Abstract
Deep brain stimulation (DBS) is a promising intervention for treatment-resistant depression (TRD). Effects on cognitive functioning are unclear since they have been studied in small samples. We aim to estimate the impact of DBS on cognitive functioning in TRD with a systematic review and meta-analyses. After systematically searching PubMed we included 10 studies which compared standardized neuropsychological tests before and after DBS or between active and sham DBS in TRD. Different random-effects meta-analyses were done for different cognitive (sub-)domains and for different follow-up time windows (<6 months, 6–18 months, and >18 months). We found no significant differences in cognitive functioning up to 6 months of DBS. After 6–18 months of DBS small to moderate improvements were found in verbal memory (Hedge’s g = 0.22, 95% CI = [0.01–0.43], p = 0.04), visual memory (Hedge’s g = 0.37, 95% CI = [0.03–0.71], p = 0.04), attention/psychomotor speed (Hedge’s g = 0.26, 95% CI = [0.02–0.50], p = 0.04) and executive functioning (Hedge’s g = 0.37, 95% CI = [0.15–0.59], p = 0.001). Not enough studies could be retrieved for a meta-analysis of effects after >18 months of DBS or for the comparison of active and sham DBS. Qualitatively, generally no differences in cognitive functioning between active and sham DBS were found. No cognitive decline was found in this meta-analysis up to 18 months of DBS in patients with TRD. Results even suggest small positive effects of DBS on cognitive functioning in TRD, although this should be interpreted with caution due to lack of controlled data.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) is one of the largest contributors to disability worldwide, with a lifetime prevalence of 14.6% in high-income countries [1, 2]. MDD is characterized by a depressed mood and/or loss of interest or experiencing pleasure and may lead to cognitive dysfunction in several domains, such as memory, attention, and executive functions [3, 4]. Despite adequate treatment with pharmacological interventions, 30% of patients with MDD do not achieve remission and are referred to as suffering from an advanced stage of treatment-resistant depression (TRD) [5, 6]. For this population, deep brain stimulation (DBS) is a promising solution [7].
DBS is a holistic treatment requiring neurosurgical, psychiatric and psychological expertise. One part consists of a surgical intervention during which electrodes are stereotactically implanted in specific areas of the brain [8]. The electrodes are subcutaneously connected to a pulse generator in the pectoral region which continuously stimulates the brain target [8]. For TRD, the most extensively studied targets are the subcallosal cingulate cortex (SCC), the ventral capsule surrounding the ventral striatum (VC/VS), and the medial forebrain bundle (MFB) [9]. Approximately half of all included patients show a clinical response (≥50% symptom reduction) after one year of open-label neurostimulation [7]. Although results from randomized, sham-controlled trials have been inconsistent, active DBS shows significantly better antidepressant effects compared to sham DBS in recent meta-analyses [7, 10, 11].
Despite its invasive nature, DBS is considered a safe and well-tolerated treatment for TRD [7]. Nevertheless, extensive research on DBS of the subthalamic nucleus in patients with Parkinson’s Disease has shown cognitive decline in several domains including general cognition, visuospatial reasoning and memory, processing speed, executive functions, verbal fluency, and verbal memory [12,13,14]. Whether such cognitive decline also occur in TRD patients treated with DBS in different target regions compared to Parkinson’s Disease, is subject of ongoing research. Several studies have examined the effects of DBS in TRD on cognitive domains including memory, attention, psychomotor speed, executive functions, visuospatial functioning, language, motor skills, visual and verbal fluency, general functioning, and intelligence [15,16,17,18,19]. Outcomes between these studies vary, with some suggesting adverse effects and others suggesting positive effects in specific cognitive domains. However, these studies lack power due to small sample sizes.
Therefore, we aim to investigate the effects of DBS on cognitive functions in patients with TRD by means of a systematic review and meta-analyses, examining both longitudinal change in cognitive function and potential differences between active and sham stimulation.
Methods
Details of the protocol for this systematic review and meta-analysis were registered in the PROSPERO International Prospective Register of Systematic Reviews with registration number CRD42022333129. This study was conducted and reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [20].
Search strategy and eligibility criteria
The PubMed/MEDLINE online database was systematically searched using terms related to DBS combined with terms related to cognitive functions on August 16, 2021 and was repeated on March 16, 2022 (See the Supplementary Materials for full search strategy). Reference lists of the included studies were also examined for additional eligible studies.
