferation (REACTOME_DNA_REPLICATION), and Stat1-signature (V$STAT1_01), and gene sets up- or downregulated in 6 tumors or 6 control glands (MG). NES normalized enrichment score; q: false discovery rate. p value was computed as implemented in GSEA. e IHC for pStats was performed on primary tumors. Scale bars, 50 µm
Histological analysis of Ret-induced mammary tumors revealed solid nodular adenocarcinomas (similar to Neu-initiated tumors [12]), with high vascularity and areas of hemorrhage. H&E-staining showed that tumors had similar morphologies, and recapitulated features of human luminal breast cancer (Fig. 2b, left) [16]. IHC analyses using anti-Ret, -ErbB2, -CK8/18, -CK14 and -αSMA antibodies showed that all tumors express Ret and ErbB2, and are of luminal epithelial origin (CK8/18+; CK14/SMA-). ErbB2 levels were similar to those in normal ducts and lower than MMTV-Neu tumor levels (ErbB2-Control), indicating no ErbB2 overexpression. Ret-induced tumors express low ER levels at the periphery of the tumor, and have no detectable progesterone receptor (PR) (Fig. 2b, right). Thus, we suggest that the Ret model resembles the specific sub-group of ER+/PR-/ErbB2- tumors. Importantly, while expression of the Ret/PTC fusion [2] generated mammary adenocarcinomas [17], our work is the first to demonstrate that WT Ret plays a causal role in mammary cancer.
We used next-generation RNA sequencing (NGS) to analyze the transcriptomes of 6 independent ER+Ret-induced tumors (T1, T11, T12, T15, T16 and T18) (Fig. 2c and Supplementary Figure S2), and control mammary glands from 3 uninduced Ret/MTB- and 3 chronically induced Ret/− females. A global gene expression analysis comparing Ret/MTB-tumors with breast cancer subgroups [18] showed that 4/6 tumors clustered with the luminal A subtype (Fig. 2c and Supplementary Table S3). We also performed correlations between signatures in Gene Set Enrichment Analysis (GSEA) using gene sets up- or downregulated by Ret overexpression. Proliferative genes were upregulated in tumors (Fig. 2d, top), and we identified enrichment of Stat1-driven genes (Fig. 2d, bottom and Supplementary Table S4); nuclear Stat1 and Stat3 phosphorylated forms were present in some tumor areas; active Stat5 was not detected (Fig. 2e). The transcription factors Stat1 and Stat3 appear to play opposite roles in tumorigenesis. While Stat3, described as oncogenic, promotes cell survival/proliferation, motility and immune tolerance [19], Stat1 is considered to be a tumor suppressor [20], which triggers anti-proliferative and pro-apoptotic responses while enhancing anti-tumor immunity [19]. Despite being activated downstream of common cytokine and growth factor receptors, perturbations in their balanced expression or phosphorylation levels may re-direct signals from proliferative to apoptotic, or from inflammatory to anti-inflammatory.
The intra-tumor heterogeneity in Stat1/3 activation suggests that local factors act as Stat activators. In the future, identification of these factors will be important to determine.
By examining TCGA data, we found that 81% (1556/1904) of human Ret+breast tumors express at least one of the therapeutic receptors (ER, PR, or ErbB2) (Supplementary Figure S3a), which is in line with our previous TMA analysis [7]. In patients with ER+tumors, PR negativity is an independent predictor of recurrence and shorter survival, hence of poorer response to hormonal therapy [21]. Thus, our model might be useful for studying therapeutic strategies targeting Ret.
Mammary carcinomas require Ret for maintenance
To examine whether established tumors require continued Ret expression for maintenance, we withdrew doxycycline from 8 tumor-bearing mice, each of which harbored one 150-400 mm3 tumor. All tumors rapidly regressed (Fig. 3a) and were undetectable after 72 h; bioluminescence and Ret-staining confirmed Ret suppression after 48 h (Fig. 3b). To gain mechanistic insight into Ret-driven tumorigenesis, we examined signaling pathway activity after 48 h of doxycycline withdrawal. In regressing tumors, IHC showed lower abundance of pT202/Y204Erk1/2 and pS240/244S6, an mTor effector that is a key component of Ret signaling [9]. Slightly lower levels of pY705Stat3 were also observed. Furthermore, we detected a significant reduction in pY701Stat1-positive nuclei and fewer pS10H3 positive proliferating cells in regressed tumors, compared to controls (Fig. 3b). Conversely, there was an increase in cleaved caspase 3 (CC3)-positive tumor cells after doxycycline removal (Fig. 3b). Thus, reduced proliferation and increased cell death, combined with signaling pathway downregulation, appear to be responsible for the initial phases of tumor regression following Ret downregulation.
