Abstract
The excitation-inhibition (E/I) imbalance is an important molecular pathological feature of major depressive disorder (MDD) as altered GABA and glutamate levels have been found in multiple brain regions in patients. Healthy subjects show topographic organization of the E/I balance (EIB) across various brain regions. We here raise the question of whether such EIB topography is altered in MDD. Therefore, we systematically review the gene and protein expressions of inhibitory GABAergic and excitatory glutamatergic signaling-related molecules in postmortem MDD brain studies as proxies for EIB topography. Searches were conducted through PubMed and 45 research articles were finally included. We found: i) brain-wide GABA- and glutamatergic alterations; ii) attenuated GABAergic with enhanced glutamatergic signaling in the cortical-subcortical limbic system; iii) that GABAergic signaling is decreased in regions comprising the default mode network (DMN) while it is increased in lateral prefrontal cortex (LPFC). These together demonstrate abnormal GABA- and glutamatergic signaling-based EIB topographies in MDD. This enhances our pathophysiological understanding of MDD and carries important therapeutic implications for stimulation treatment.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) is a mental disorder that features a variety of different symptoms including affective, cognitive, sensory-perceptual, motor, and vegetative [1]. The symptomatic complexity of MDD is mirrored by an almost analogous complexity on the level of the brain regions, including limbic regions [2, 3], regions that comprise default mode network (DMN) and lateral prefrontal cortex (LPFC) [4,5,6], as well as lower-order regions like motor cortex [7] and visual cortex [8, 9]. The hierarchical architecture of functional brain networks in the healthy brains is altered in MDD and linked with gene expression profiles [10]. Excitation-inhibition (E/I) imbalance is an important molecular pathological feature of MDD as alterations in GABA and glutamate levels have been found in multiple brain regions in MDD patients [11, 12]. For instance, increases in glutamate concentrations in the medial prefrontal cortex positively correlate with the anxiety levels in women with MDD [13]. Yet another study observed inverse correlation between glutamate levels in the dorsal anterior cingulate cortex (ACC) and anhedonia ratings [14]. Together, these observations raise the question whether there are brain-wide changes in E/I balance (EIB) pattern in MDD following a particular topographic pattern in comparison to healthy brains. Addressing this yet to be answered question is the main goal of our paper.
For that purpose, we hereby review the changes in the expression levels of molecules associated with the inhibitory GABA and excitatory glutamate systems throughout the whole brain relying on postmortem brain studies of MDD patients compared to healthy controls. Following recent results on the topographic distribution of EIB in healthy subjects [15], we focus on three key topographic features in the present study: i) general view of whole brain involvement considering all regions; ii) cortical and subcortical limbic regions; iii) comparison between DMN regions and LPFC as they are known to stand in a negative relationship [16]. Accordingly, unlike most of the postmortem and imaging studies that focus on specific regions or networks, we here pursue an explicit whole-brain topographic approach. This contributes to a better understanding of the pathophysiological mechanisms of MDD, and moreover to future therapeutic stimulation interventions as for instance transcranial magnetic stimulation or deep brain stimulation, as they operate through modifying the EIB [17].
Methods
Retrieval strategies
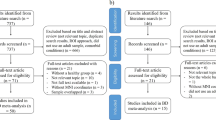
This study was conducted in accordance with the 2020 PRISMA guidelines (see Fig. 1 for schematic representation). Journal articles written in English on human brain research were systematically searched in PubMed from the earliest record to 05th November 2022. The following terms were searched as keywords in the title and abstract sections: (Major-depressive-disorder OR depression) AND (GABA* OR glutam*) AND (postmortem OR post-mortem). Articles other than original research, such as review and case report, were excluded. Included studies quantified gene or protein expression levels of the following molecules in postmortem MDD brains by comparison with the healthy postmortem brains: i) glutamic acid decarboxylase (GAD) and glutaminase (GLS); ii) calcium-binding proteins parvalbumin (PV), calbindin (CB), calretinin (CR), and neuropeptide somatostatin (SST); iii) subunits of GABA-A and -B receptors, and of ionotropic glutamate receptor AMPA, NMDA, and kainite; iv) GABA transporter 1 (GAT1), vesicular glutamate transporter (VGLUT), and excitatory amino acid transporter (EAAT). Molecules were summarized in Supplementary Table 1. Search and selection were done by two independent reviewers.