Articles were included if they were original studies published in English or Dutch in a peer-reviewed journal. The studies included human adults (≥18 years of age) diagnosed with MDD who were being treated with DBS. To reduce the risk of an overly stringent study selection we also included studies with samples with a maximum of 15% bipolar disorder (BP) diagnoses. All studies used neuropsychological tests to measure cognitive function at one pre-DBS baseline assessment and at least one post-DBS follow-up assessment or after active and sham stimulation. Exclusion criteria were studies involving patients with comorbid neurological or psychotic disorders, case studies, or series with four participants or less.
Study selection and quality assessment
Titles and abstracts of the articles that resulted from the initial electronic search on August 16, 2021 were screened and scrutinized by two reviewers (ZH and TH) independently. Articles that clearly did not fulfill the eligibility criteria were excluded. Next, the full texts of the remaining articles were reviewed and ineligible studies were excluded. Additional articles that were identified after the repeated search on March 16, 2022 were similarly reviewed by two reviewers (GM and NR). Any persisting disagreements on eligibility were resolved with the help of a third reviewer (IB). One reviewer (TH) critically appraised all included studies with The Joanna Briggs Institute Critical Appraisal checklist for Case Control studies [21].
Data extraction and outcome measures
Data was extracted by one reviewer (TH) and verified by another reviewer (NR). All data were collected in an SQLite database [22]. The following descriptive variables were extracted from the included studies: author, year, number of included patients (full sample), patient characteristics (age and sex) and stimulation target. If reported, the name of the neuropsychological test, the number of patients taking the test and the outcome (mean and standard deviation (SD)) were extracted for each test assessing cognitive functioning at each individual assessment. Neuropsychological tests were categorized into cognitive domains and subdomains based on generally accepted domains [23], large factor analytical studies [24], or descriptions of the tests in the included articles. The primary outcomes were pre- and post-DBS (baseline vs. follow-up) cognitive functioning. For this, each follow-up assessment was categorized into three follow-up time windows: short-term (<6 months postoperatively), medium-term (6–18 months postoperatively), or long-term (>18 months postoperatively). We based these time windows on the different stages of the DBS treatment: during the first 6 months DBS parameters are frequently adapted to optimize effect and cognitive functioning may be subject to fluctuations. In the 6–18 month range optimization is usually finished and can be considered the initial stabilization phase. The >18-month range can be regarded as the maintenance phase and results as long-term effects. If two or more assessments from one study fell into the same follow-up time window, only the longest follow-up assessment was included. The secondary outcome was cognitive functioning during active- compared to sham stimulation.
Statistical analysis
Meta-analyses were performed (baseline vs. short-term follow-up, baseline vs. medium-term follow-up, baseline vs. long-term follow-up and active vs. sham) for all cognitive domains and subdomains tested by at least three different studies. Studies and cognitive domains that were not included in the meta-analyses were reviewed qualitatively. When a study reported on multiple outcome measures belonging to the same cognitive subdomain, or the same domain in case a domain had no subdomains, we included the test outcome that we considered the best fit for that (sub)domain. If a lower score represents a better outcome (e.g., reaction time or the total number of errors), these values were multiplied by −1.0 ensuring a positive effect size always translates into cognitive improvement after DBS. Meta-analyses were performed using random-effects models to account for small sample sizes and expected statistical heterogeneity across studies [25]. As the studies in the quantitative analysis used different neuropsychological tests, a standardized mean difference was computed as Hedges g. Weights of the individual effect sizes were computed using the inverse variance method and the respective 95% CI’s were computed using Jackson’s method. All analyses were performed using R Statistical Software, version 4.1.0 with the “meta” package [26,27,28]. The ‘Trim-and-Fill’ function was used to estimate and adjust the meta-analyses results for publication bias. Forest and funnel plots were generated to visualize the meta-analyses results and publication bias estimates respectively. We considered p < 0.05 as significant and effect sizes of 0–0.19 as negligible, 0.2–0.49 as small, 0.5–0.79 as medium and >0.8 as large effects.
Results
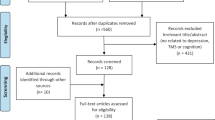
A total of 620 articles were identified of which 20 were potentially eligible for inclusion. After subsequent full-text screening ten articles met the inclusion criteria and were included in the meta-analysis/review. The study selection process is shown in a PRISMA flow diagram (Fig. 1).