Ret/MTB-evoked tumors are dependent on Ret expression for growth. a Mice were monitored for tumor formation. In some cases, doxycycline-food was withdrawn and tumors were monitored for regression. Representative growth curves of Ret/MTB primary tumors: 12° growing under doxycycline -food and 6° regressing after doxycycline withdrawal. Tumor volumes were determined according to the formula: length × diameter 2 × π/6. Tumor tissue was collected before or after doxycycline withdrawal and stored to perform subsequent analyses. b Luciferase activity measured in Ret/MTB mice. Representative IHC images and corresponding quantifications are shown for the indicated antibodies. Plots, median of the values ± s.d. from independent females. Scale bars, 50 µm. p value by Mann–Whitney test. c GSEA shows the correlation between indicators of a Stat1-signature (V$STAT1_01) and an mTor-signature (MTOR_UP.V1_UP) and gene sets regulated in Ret/MTB-tumors (TumorDox) after 48 h of doxycycline withdrawal (TumorDox-OUT). n = 3 each group. NES: normalized enrichment score; q: false discovery rate. p value was computed on the basis of comparison against random stimulation as implemented in GSEA. d Ret/MTB tumor fragments (1–2 mm in diameter) were engrafted in female Balb/c nude mice. Recipient animals were maintained under doxycycline-food starting 2 days before performing implants, to ensure continued tumor graft growth. Tumor growth was monitored. In the experimental group, doxycycline-food was withdrawn and tumors were monitored for regression. Growth curves of tumour grafts in Balb/c Nude mice growing under the indicated conditions. Tumor mass from 2 independent experiments is represented in a bar graph. p value by t test
We performed NGS on RNA from 48 h-regressing tumors and compared differential gene expression with tumors growing on doxycycline. Supporting the previous results, Ret downregulation was accompanied by downregulation of Stat1- and mTor-regulated genes and a decrease in DNA replication signature (Fig. 3c and Supplementary Figure S3b).
To analyze the impact of Ret inhibition on tumor growth, in a reproducible manner with larger cohorts, we engrafted pieces of Ret‐induced tumors onto flanks of Balb/c nude females. The grafts show less vascularity than primary tumors, importantly, however, they retain all other characteristics of the primary (Supplementary Figure S4). When tumors reached approximately 100–400 mm3, mice were subjected to different treatments. First, we determined that doxycycline-withdrawal produced tumor regression in 48 h (Fig. 3d), verifying their Ret-dependency. Second, we tested the in vivo response to an ErbB2 inhibitor and as anticipated from the low ErbB2 levels, no effects were found, confirming ErbB2 independence (Supplementary Figure S5a). Third, growth of tumor grafts was insensitive to hormone deprivation, mimicked by ovariectomy (Supplementary Figure S5b), and intact tumor-bearing mice did not respond to fulvestrant (Supplementary Figure S5c), confirming their estrogen-independence. Taken together, these data show that Ret/MTB tumors are functionally similar to human ER+/PR-/ErbB2- breast cancers.
Systemic inhibition of Ret signaling causes tumor reduction
We tested the role of Ret in signaling and tumor growth by treating mice bearing Ret-induced tumor transplants with NVP-AST487, a tool compound targeting Ret and additional kinases that we and others have successfully used [7, 22]. Of note, several commercially available multi-kinase inhibitors, such as vandetanib or cabozantinib, also have activity against the Ret kinase, however, selective Ret inhibitors have not yet been developed for clinical use. Several phase II clinical trials have been initiated to investigate the therapeutic effects of these multi-kinase inhibitors in patients with advanced Ret fusion-positive NSCLC [23]. We show here that treatment with NVP-AST487 significantly reduced tumor growth (Fig. 4a) and WB analysis showed that tumor grafts from these mice had decreased levels of phosphorylated-Ret, accompanied by modest lower abundance of active-Erk and -mTor (Fig. 4b), as well as a reduction in phospho-S6, –Stat3 and -Stat1 (Fig. 4c).