Brain region grouping
The cortical regions investigated were defined by the Brodmann area (BA) as it is the basis for defining postmortem anatomical human brain structures. For grouping the brain regions, we followed the seven-network parcellation [18, 19]. Among which, three profiles, i.e., the limbic regions, regions that comprise DMN and LPFC were highlighted. In brief, limbic regions include BA11, 24, 25 and 28, as well as the hippocampus and amygdala [20, 21]; DMN regions includes the medial part of BA8~10, BA21, 24 and 28 [22]; LPFC includes BA44, 46 and 47. Involved brain regions and grouping details are shown in Supplementary Table 2.
Semi-quantification
Each study that reveals a significant increase in MDD patients compared with healthy controls in the expression level of one or more GABA and glutamate neurotransmission-related molecules will be counted as one entry and represented by a red rectangle in the figures, indicating an activation in the corresponding system; instead, studies that found a significant decrease in expression will be indicated in blue. Results for those molecules whose expression was not significantly different between MDD patients and healthy controls will not be shown in the figures while will be listed in Table 1. Of note, for studies measuring transporters that remove neurotransmitters from the synaptic cleft, namely GAT1 and EAAT1~3 in this review, lower levels of transporters imply higher neurotransmitter retention in the synaptic cleft, this would be seen as an increase in corresponding neurotransmission (red rectangle); conversely, an increase in the transporter level would be considered as a reduction in the transmission.
Results
Search results
Searching identified a total of 202 records from PubMed, of which 40 review articles and 40 articles on animals were removed before screening. Of these, 77 records that didn’t meet the inclusion criteria were excluded. See the PRISMA flow chart of review process presented in Fig. 1 for details. Characteristics and main findings of the included studies are shown in Table 1.
Global whole brain EIB alterations in postmortem MDD brains
We first provide a global overview of GABA- and glutamatergic changes throughout the whole brain in MDD. Despite the disparity in the number of studies across different cortical regions, this overview shows a brain-wide variation in EIB-related markers (Fig. 2). This suggests a more global rather than localized changes in the EIB related to glutamatergic excitation and GABAergic inhibition in MDD brain. We next raise the question whether there are specific topographic patterns in the EIB changes of MDD, that is, balances between different set of regions, within such global change.
Global GABAergic (upper) and glutamatergic (lower) signaling changes. Each rectangle represents a study, with red indicating an increase in the system activity and blue a decrease. The numbers in the circles represent the corresponding Brodmann areas. Circles with light green background indicate that there were studies measuring the transporters which remove the neurotransmitters from the synaptic cleft, namely GAT1 or EAAT1~3. am = amygdala, hp = hippocampus.
E/I imbalance within cortical-subcortical limbic regions
GABAergic alterations
Higher mRNA levels of GAD65 and -67 were found in BA24 [23]; GAD65/67-ir neuronal density was increased in both the orbitofrontal cortex (BAs not specified) and the hippocampus [24]. However, studies have also reported that both mRNA [25] and protein levels of GAD67 [26] were reduced in BA25; lower GAD67 mRNA was also found in the hippocampus [27]. Lower PV mRNA levels were identified in BA25 [25]. The mRNA [25, 28, 29] and protein [28] levels of SST were also reduced in BA25. Decreased SST gene expression [30] and density of SST-ir neurons [31] were also observed in the amygdala. Gene expression of many GABA-A receptor subunits has been found elevated in BA24 [23, 32], namely α1, α2, α4, α5, β1~3, δ, ε, γ2, and θ; while diminished α1 expression has also been reported [32]. Higher levels of GABA-B receptor subunits 1 and 2 were observed in BA24 [23]. In sum, evidence points strongly towards reduced GABAergic signaling in MDD in the limbic regions (Fig. 3).
Glutamatergic alterations
GLS gene expression levels were increased in BA24 [23]. Higher gene expression of AMPA 1 [23, 33], 2~4 [23], NMDA 1~2C [23], kainate 1 [23], 5 [33]; and elevated [3H] AMPA binding density were observed in BA24 [34]. The mRNA level of AMPA 1, 3, 4 was found lower in the hippocampus [35]; while AMPA 4 levels were higher in the amygdala [32]. NMDA 2A protein levels were increased in amygdala [36]. VGLUT1~2 mRNA levels were higher in BA24 [23]; and VGLUT1 was also higher in the hippocampus [37]; while VGLUT1 was lower in BA28 [38]. Diminished levels of EAAT1~2 gene expression was reported in both BA24 [33] and hippocampus [37]; higher levels of EAAT3 [39] were found in BA24; whereas decreased EAAT3 protein levels were validated in BA25 [26]. In sum, there is strong evidence for increased glutamatergic signaling in the limbic regions of MDD (Fig. 3).