The following cognitive domains and their respective subdomains were assessed in the included articles: verbal memory (subdomains: immediate recall, immediate recognition, delayed recall, and delayed recognition), visual memory (subdomains: immediate recall, delayed recall, immediate recognition, and delayed recognition), executive functioning (subdomains: cognitive flexibility, cognitive inhibition, decision making, and planning), verbal fluency (subdomains: general, letter and categorical fluency), visual fluency, working memory, attention/psychomotor speed, visuospatial functioning, language, motor skills, general functioning, concentration and intelligence. For a list of neuropsychological tests included in each study see Supplementary Table 1.
Study and patient characteristics
Study characteristics are summarized in Table 1. The included studies reported on 137 patients (64 male, 73 female) with TRD who received active (n = 125) or only sham (n = 12) DBS. Across all meta-analyses, 87 unique patients were included (86 MDD/1 BP). The mean age in included studies varied from 40.0 to 53.4 years. Four studies targeted the SCC, two the Nucleus Accumbens (NAcc), two the superolateral branch of the medial forebrain bundle (slMFB), one the ventral anterior limb of the internal capsule (vALIC), and one the VC/VS.
Critical appraisal
Quality assessment of the included studies, according to The Joanna Briggs Institute Critical Appraisal checklist for Case Control studies [21], is provided in Supplementary Table 2.
Baseline vs. follow-up
Meta-analyses
All effect sizes, computed for the short-term (<6 months) and medium-term (6–18 months) assessments on cognitive functioning, are summarized in Table 2. Only one study reported a long-term assessment (>18 months) of cognitive functioning. Therefore, no meta-analyses were performed for this follow-up time window. An overview of the forest and funnel plots of each meta-analysis is provided in the Supplementary Materials.
Short-term vs Baseline (<6 months)
For the short-term assessment only two domains were reported on by at least three studies. Both verbal memory and executive functioning did not change significantly after up to 6 months of DBS with negligible to small effect sizes (p > 0.05).
Medium-term vs Baseline (6–18 months)
For the medium-term assessment eight domains were reported on by at least three studies. A small, significant improvement was found for the verbal memory domain (Hedges’ g = 0.22, 95% CI = [0.01–0.43], p = 0.04; Fig. 2). Trim-and-fill in the overall verbal memory domain did not alter results (Hedges’ g = 0.22, 95% CI = [0.01–0.43], p = 0.04). Zooming in into subdomains, we found a small, significant improvement in delayed recall (Hedges’ g = 0.45, 95% CI = [0.05–0.86], p = 0.03) and negligible to small, non-significant effects in the other subdomains (p > 0.05).
A small, significant, positive effect was found for the overall domain (p = 0.04) comprised of a small, significant, positive effect in the subdomain of delayed recall (p = 0.027) and a small, non-significant, positive effect in immediate recall (p > 0.05). Abbreviations: SD standard deviation, SMD standardized mean difference (Hedges’ g), CI confidence interval, VLMT Verbal Learning and Memory Test, HVLT Hopkins Verbal Learning Test, VRM Verbal Recognition Memory, CVLT California Verbal Learning Test, RAVLT Rey Auditory Verbal learning Test, recog recognition, i immediate, d delayed.
A small and significant improvement was found for visual memory (Hedges’ g = 0.37, 95% CI = [0.03–0.71], p = 0.04; Fig. 3). With trim-and-fill the effect increased and remained significant (Hedges’ g = 0.46, 95% CI = [0.13–0.79], p = 0.006). A moderate and significant improvement in the subdomain immediate recall was found (Hedges’ g = 0.57, 95% CI = [0.10–1.05], p = 0.02). All other subdomains showed non-significant effects.
A small, significant, positive effect was observed for the overall domain (p = 0.035) comprised of a moderate, significant, positive effect in the subdomain immediate recall (p = 0.018), and a small, non-significant, positive effect for subdomain delayed recall (p > 0.05). Abbreviations: SD standard deviation, SMD standardized mean difference (Hedges’ g), CI confidence interval, PAL Paired Associates Learning, BLT Brown Location Test, RVDLT Rey Visual Design Learning Test, BVMT-R Brief Visual Memory test Revised, i immediate, d delayed, recog recognition.
A small and significant improvement was found for executive functioning (Hedges’ g = 0.37, 95% CI = [0.15–0.59], p = 0.001; Fig. 4). With trim-and-fill the effect slightly increased and remained significant (Hedges’ g = 0.40, 95% CI = [0.18–0.61], p < 0.001). A moderate and significant improvement was found for the subdomain planning (Hedges’ g = 0.54, 95% CI = [0.09–0.99], p = 0.02). Small improvements were found for subdomains cognitive flexibility (p = 0.05) and cognitive inhibition (p = 0.07), which trended towards significance. Decision making showed a negligible and non-significant change.