Treatment with a Ret kinase inhibitor reduces tumor growth. a One-2 mm fragments of Ret/MTB tumors were engrafted in female Balb/c nude mice (4–10 mice per group). Starting 2 days before implantation, recipient animals were started on doxycycline -food. When tumors reached ~ 100 mm3, mice were randomized by Stratified Sample randomization method into groups that received vehicle (N-methylpyrrolidone/PEG300; 1:10 v/v) or NVP-AST487 (50 mg/Kg per day, Novartis), as described [7]. A fresh solution of Ret inhibitor was prepared every other day and administered orally once daily. Tumor growth and body weight were monitored every 2–3 days. One of 3 experiments is shown. Dots represent the mean of tumor volume ± s.e.m. Tumor mass was determined at the end of the experiment. p value by Mann–Whitney test. b Lysates from tumors collected 1 and 3 h after the last treatment were analyzed by WB with the indicated antisera. On the right, quantification of additional western analyses with the indicated phospho-protein/protein was performed using imageJ in 4–9 independent tumors for group, from 2 independent experiments. Data shown are the mean ± s.e.m. p value by unpaired t test. c IHC for pS240/244S6, pY705Stat3 and pY701Stat1 on treated-tumors and quantification from 2 independent experiments. Scale bars, 50 µm. p value by Mann–Whitney test
We show here that WT Ret expression in the mammary epithelium potently evokes mammary tumors and, we end by summarizing and discussing the important conclusions. Our finding that Ret causes ER+tumors suggests that Ret activity expands ER+mammary cells, which is consistent with Ret expression in human ER+tumors [7,8,9]. Moreover, in our model, we show that these Ret/MTB-evoked tumors are PR- and do not respond to endocrine treatment. This is consistent with the observation that Ret expression in human tumors is associated with tamoxifen resistance [9, 24], which has been proposed to be due to Ret-driven mTor pathway activity [9]. Interestingly, we show that proliferation of Ret/MTB-evoked tumors is blocked by Ret-targeted therapy, which is accompanied by a modulation in mTor signaling, suggesting that our model could be useful for studying hormone resistance. In the clinic, treatment with mTor inhibitors reverts many but not all cases of established resistance [25] and new biomarkers to guide therapeutic decisions are being sought. Based on our results, Ret expression in ER+/PR-/ErbB2- breast tumors might be a relevant biomarker predicting hormone-resistance. In the future blocking Ret alone or in combination with an mTor inhibitor might be an alternative therapy.
References
Hynes NE, Watson CJ. Mammary gland growth factors: roles in normal development and in cancer. Cold Spring Harb Perspect Biol. 2010;2:a003186.
Grieco M, Santoro M, Berlingieri MT, Melilo RM, Donghi R, Bongarzone I, et al. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell. 1990;60:557–63.
Kohno T, Ichikawa H, Totoki Y, Yasuda K, Hiramoto M, Nammo T. et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med. 2012;18:375–7.
Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846.
Nik-Zainal S, Davies H, Staaf J, Ramakrishna M, Glodzik D, Zou X. et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature. 2016;534:47–54.
Boulay A, Breuleux M, Stephan C, Fux C, Brisken C, Fiche M, et al. The Ret receptor tyrosine kinase pathway functionally interacts with the ERalpha pathway in breast cancer. Cancer Res. 2008;68:3743–51.
Gattelli A, Nalvarte I, Boulay A, Roloff TC, Schreiber M, Carragher N, et al. Ret inhibition decreases growth and metastatic potential of estrogen receptor positive breast cancer cells. EMBO Mol Med. 2013;5:1335–50.
Esseghir S, Todd SK, Hunt T, Poulsom R, Plaza-Menacho I, Reis-Filho JS, et al. A role for glial cell derived neurotrophic factor induced expression by inflammatory cytokines and RET/GFR alpha 1 receptor up-regulation in breast cancer. Cancer Res. 2007;67:11732–41.
Plaza-Menacho I, Morandi A, Robertson D, Pancholi S, Drury S, Dowsett M, et al. Targeting the receptor tyrosine kinase RET sensitizes breast cancer cells to tamoxifen treatment and reveals a role for RET in endocrine resistance. Oncogene. 2010;29:4648–57.
Spanheimer PM, Park JM, Askeland RW, Kulak MV, Woodfield GW, De Andrade JP, et al. Inhibition of Ret increases the efficacy of antiestrogen and is a novel treatment strategy for luminal breast cancer. Clin Cancer Res. 2014;20:2115–25.