Together, this opposing change between the levels of molecules associated with GABA and glutamate neurotransmission supposedly leads to E/I imbalance with abnormally increased excitation and reduced inhibition in the cortical-subcortical limbic regions (Fig. 3).
E/I imbalance between default mode network (DMN) and lateral prefrontal cortex (LPFC)
GABAergic alterations
DMN regions: GAD67 protein levels were significantly reduced in BA9 [40], while higher mRNA levels of GAD65 and -67 were found in BA24 [23]. The mRNA levels of SST [41], and the density of CB- [42] and CR-ir [43] neurons were decreased in BA9. Diminished gene expression of GABA-A receptor subunits was found, including α1 (BA10 [44] and 24 [32]), α3 in BA10 [44], α4 (BA8 [32], 9 [32], and 10 [44]); γ1 in BA21 [32], and δ in BA10 [44]. Elevated gene expression of GABA-A receptor subunits has also been reported, including β3 in BA10 [32], and α1, α2, α4, α5, β1~3, δ, ε, γ2, and θ in BA24 [23, 32]. Higher levels of GABA-B receptor subunits 1 and 2 were observed in BA24 [23]. In sum, strong evidence shows reductions in the levels of various molecules associated with GABA neurotransmission in the DMN regions of MDD patients (Fig. 4).
LPFC: Increased GAD65 and -67 mRNA levels were found in mainly dorsolateral prefrontal BA46 [23]. GAD65/67-ir neuronal densities have been reported to be higher in the dorsolateral prefrontal cortex (BAs not specified) [24]. Higher levels of gene expression levels were observed for GABA-A receptor subunits, including α1~5, β1~3, δ, γ2, and ε in BA46 [23, 32]; β3, δ, and γ2 in the dorsal-LPFC [33]; as well as δ in BA44 [32, 45]. Whereas reduced levels of subunit γ1 were found in BA46 [32]. In addition, higher GABA-B receptor subunit 1 was identified in BA46 [23]; higher subunit 2 was seen in both BA44 [45] and 46 [23]. Gene expression level of GAT1 was decreased in BA46 [32]. In sum, there is strong evidence for the increased level of GABA neurotransmission-related molecules in the LPFC of MDD patients (Fig. 4).
Glutamatergic alterations
DMN regions: GLS gene expression levels were increased in BA24 [23]. Higher gene expression of AMPA 1 [23, 33], 2~4 [23], NMDA 1~2C [23], kainate 1 [23], 5 [33]; and elevated [3H] AMPA binding density were observed in BA24 [34]. Gene expression levels of AMPA subunits 2 and 4 were also increased in BA10 [32], so was the level of subunit 1 in BA21 [32]. While there were studies that found a significant decrease in the protein level of AMPA 1 [46] and NMDA 2A~2B [46, 47] in BA10. The gene expression of VGULT1 was found elevated in BA10 [48] and BA24 [23], while was found decreased in BA28 [38]; its protein level was observed to reduce in BA9 [49]. VGLUT2 gene expression was higher in BA24 [23] but lower in BA21 [38]. Diminished gene expression level of EAAT1~2 [33] and higher EAAT3 levels [39] were reported in BA24. In sum, there is some evidence for increase in glutamatergic molecules in DMN of MDD albeit, unlike in the case of GABAergic decrease in DMN, the evidence is not as uniform across all measures (Fig. 4).
LPFC: GLS mRNA levels were elevated in BA46 [23]. In BA46, gene expression levels of AMPA 2~4, kainate 1, and NMDA 1~2B were reported to be increased [23], while AMPA 3 [32, 45] was decreased. Higher gene expression levels of AMPA 2, 3 [33, 50] and kainate 1, 2, 5 [33, 50] were detected in the dorsal-LPFC, while NMDA 1~2A reduced [51]. EAAT1~2 mRNA levels were diminished in dorsal-LPFC [33]; and their protein levels were lower in BA47 [52]. In sum, there is evidence for changes in glutamatergic molecules in LPFC of MDD (Fig. 4).