A small, significant, positive effect was found for the overall domain (p = 0.001) comprised of a moderate, significant, positive effect for the subdomain planning (p = 0.019) and small, non-significant, positive effects for subdomains cognitive flexibility (p = 0.053) and cognitive inhibition (p = 0.073). Abbreviations: SD standard deviation, SMD standardized mean difference (Hedges’ g), CI confidence interval, TMT Trail Making Test, WCST-perserr Wisconsin Card-Sorting Test – perseverative errors, OA Object Alternation, IED-toterrorad Intra/Extradimensional Shift – total errors, adjusted, IGT Iowa Gambling task, CGT-qod Cambridge Gambling Test – quality of decision making, SOC-problemsmin/exceedmin Stockings of Cambridge – problems solved in minimal moves/number of moves exceeding the minimum needed to solve the exercises, DKEFS-tower Delis-Kaplan Executive Function System – tower test, TOL Tower of London.
All other domains showed negligible to small, non-significant changes (working memory, attention/psychomotor speed, language, verbal fluency, and intelligence).
Qualitative review
For the short-term assessment the following domains were reported on by less than three studies: visual memory (k = 2), verbal fluency (k = 1), visual fluency (k = 1), working memory (k = 1), visuospatial functioning (k = 1), attention/psychomotor speed (k = 2), language (k = 1), concentration (k = 1), general functioning (k = 1), and intelligence (k = 1). They reported no statistical tests to assess changes after DBS. Based on the reported means and SDs by Coenen et al. [29], Moreines et al. [30], and McNeely et al. [31], generally no substantial changes in cognitive functioning in the above mentioned domains up to 6 months of DBS were observed, though a minor decline in verbal fluency was found by Coenen et al. [29].
For the medium-term assessment the following domains were reported on by less than three studies: motor skills (k = 2), visual fluency (k = 2), visuospatial functioning (k = 2), and general functioning (k = 2). McInerney et al. [32] and Fenoy et al. [33] reported no significant differences in motor skills after 12 months of DBS. Grubert et al. [17] reported significant improvements in visual fluency and visuospatial functioning after 12 months of DBS whereas Fenoy et al. [33] found no differences in these domains. Additionally, Grubert et al. [17] and Fenoy et al. [33] found no significant difference in general functioning.
Only one study reported a long-term assessment (>18 months) of cognitive functioning. Bewernick et al. [34] reported no significant differences in cognitive functioning after 24 to 36 months of DBS, except for a significant improvement in visual fluency.
Active vs. sham DBS
Three studies compared cognitive functioning during active vs. sham stimulation [15, 19, 29]. Due to differences in study design, outcome measures, follow-up- and stimulation time, data was too heterogeneous for meta-analyses. Therefore, available evidence was reviewed qualitatively per neurocognitive (sub)domain. Studies compared outcomes in the domains of general functioning (k = 1), verbal memory (k = 3), visual memory (k = 3), working memory (k = 2), attention/psychomotor speed (k = 3), executive functioning (k = 3), visual fluency (k = 1), verbal fluency (k = 1), visuospatial functioning (k = 1), language (k = 1), intelligence (k = 2) and motor skills (k = 1).
Bergfeld et al. [15] randomized 16 patients with vALIC DBS to a 12-week cross-over phase during which patients received 6 weeks of active and six weeks of sham stimulation. No significant differences in cognitive functioning after active vs. sham stimulation were found for any of the assessed domains. However, a trend (defined as 0.01 < p < 0.05) was reported towards better functioning in the domains of language and attention/psychomotor speed after active stimulation. Domains of executive functioning and visual memory and verbal memory showed no significant differences.
Kubu et al. [19] randomized 25 participants to active (n = 13) vs. sham (n = 12) stimulation for 16 weeks in a double-blind study. None of the tests assessing verbal, visual and working memory, attention/psychomotor speed, verbal fluency, motor skills or intelligence showed significant differences between the active and sham groups over time. A measure assessing executive functioning (cognitive flexibility and cognitive inhibition) did show a trend towards decline in the active group compared to the sham group, but this did not survive multiple comparisons correction.
Coenen et al. [29] had a single-blind, randomized sham lead-in phase during which eight of their participants received sham stimulation and eight others received active stimulation. Over a period of eight weeks, no significant differences were found between groups for any of the cognitive domains (general functioning, executive functioning, verbal-, visual-, or working memory, attention/psychomotor speed, visual fluency, visuospatial functioning, language or intelligence).