Gunther EJ, Belka GK, Wertheim GB, Wang J, Hartman JL, Boxer RB, et al. A novel doxycycline-inducible system for the transgenic analysis of mammary gland biology. FASEB J. 2002;16:283–92.
Moody SE, Sarkisian CJ, Hahn KT, Gunther EJ, Pickup S, Dugan KD, et al. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell. 2002;2:451–61.
Sarkisian JC, Keister BA, Stairs DB, Boxer RB, Moody SE, Chodosh LA. Dose-dependent oncogene-induced senescence in vivo and its evasion during mamary tumorigenesis. Nat Cell Biol. 2007;9:493–505.
Woodfield GW, Chen Y, Bair TB, Domann FE, Weigel J. Identification of primary gene targets of TFAP2C in hormone responsive breast carcinoma cells. Genes Chrom Cancer. 2010;49:948–62.
Morandi A, Martin LA, Gao Q, Pancholi S, Mackay A, Robertson D. et al. GDNF-Ret signalling in ER-positive breast cancers is a key determinant of response and resistance to aromatase inhibitors. Cancer Res. 2013;73:3783–95.
Cardiff RD, Wellings SR. The comparative pathology of human and mouse mammary glands. J Mammary Gland Biol Neoplasia. 1999;4:105–22.
Iwamoto T, Takahashi M, Ito M, Hamaguchi M, Isobe K, Misawa N, et al. Oncogenicity of the ret transforming gene in MMTV/ret transgenic mice. Oncogene. 1990;5:535–42.
Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–7.
Avalle L, Pensa S, Regis G, Novelli F, Poli V. Stat1 and Stat3 in tumorigenesis. A matter of balance. JAK-STAT. 2012;1:65–72.
Chan SR, Vermi W, Luo J, Lucini L, Rickert C, Fowler AM, et al. Stat1-deficient mice spontaneously develop estrogen receptor a-positive luminal mammary carcinomas. Brest Cancer Res. 2012;14:R16.
Rakha EA, El-Sayed ME, Green AR, Paish EC, Powe DG, Gee J, et al. Biologic and clinical characteristics of breast cancer with single hormone receptor positive phenotype. J Clin Oncol. 2007;25:4772–8.
Akeno-Stuart N, Croyle M, Knauf JA, Malaguarnera R, Vitagliano D, Santoro M, et al. The RET kinase inhibitor NVP-AST487 blocks growth and calcitonin gene expression through distinct mechanisms in medullary thyroid cancer cells. Cancer Res. 2007;67:6956–64.
Kohno T, Tsuta K, Tsuchihara K, Nakaodu T, Yoh K, Goto K. Ret fusion gene: Translation to personalized lung cancer therapy. Cancer Sci. 2013;104:1396–1400.
Kang J, Qian PX, Pandey V, Perry JK, Miller LD, Liu ET, et al. Artemin is estrogen regulated and mediates antiestrogen resistance in mammary carcinoma. Oncogene. 2010;31:402.
Baselga J, Campone M, Piccart M, Burris HA, Ruggo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9.
Acknowledgements
We thank Dr. Giorgio Caravatti (Novartis Institutes for Biomedical Research, Basel, Switzerland) and all members of Hynes and Gattelli labs for helpful discussions.
Funding
AG was supported by grant KG101234 from Susan G. Komen for the Cure® and grant GF N° 02 from Fundación para el Progreso de la Medicina Córdoba. NEH was supported by grants from Susan G. Komen for the Cure® SAC110041, from Krebsliga beider Basel 10-2030 and by the Novartis Research Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Gattelli, A., García Solá, M.E., Roloff, T.C. et al. Chronic expression of wild-type Ret receptor in the mammary gland induces luminal tumors that are sensitive to Ret inhibition. Oncogene 37, 4046–4054 (2018). https://doi.org/10.1038/s41388-018-0235-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0235-y
- Springer Nature Limited
This article is cited by
-
RET signaling in breast cancer therapeutic resistance and metastasis
Breast Cancer Research (2023)
-
Ret Receptor Has Distinct Alterations and Functions in Breast Cancer
Journal of Mammary Gland Biology and Neoplasia (2020)
-
RET rearrangements are actionable alterations in breast cancer
Nature Communications (2018)