Together, GABAergic signaling seems to be abnormally strong in LPFC relative to DMN in MDD. In contrast, the glutamatergic evidence in both DMN and LPFC is mixed when considering all markers as they show both increases and decreases. Therefore, more research is needed to develop a clearer picture of the balance of glutamatergic changes between DMN and LPFC in MDD.
Other EIB alterations
GABAergic alterations
Motor-related regions: Higher gene expressions of GABA-A receptor subunit δ and β3 were observed in BA6 [32]; protein levels of GABA-A receptor subunit α5 [53] and GABA-B receptor subunit 1 [54] were both significantly increased in BA7. The gene expression level of GAT1 was decreased in BA4 [32]. Figure not shown.
Primary sensory regions: CB-ir neuron densities were significantly decreased in the visual cortex (BA17) [55]; the densities of both CB- and CR-ir neurons were lower in the auditory cortex (BA41 and 42) [56]. Figure not shown.
Other regions: Increased GAD 65/67-ir neuronal density was observed in BA22 [24]. Gene expressions of the GABA-A receptor subunit α5 and -γ2 were elevated in BA20 [32]; and levels of subunit β3 were higher in BA38 [32]. Figure not shown.
Glutamatergic alterations
Motor-related regions: Decreased gene expression of kainate subunit 1 [32] and protein level of NMDA subunit 2A [57] were found in BA6. Figure not shown.
Other regions: Increased gene expressions of GLS and NMDA 2A were observed in BA20 [32]. Figure not shown.
Summary – Topographic structure of EIB changes
By summarizing the EIB changes, three key observations emerge in the brain topography of MDD patients (Fig. 5).
-
i.
EIB reflected by glutamatergic excitation and GABAergic inhibition in MDD brains shows general brain-wide changes across the whole brain regions rather than regionally localized changes.
-
ii.
Opposite alteration is found between GABAergic and glutamatergic signaling within the cortical-subcortical limbic regions: GABAergic signaling is attenuated, whereas glutamatergic signaling is elevated.
-
iii.
Opposite GABAergic signaling is found between the DMN regions and LPFC: increased in the LPFC, while, on the contrary, diminished in the DMN regions. On the other hand, the glutamatergic signaling is relatively unchanged in both LPFC and DMN regions.
Discussion
We here reviewed the brain-wide topographic pattern of E/I imbalances in MDD as operationalized by changes of inhibitory GABAergic and excitatory glutamatergic markers in postmortem tissue. Our findings of brain-wide E/I imbalance are further supported by in vivo magnetic resonance spectroscopy (MRS) studies in MDD patients. Reduction in GABA levels were observed in multiple brain regions, especially in the medial prefrontal regions including the ACC in MDD patients [11, 58,59,60]. These support our observation of decreased GABAergic signaling in especially cortical-subcortical limbic and DMN regions in postmortem studies as reviewed here.
One key system with changes in E/I imbalance in MDD are the regions of the cortical-subcortical limbic system with especially its anterior regions like the perigenual ACC (PACC). In MDD patients, significantly reduce negative BOLD responses during external emotional tasks have been demonstrated in PACC [61], together with enhanced resting-state activities [60, 62]; and increased functional connectivity of the rostral ACC with subcortical limbic regions has been reported [16]. These are in line with our observation of reduced GABAergic and elevated glutamatergic signaling in cortical-subcortical limbic regions.
Yet another key network is the default-mode network (DMN). Our current data suggest that increased DMN activity may be related to reduced GABAergic inhibition in DMN as suggested by our previous study [16]. In contrast, the LPFC in MDD patients is characterized by decreased activity [16], and our present data suggest such deficient LPFC activity to be related to increased GABAergic inhibition. These changes amount to an abnormal reciprocal balance of decreased-increased GABAergic inhibition in DMN-LPFC which well mirrors an analogous pattern of their reciprocal modulation on the more systemic-macroscopic level, e.g., hyperactivity in DMN and hypoactivity in LPFC [5, 16]. This is supported by a recent study that showed how deficits in inhibitory GABAergic interneurons, through modulating excitatory neuronal input/output and local cell circuit processing of information in key brain regions, may underlie the shift in the balance of DMN and LPFC [16]. Such switch in the supposedly GABAergic mediated reciprocal activity balance of DMN and LPFC in MDD patients is closely associated with a shift in awareness. Rather than focusing on the external environment, people with MDD show increased self-focused awareness of their own physical conditions or thoughts generally coupled with negative aspects [63, 64]. Increased self-awareness is related to increased neural activity in DMN as has been revealed by functional imaging studies in MDD patients [5, 61, 65].