In conclusion, none of the included studies found statistically significant differences in cognitive functioning between active and sham stimulation.
Discussion
To our knowledge, this is the first systematic review and meta-analysis aiming assessing the effect of DBS on cognitive outcomes in patients suffering from TRD. Our meta-analyses showed significant, small improvements in verbal memory (predominantly delayed recall), visual memory (predominantly immediate recall), and executive functioning (predominantly planning) after 6–18 months of stimulation. The short term (<6 months) meta-analysis did not yield any significant changes, but only two domains were assessed. One study with a follow-up of over 18 months was available, which did not find cognitive decline. Additionally, studies comparing sham versus active DBS did not show significant differences in cognitive functioning.
These results are reassuring for clinical practice as they provide further support for DBS as a safe treatment for TRD. Concerns of cognitive decline following DBS [14] should be attenuated in patients with TRD since no indication for decline was found in any of the cognitive domains. Moreover, our meta-analysis shows a significant improvement in verbal memory, visual memory, and executive functioning, further substantiating the safety of DBS for TRD. These improvements may be observed secondary to a depressive symptom decrease as a direct result of DBS. However, multiple studies found no correlation between cognitive improvement and symptom improvement [17, 19, 32]. This might suggest that the cognitive improvement may be a direct effect of DBS and partly independent of symptom improvement. On the other hand, these cognitive improvements can likely be explained by practice effects, a phenomenon that is observed across tests of many cognitive domains, including verbal memory, visual memory, and executive functioning [35]. Indeed, the significant cognitive improvements after DBS that were found in the two included studies that did incorporate a (healthy) control group found the same improvements in the control group [15, 36]. Future studies should incorporate (healthy) control groups and compare cognitive changes in responders with non-responders to differentiate cognitive improvement from practice effects and symptom improvement.
Our findings are in contrast with the cognitive decline on some domains in patients with Parkinson’s disease after DBS, which is the main source of concern for cognitive decline following DBS [12,13,14]. This difference may be explained by three inherent population and treatment characteristics that could potentially coincide. First, cognitive decline in DBS studies on Parkinson’s could include more degenerative effects related to aging compared to our study [37]. Mean age at diagnosis of Parkinson’s Disease is 70 years [38] whereas studies included in our meta-analysis report substantially lower average ages between 40 and 50 years. Additionally, advancing age is a risk factor for postoperative cognitive decline in Parkinson’s Disease [39,40,41], further substantiating the role of age related differences. Second, Parkinson’s Disease is a progressive, neurodegenerative movement disorder known to cause cognitive decline and even dementia in later stages of the disease [42,43,44]. Conversely, MDD has been associated with moderate dysfunction in several cognitive domains, but is not typically associated with a faster cognitive decline over time [45, 46]. Cognitive dysfunction in MDD may lead to an earlier cross of the threshold for clinical dementia due to lower cognitive reserve [37, 45, 46], but this association is predominantly found in late onset depression (>60 years) [45, 46]. Finally, the difference in cognitive changes following DBS may be related to the stimulation site. Target regions for Parkinson’s Disease are located in the motor circuitry of the basal ganglia, the subthalamic nucleus or globus pallidus internus [47, 48], whereas targets for TRD typically reside in distributed frontolimbic networks associated with the processing of emotion and reward [49,50,51,52,53]. Although little is known about the exact mechanisms behind cognitive effects following DBS, stimulation induced engagement of (pre)frontal regions [53,54,55,56,57] may contribute to the observed cognitive improvement following DBS in targets for TRD [32, 55].
In this regard, it would be interesting to consider possible differences between various DBS targets or exact stimulation sites. The limited number of existing studies does not justify a formal meta-regression with DBS targets as a predictor. However, existing data could be used to explore whether stimulation of specific tracts are associated with changes in specific cognitive domains. A similar approach has resulted in specification of bundles that result in more symptom improvement [58,59,60].
Our study has several limitations. First, due to the relatively small patient samples included in our analyses there is limited statistical power. Therefore, it is not possible to draw definitive conclusions. After pooling all available data, only 87 unique patients were included for formal meta-analyses and effect sizes should, therefore, be regarded as preliminary. Second, as discussed above, since most studies did not include a (healthy) control group, it is difficult to differentiate between actual cognitive improvements due to DBS and practice effects. Finally, we defined rather liberal time windows for our meta-analyses. Our main findings come from studies with a follow-up assessment between 6–18 months of DBS. However, the effect of DBS on cognitive functioning at 6 months may be different compared to 18 months. Future research should expand on our meta-analysis with more narrow time windows in order to draw specific conclusions on the timing of the effects on cognitive functioning following DBS.
In conclusion, no cognitive decline was found in our meta-analyses after 6–18 months of DBS in patients with TRD. This provides further support for DBS as a safe treatment for TRD. Notably, our analysis did show small, significant positive effects after 6–18 months of DBS in the cognitive domains of verbal memory, visual memory, and executive functioning. It currently remains difficult to conclude whether these improvements are due to practice effects, a direct effect of DBS, or a secondary to depressive symptom improvement. Future studies with larger sample sizes and (healthy) control groups are necessary to corroborate these preliminary results, identify potential differences between target sites and control for depressive symptom improvement and practice effects.
References
Herrman H, Patel V, Kieling C, Berk M, Buchweitz C, Cuijpers P, et al. Time for united action on depression: a Lancet–World Psychiatric Association Commission. Lancet. 2022;399:957–1022.
James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858.
Kaser M, Zaman R, Sahakian BJ. Cognition as a treatment target in depression. Psychol Med. 2017;47:987–9.
American Psychiatric Association. American Psychiatric Association: Diagnostic and statistical manual of mental disorders (DSM). 5th edition. American Psychiatric Association; 2013.
Salloum NC, Papakostas GI. Staging treatment intensity and defining resistant depression: historical overview and future directions. J Clin Psychiatry. 2019;80:21624.
Rush JA, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163:1905–17.
Dandekar MP, Fenoy AJ, Carvalho AF, Soares JC, Quevedo J. Deep brain stimulation for treatment-resistant depression: an integrative review of preclinical and clinical findings and translational implications. Mol Psychiatry. 2018;23:1094–112.
Temel, Y, Leentjens, AF, de Bie, RM, Chabardes, S, & Fasano, A (Eds.). Fundamentals and clinics of deep brain stimulation: an interdisciplinary approach. Springer Nature; 2020.
Bergfeld, I.O., Figee, M. Deep Brain Stimulation for Depression. In: Temel, Y., Leentjens, A., de Bie, R., Chabardes, S., Fasano, A. (eds). Fundamentals and Clinics of Deep Brain Stimulation. Springer Nature; 2020. pp 279–90
Hitti FL, Yang AI, Cristancho MA, Baltuch GH. Deep brain stimulation is effective for treatment-resistant depression: a meta-analysis and meta-regression. J Clin Med. 2020;9:2796.
Holtzheimer PE, Husain MM, Lisanby SH, Taylor SF, Whitworth LA, McClintock S, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4:839–49.
Zangaglia R, Pacchetti C, Pasotti C, Mancini F, Servello D, Sinforiani E, et al. Deep brain stimulation and cognitive functions in Parkinson’s disease: A three-year controlled study. Mov Disord. 2009;24:1621–8.
Witt K, Daniels C, Reiff J, Krack P, Volkmann J, Pinsker MO, et al. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: a randomised, multicentre study. Lancet Neurol. 2008;7:605–14.
Bucur M, Papagno C Deep Brain Stimulation in Parkinson Disease: a meta-analysis of the long-term neuropsychological outcomes. Neuropsychol Rev. 2022. https://doi.org/10.1007/s11065-022-09540-9
Bergfeld IO, Mantione M, Hoogendoorn MLC, Ruhé HG, Horst F, Notten P, et al. Impact of deep brain stimulation of the ventral anterior limb of the internal capsule on cognition in depression. Psychol Med. 2017;47:1647–58.
Bergfeld IO, Mantione M, Hoogendoorn MLC, Horst F, Notten P, Schuurman PR, et al. Episodic memory following deep brain stimulation of the ventral anterior limb of the internal capsule and electroconvulsive therapy. Brain Stimul. 2017;10:959–66.
Grubert C, Hurlemann R, Bewernick BH, Kayser S, Hadrysiewicz B, Axmacher N, et al. Neuropsychological safety of nucleus accumbens deep brain stimulation for major depression: effects of 12-month stimulation. World J Biol Psychiatry. 2011;12:516–27.
Bergfeld IO, Mantione M, Hoogendoorn MLC, Denys D. Cognitive functioning in psychiatric-disorders following deep brain stimulation. Brain Stimul. 2013;6:532–7.