In addition to limbic and DMN-LPFC regions, we also demonstrate abnormal E/I imbalance in more primary regions. For instance, in the occipital cortex of MDD patients, GABA levels were found to be unchanged [66] or lower [11, 58, 59] compared to healthy controls; the latter is consistent with the reduced CB-ir neuron density that has been found in the postmortem study [55].
Beyond demonstrating global topographic pattern of EIB changes in MDD, our findings carry important therapeutic implications. The current practice mostly focuses on stimulating one region in isolation as for instance the dorsal LPFC with transcranial magnetic stimulation [67] or the ACC with deep brain stimulation [68, 69]. Our results show that we may need to extend our therapeutic approach beyond single regions: we need to change the topographic pattern of EIB rather than just targeting the EIB in one single region or network. Our previous clinical study demonstrated the therapeutic potential and good tolerance of transcranial magnetic stimulation in the visual cortex of patients with MDD [17]. Due to the co-occurrence of different symptoms with different balances or constellations among sensory, motor, cognitive, affective, social, and vegetative functions in MDD [1], one can thus speak of “symptom coupling” or “co-occurrence of symptom” which, as we assume, can be traced to the brain-wide topography changes in EIB across different regions and their respectively associated functions [70]. In that case topography, indexing the spatial relations between different regions’ EIB, would also be manifest on the psychological or mental level in form of the relationship of the different symptoms – topography may then provide the “common currency” of cellular/E/I imbalance, neural and mental levels [71].
Conclusion
Recent studies of the healthy brain have shown global topographic patterns of EIB. We here ask the question whether there are abnormal topographic EIB patterns in MDD compared with healthy brains. We show that GABA- and glutamatergic changes in postmortem MDD brains exhibit i) brain-wide changes; ii) disbalance in cortical-subcortical limbic regions with decreased GABAergic signaling and increased glutamatergic signaling; iii) reciprocal modulation of GABA neurotransmission in DMN (reduced GABAergic signaling) and LPFC (elevated GABAergic signaling). Together, we demonstrate global-topographic E/I imbalances in MDD, supported by in vivo MRS findings of changes in especially inhibitory GABAergic system of MDD. Beyond providing novel insight into pathophysiological mechanisms, these findings carry important implications for stimulation therapy that may target topographic patterns of EIB rather than individual regions of EIB.
References
Diagnostic and statistical manual of mental disorders. 5th ed. American Psychiatric Association: Washington; 2013.
Bennett MR. The prefrontal-limbic network in depression: modulation by hypothalamus, basal ganglia and midbrain. Prog Neurobiol. 2011;93:468–87.
Li G, Liu Y, Zheng Y, Wu Y, Li D, Liang X, et al. Multiscale neural modeling of resting-state fMRI reveals executive-limbic malfunction as a core mechanism in major depressive disorder. NeuroImage Clin. 2021;31:102758.
Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–11.
Scalabrini A, Vai B, Poletti S, Damiani S, Mucci C, Colombo C, et al. All roads lead to the default-mode network-global source of DMN abnormalities in major depressive disorder. Neuropsychopharmacology. 2020;45:2058–69.
Lu X, Zhang JF, Gu F, Zhang HX, Zhang M, Zhang HS, et al. Altered task modulation of global signal topography in the default-mode network of unmedicated major depressive disorder. J Affect Disord. 2022;297:53–61.
Northoff G, Hirjak D, Wolf RC, Magioncalda P, Martino M. All roads lead to the motor cortex: psychomotor mechanisms and their biochemical modulation in psychiatric disorders. Mol Psychiatry. 2021;26:92–102.
Song XM, Hu XW, Li Z, Gao Y, Ju X, Liu DY, et al. Reduction of higher-order occipital GABA and impaired visual perception in acute major depressive disorder. Mol Psychiatry. 2021;26:6747–55.
Liu DY, Ju X, Gao Y, Han JF, Li Z, Hu XW, et al. From molecular to behavior: higher order occipital cortex in major depressive disorder. Cereb Cortex. 2022;32:2129–39.