Kubu CS, Brelje T, Butters MA, Deckersbach T, Malloy P, Moberg P, et al. Cognitive outcome after ventral capsule/ventral striatum stimulation for treatment-resistant major depression. J Neurol Neurosurg Psychiatry. 2017;88:262.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647.
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z (Editors). JBI Manual for Evidence Synthesis. JBI, 2020. Available from https://synthesismanual.jbi.global
Hipp RD SQLite [Internet]. 2020. Available from: https://www.sqlite.org/index.html
Bouma A, Mulder J, Lindeboom J, Schmand B (Eds.). Handboek neuropsychologische diagnostiek. Second Edition. Pearson Assessment and Information B.V.; 2012.
van Rentergem JA, de Vent NR, Schmand BA, Murre JMJ, Staaks JPC, Huizenga HM. The factor structure of cognitive functioning in cognitively healthy participants: a meta-analysis and meta-analysis of individual participant data. Neuropsychol Rev. 2020;30:51–96.
Nikolakopoulou A, Mavridis D, Salanti G. Demystifying fixed and random effects meta-analysis. Evid Based Ment Health. 2014;17:53–7.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–60.
R Core Team (2022). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
RStudio Team (2022). RStudio: integrated development environment for R. RStudio, PBC, Boston, MA. http://www.rstudio.com/
Coenen VA, Bewernick BH, Kayser S, Kilian H, Boström J, Greschus S, et al. Superolateral medial forebrain bundle deep brain stimulation in major depression: a gateway trial. Neuropsychopharmacology. 2019;44:1224–32.
Moreines JL, McClintock SM, Kelley ME, Holtzheimer PE, Mayberg HS. Neuropsychological function before and after subcallosal cingulate deep brain stimulation in patients with treatment-resistant depression. Depress Anxiety. 2014;31:690–8.
McNeely HE, Mayberg HS, Lozano AM, Kennedy SH. Neuropsychological impact of Cg25 deep brain stimulation for treatment-resistant depression: preliminary results over 12 months. J Nerv Ment Dis. 2008;196:405–10.
McInerney SJ, McNeely HE, Geraci J, Giacobbe P, Rizvi SJ, Ceniti AK, et al. Neurocognitive predictors of response in treatment resistant depression to subcallosal cingulate gyrus deep brain stimulation. Front Hum Neurosci. 2017;11:74.
Fenoy AJ, Schulz PE, Selvaraj S, Burrows CL, Zunta-Soares G, Durkin K, et al. A longitudinal study on deep brain stimulation of the medial forebrain bundle for treatment-resistant depression. Transl Psychiatry. 2018;8:111.
Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology. 2012;37:1975–85.
Calamia M, Markon K, Tranel D. Scoring higher the second time around: meta-analyses of practice effects in neuropsychological assessment. Clin Neuropsychologist. 2012;26:543–70.
Serra-Blasco M, de Vita S, Rodríguez MR, de Diego-Adeliño J, Puigdemont D, Martín-Blanco A, et al. Cognitive functioning after deep brain stimulation in subcallosal cingulate gyrus for treatment-resistant depression: an exploratory study. Psychiatry Res. 2015;225:341–6.
Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med. 2013;29:737–52.
Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, et al. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–22.
Alegret M, Junqué C, Valldeoriola F, Vendrell P, Pilleri M, Rumià J, et al. Effects of bilateral subthalamic stimulation on cognitive function in Parkinson disease. Arch Neurol. 2001;58:1223–7.
Smeding HMM, Speelman JD, Koning-Haanstra M, Schuurman PR, Nijssen P, van Laar T, et al. Neuropsychological effects of bilateral STN stimulation in Parkinson disease: a controlled study. Neurology. 2006;66:1830–6.
Trépanier LL, Kumar R, Lozano AM, Lang AE, Saint-Cyr JA. Neuropsychological outcome of GPi pallidotomy and GPi or STN deep brain stimulation in Parkinson’s disease. Brain Cogn. 2000;42:324–47.
Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson’s disease. Mov Disord. 2005;20:1255–63.
Cummings JL. Intellectual impairment in Parkinson’s disease: clinical, pathologic, and biochemical correlates. J Geriatr Psychiatry Neurol. 1988;1:24–36.
Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sørensen P. Risk of dementia in Parkinson’s disease: a community-based, prospective study. Neurology 2001;56:730–6.
Ly M, Karim HT, Becker JT, Lopez OL, Anderson SJ, Aizenstein HJ, et al. Late-life depression and increased risk of dementia: a longitudinal cohort study. Transl Psychiatry. 2021;11:147.
Stafford J, Chung WT, Sommerlad A, Kirkbride JB, Howard R. Psychiatric disorders and risk of subsequent dementia: systematic review and meta-analysis of longitudinal studies. Int J Geriatr Psychiatry. 2022;37.
Johnson MD, Miocinovic S, McIntyre CC, Vitek JL. Mechanisms and targets of deep brain stimulation in movement disorders. Neurotherapeutics.2008;5:294–308.
Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal “hyperdirect” pathway. Neurosci Res. 2002;43:111–7.
Figee M, Riva-Posse P, Choi KS, Bederson L, Mayberg HS, Kopell BH. Deep brain stimulation for depression. Neurotherapeutics. 2022;19:1229–45.
Coenen VA, Schlaepfer TE, Maedler B, Panksepp J. Cross-species affective functions of the medial forebrain bundle-Implications for the treatment of affective pain and depression in humans. Neurosci Biobehav Rev. 2011;35:1971–81.
Yu Q, Guo X, Zhu Z, Feng C, Jiang H, Zheng Z. White matter tracts associated with deep brain stimulation targets in major depressive disorder: a systematic review. Front Psychiatry. 2022;13:1–12.
Coenen VA, Panksepp J, Hurwitz TA, Urbach H, Mädler B. Human medial forebrain bundle (MFB) and anterior thalamic radiation (ATR): Imaging of two major subcortical pathways and the dynamic balance of opposite affects in understanding depression. J Neuropsychiatry Clin Neurosci. 2012;24:223–36.
Coenen VA, Schumacher LV, Kaller C, Schlaepfer TE, Reinacher PC, Egger K, et al. The anatomy of the human medial forebrain bundle: Ventral tegmental area connections to reward-associated subcortical and frontal lobe regions. NeuroImage Clin. 2018;18:770–83.
Laxton AW, Lipsman N, Lozano AM. Deep brain stimulation for cognitive disorders. 1st ed. Vol. 116, Handbook of clinical neurology. Elsevier B.V.; 2013. p. 307–11.
Broadway JM, Holtzheimer PE, Hilimire MR, Parks NA, Devylder JE, Mayberg HS, et al. Frontal theta cordance predicts 6-month antidepressant response to subcallosal cingulate deep brain stimulation for treatment-resistant depression: a pilot study. Neuropsychopharmacology. 2012;37:1764–72.
Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60.
Conen S, Talbot PS, Matthews JC, Anton-Rodriguez J, Patel NK. Acute and chronic changes in brain activity with deep brain stimulation for refractory depression. J Psychopharmacol. 2018;32:430–40. http://www.sagepub.co.uk/journal.aspx?pid=105678
Liebrand LC, Natarajan SJ, Caan MWA, Schuurman PR, van den Munckhof P, de Kwaasteniet B, et al. Distance to white matter trajectories is associated with treatment response to internal capsule deep brain stimulation in treatment-refractory depression. NeuroImage Clin. 2020;28:102363.
Riva-Posse P, Choi KS, Holtzheimer PE, Crowell AL, Garlow SJ, Rajendra JK, et al. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol Psychiatry. 2018;23:843–9.
Coenen VA, Sajonz B, Reisert M, Bostroem J, Bewernick B, Urbach H, et al. Tractography-assisted deep brain stimulation of the superolateral branch of the medial forebrain bundle (slMFB DBS) in major depression. NeuroImage Clin. 2018;20:580–93.
Author information
Authors and Affiliations
Contributions
NR, GM, and TH: these authors contributed equally to this work. Conceptualization: TH and IB. Investigation: NR, GM, TH, ZH, and IB. Project administration: IB. Methodology: NR, GM, TH, and IB. Formal analysis: NR and TH. Writing—original draft and visualization: NR, GM, and TH. Supervision: DD and IB. Writing—review & editing: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: NR, GM, IB, and DD currently execute an investigator-initiated clinical trial on deep brain stimulation for depression, which is funded by Boston Scientific (24 DBS systems in kind) and a grant of ZonMw (nr. 636310016). All other authors do not declare any conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Runia, N., Mol, G.J.J., Hillenius, T. et al. Effects of deep brain stimulation on cognitive functioning in treatment-resistant depression: a systematic review and meta-analysis. Mol Psychiatry 28, 4585–4593 (2023). https://doi.org/10.1038/s41380-023-02262-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02262-1
- Springer Nature Limited