Xia M, Liu J, Mechelli A, Sun X, Ma Q, Wang X, et al. Connectome gradient dysfunction in major depression and its association with gene expression profiles and treatment outcomes. Mol Psychiatry. 2022;27:1384–93.
Godfrey KEM, Gardner AC, Kwon S, Chea W, Muthukumaraswamy SD. Differences in excitatory and inhibitory neurotransmitter levels between depressed patients and healthy controls: a systematic review and meta-analysis. J Psychiatr Res. 2018;105:33–44.
Duman RS, Sanacora G, Krystal JH. Altered connectivity in depression: GABA and Glutamate neurotransmitter deficits and reversal by novel treatments. Neuron. 2019;102:75–90.
Zhang X, Tang Y, Maletic-Savatic M, Sheng J, Zhang X, Zhu Y, et al. Altered neuronal spontaneous activity correlates with glutamate concentration in medial prefrontal cortex of major depressed females: an fMRI-MRS study. J Affect Disord. 2016;201:153–61.
Benson KL, Bottary R, Schoerning L, Baer L, Gonenc A, Eric Jensen J. et al. 1H MRS measurement of cortical GABA and glutamate in primary insomnia and major depressive disorder: relationship to sleep quality and depression severity. J Affect Disord. 2020;274:624–31.
Wang XJ. Macroscopic gradients of synaptic excitation and inhibition in the neocortex. Nat Rev Neurosci. 2020;21:169–78.
Northoff G, Sibille E. Why are cortical GABA neurons relevant to internal focus in depression? A cross-level model linking cellular, biochemical and neural network findings. Mol Psychiatry. 2014;19:966–77.
Zhang Z, Zhang H, Xie CM, Zhang M, Shi Y, Song R, et al. Task-related functional magnetic resonance imaging-based neuronavigation for the treatment of depression by individualized repetitive transcranial magnetic stimulation of the visual cortex. Sci China Life Sci. 2021;64:96–106.
Margulies DS, Ghosh SS, Goulas A, Falkiewicz M, Huntenburg JM, Langs G, et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc Natl Acad Sci USA. 2016;113:12574–79.
Ito T, Hearne LJ, Cole MW. A cortical hierarchy of localized and distributed processes revealed via dissociation of task activations, connectivity changes, and intrinsic timescales. NeuroImage. 2020;221:117141.
Rolls ET. Limbic systems for emotion and for memory, but no single limbic system. Cortex. 2015;62:119–57.
Rolls ET. The cingulate cortex and limbic systems for action, emotion, and memory. Handb Clin Neurol. 2019;166:23–37.
Thatcher RW, North DM, Biver CJ. LORETA EEG phase reset of the default mode network. Front Hum Neurosci. 2014;8:529.
Zhao J, Verwer RWH, Gao SF, Qi XR, Lucassen PJ, Kessels HW, et al. Prefrontal alterations in GABAergic and glutamatergic gene expression in relation to depression and suicide. J Psychiatr Res. 2018;102:261–74.
Bielau H, Steiner J, Mawrin C, Trübner K, Brisch R, Meyer-Lotz G, et al. Dysregulation of GABAergic neurotransmission in mood disorders: a postmortem study. Ann N.Y. Acad Sci. 2007;1096:157–69.
Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, Sibille E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry. 2012;169:1194–202.
Scifo E, Pabba M, Kapadia F, Ma T, Lewis DA, Tseng GC, et al. Sustained molecular pathology across episodes and remission in major depressive disorder. Biol Psychiatry. 2018;83:81–89.
Thompson Ray M, Weickert CS, Wyatt E, Webster MJ. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci. 2011;36:195–203.
Tripp A, Kota RS, Lewis DA, Sibille E. Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol Dis. 2011;42:116–24.
Seney ML, Tripp A, McCune S, Lewis DA, Sibille E. Laminar and cellular analyses of reduced somatostatin gene expression in the subgenual anterior cingulate cortex in major depression. Neurobiol Dis. 2015;73:213–9.
Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012;17:1130–42.
Douillard-Guilloux G, Lewis D, Seney ML, Sibille E. Decrease in somatostatin-positive cell density in the amygdala of females with major depression. Depress Anxiety. 2017;34:68–78.
Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, et al. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PloS One. 2009;4:e6585.
Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci USA. 2005;102:15653–8.
Gibbons AS, Brooks L, Scarr E, Dean B. AMPA receptor expression is increased post-mortem samples of the anterior cingulate from subjects with major depressive disorder. J Affect Disord. 2012;136:1232–7.
Duric V, Banasr M, Stockmeier CA, Simen AA, Newton SS, Overholser JC, et al. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int J Neuropsychopharmacol. 2013;16:69–82.
Karolewicz B, Szebeni K, Gilmore T, Maciag D, Stockmeier CA, Ordway GA. Elevated levels of NR2A and PSD-95 in the lateral amygdala in depression. Int J Neuropsychopharmacol. 2009;12:143–53.
Medina A, Burke S, Thompson RC, Bunney W Jr., Myers RM, Schatzberg A, et al. Glutamate transporters: a key piece in the glutamate puzzle of major depressive disorder. J Psychiatr Res. 2013;47:1150–6.
Uezato A, Meador-Woodruff JH, McCullumsmith RE. Vesicular glutamate transporter mRNA expression in the medial temporal lobe in major depressive disorder, bipolar disorder, and schizophrenia. Bipolar Disord. 2009;11:711–25.
Zhao J, Verwer RW, van Wamelen DJ, Qi XR, Gao SF, Lucassen PJ, et al. Prefrontal changes in the glutamate-glutamine cycle and neuronal/glial glutamate transporters in depression with and without suicide. J Psychiatr Res. 2016;82:8–15.
Karolewicz B, Maciag D, O'Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G. Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol. 2010;13:411–20.
Sibille E, Morris HM, Kota RS, Lewis DA. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol. 2011;14:721–34.
Rajkowska G, O'Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacol. 2007;32:471–82.
Oh DH, Son H, Hwang S, Kim SH. Neuropathological abnormalities of astrocytes, GABAergic neurons, and pyramidal neurons in the dorsolateral prefrontal cortices of patients with major depressive disorder. Eur Neuropsychopharmacol. 2012;22:330–8.
Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO. et al. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region.J Neurosci.2004;24:1478–85.
Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, ffrench-Mullen J, et al. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol psychiatry. 2009;14:175–89.
Rafalo-Ulinska A, Piotrowska J, Kryczyk A, Opoka W, Sowa-Kucma M, Misztak P, et al. Zinc transporters protein level in postmortem brain of depressed subjects and suicide victims. J Psychiatr Res. 2016;83:220–29.
Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuro-Psychopharmacol Biol psychiatry. 2009;33:70–5.
Gottschalk MG, Wesseling H, Guest PC, Bahn S. Proteomic enrichment analysis of psychotic and affective disorders reveals common signatures in presynaptic glutamatergic signaling and energy metabolism. Int J Neuropsychopharmacol. 2014;18:pyu019.
Gilabert-Juan J, Varea E, Guirado R, Blasco-Ibáñez JM, Crespo C, Nácher J. Alterations in the expression of PSA-NCAM and synaptic proteins in the dorsolateral prefrontal cortex of psychiatric disorder patients. Neurosci Lett. 2012;530:97–102.
Gray AL, Hyde TM, Deep-Soboslay A, Kleinman JE, Sodhi MS. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol psychiatry. 2015;20:1057–68.
Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacol. 2008;33:2175–86.
Miguel-Hidalgo JJ, Waltzer R, Whittom AA, Austin MC, Rajkowska G, Stockmeier CA. Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. J Affect Disord. 2010;127:230–40.
Xiong Z, Zhang K, Ishima T, Ren Q, Chang L, Chen J, et al. Comparison of rapid and long-lasting antidepressant effects of negative modulators of α5-containing GABA(A) receptors and (R)‑ketamine in a chronic social defeat stress model. Pharmacol, Biochem, Behav. 2018;175:139–45.
Xiong Z, Zhang K, Ren Q, Chang L, Chen J, Hashimoto K. Increased expression of inwardly rectifying Kir4.1 channel in the parietal cortex from patients with major depressive disorder. J Affect Disord. 2019;245:265–69.
Maciag D, Hughes J, O'Dwyer G, Pride Y, Stockmeier CA, Sanacora G, et al. Reduced density of calbindin immunoreactive GABAergic neurons in the occipital cortex in major depression: relevance to neuroimaging studies. Biol psychiatry. 2010;67:465–70.
Smiley JF, Hackett TA, Bleiwas C, Petkova E, Stankov A, Mann JJ, et al. Reduced GABA neuron density in auditory cerebral cortex of subjects with major depressive disorder. J Chem Neuroanat. 2016;76:108–21.
Matas E, John Francis William D, Toro CT. Abnormal expression of post-synaptic proteins in prefrontal cortex of patients with schizophrenia. Neurosci Lett. 2021;745:135629.
Schür RR, Draisma LW, Wijnen JP, Boks MP, Koevoets MG, Joëls M, et al. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of (1) H-MRS studies. Hum brain Mapp. 2016;37:3337–52.
Romeo B, Choucha W, Fossati P, Rotge JY. Meta-analysis of central and peripheral γ-aminobutyric acid levels in patients with unipolar and bipolar depression. J psychiatry Neurosci. 2018;43:58–66.
Kantrowitz JT, Dong Z, Milak MS, Rashid R, Kegeles LS, Javitt DC, et al. Ventromedial prefrontal cortex/anterior cingulate cortex Glx, glutamate, and GABA levels in medication-free major depressive disorder. Transl psychiatry. 2021;11:419.
Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34:932–43.
Abdallah CG, Hannestad J, Mason GF, Holmes SE, DellaGioia N, Sanacora G, et al. Metabotropic glutamate receptor 5 and glutamate involvement in major depressive disorder: a multimodal imaging study. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:449–56.
Northoff G. Psychopathology and pathophysiology of the self in depression - neuropsychiatric hypothesis. J Affect Disord. 2007;104:1–14.
Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol psychiatry. 2015;78:224–30.
Grimm S, Ernst J, Boesiger P, Schuepbach D, Boeker H, Northoff G. Reduced negative BOLD responses in the default-mode network and increased self-focus in depression. World J Biol Psychiatry. 2011;12:627–37.
Truong V, Cheng PZ, Lee HC, Lane TJ, Hsu TY, Duncan NW. Occipital gamma-aminobutyric acid and glutamate-glutamine alterations in major depressive disorder: an mrs study and meta-analysis. Psychiatry Res Neuroimaging. 2021;308:111238.
Leuchter AF, Cook IA, Jin Y, Phillips B. The relationship between brain oscillatory activity and therapeutic effectiveness of transcranial magnetic stimulation in the treatment of major depressive disorder. Front Hum Neurosci. 2013;7:37.
Sakas DE, Panourias IG. Rostral cingulate gyrus: a putative target for deep brain stimulation in treatment-refractory depression. Med hypotheses. 2006;66:491–4.
Broadway JM, Holtzheimer PE, Hilimire MR, Parks NA, Devylder JE, Mayberg HS, et al. Frontal theta cordance predicts 6-month antidepressant response to subcallosal cingulate deep brain stimulation for treatment-resistant depression: a pilot study. Neuropsychopharmacology. 2012;37:1764–72.
Northoff G, Hirjak D, Wolf RC, Magioncalda P, Martino M. Why is there symptom coupling of psychological and motor changes in psychomotor mechanisms? Insights from the brain’s topography. Mol Psychiatry. 2021;26:3669–71.
Northoff G, Wainio-Theberge S, Evers K. Is temporo-spatial dynamics the "common currency" of brain and mind? In quest of "Spatiotemporal Neuroscience". Phys life Rev. 2020;33:34–54.
Acknowledgements
The authors thank Dr. Dick Swaab (Netherlands Institute for Neuroscience) for his academic advice on this work. There is no funding support for this research.
Author information
Authors and Affiliations
Contributions
Y.H. and G.N. designed the study. Y.H. and Z.T. contributed to the reference retrieval. Y.H. and G.N. made the figures. Y.H., Z.T., D.H., and G.N. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, YT., Tan, ZL., Hirjak, D. et al. Brain-wide changes in excitation-inhibition balance of major depressive disorder: a systematic review of topographic patterns of GABA- and glutamatergic alterations. Mol Psychiatry 28, 3257–3266 (2023). https://doi.org/10.1038/s41380-023-02193-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02193-x
- Springer Nature Limited
This article is cited by
-
Is depression a global brain disorder with topographic dynamic reorganization?
Translational Psychiatry (2024)
-
Major depressive disorder: hypothesis, mechanism, prevention and treatment
Signal Transduction and Targeted Therapy (2024)
-
Elevated body temperature is associated with depressive symptoms: results from the TemPredict Study
Scientific Reports (2024)