Abstract
Depression has been associated with inflammatory pathophysiological mechanisms, including alterations in amount of circulating immune cells. However, no meta-analysis within the past 20 years have reevaluated the circulating immune cells in blood and cerebrospinal fluid (CSF) from patients with depression compared to healthy controls. The aim of this study was to systematically evaluate the circulating immune cells in blood and CSF from patients with unipolar depression compared to healthy controls. Databases were searched up until February 12, 2021. Data-extraction was performed by two independent reviewers. 104 studies were included in the meta-analysis using fixed and random-effects models. Patients with depression had a significantly higher overall leukocyte count (35 studies; SMD, 0.46; 95% CI: 0.31–0.60, I2 = 68%), higher neutrophil count (24 studies; SMD, 0.52; 95% CI: 0.33–0.71, I2 = 77%) and higher monocyte count (27 studies; SMD, 0.32; 95% CI: 0.11–0.53, I2 = 77%) compared to healthy controls. Leukocyte counts were higher in inpatients, indicating a relation to depression severity. Furthermore, there were significant alterations in several lymphocyte subsets, including higher natural killer cells and T cell subsets. Higher neutrophil/lymphocyte ratio (11 studies; SMD = 0.24; 95% CI: 0.06–0.42, I2 = 73%), CD4/CD8 cell-ratio (26 studies; SMD = 0.14; 95% CI: 0.01–0.28, I2 = 42%) and T helper 17/T regulatory ratio (2 studies; SMD = 1.05; 95% CI: 0.15–1.95, I2 = 86%) were found in patients compared to healthy controls. CSF white cell count was higher in patients compared to controls (3 studies; SMD = 0.20; 95% CI: 0.01–0.38, I2 = 0%). There were no data for CSF cell subsets. This study suggests that there are several blood immune cell alterations in patients with unipolar depression compared to healthy controls, both in major leukocyte subsets and more specialized immune cell subsets.
Similar content being viewed by others
Introduction
Depression is estimated to affect more than 260 million people on a global scale [1]. Despite the immense impact of depression, our understanding of the etiology and pathophysiology remains inadequate - emphasized by the fact that approximately a third of patients with depression do not respond adequately to antidepressant treatment [2,3,4]. The past decades, immunological alterations have increasingly been associated with depression. Epidemiological studies have demonstrated that immune exposures, such as infections and autoimmune disorders, are associated with an increased risk of subsequent depression [5]. Moreover, studies have found that therapeutic treatment with pro-inflammatory medicine can induce depressive symptomatology [6], and anti-inflammatory treatment has shown to reduce depressive symptoms [7].
Several meta-analyses have shown alterations of inflammatory biomarkers in the blood and cerebrospinal fluid (CSF) from patients with depression [8,9,10]; however, no meta-analysis within the past 20 years have been conducted regarding subsets of circulating immune cells in blood or CSF from patients with unipolar depression. The most recent meta-analysis from 2018 focused exclusively on ratios between different immune cells in blood from patients with mood disorders compared to healthy controls and revealed an increased neutrophil-lymphocyte ratio among patients with major depressive disorder [11]. Two meta-analyses, published in 1993 and 2001, reported leukocytosis in blood from patients with depression compared to healthy controls [12, 13]. In 1993, a significant increase in the absolute neutrophil count and decreased absolute lymphocyte count were reported [12], whereas the meta-analysis from 2001 only reported significant changes in the relative proportions of these cells [13]. In regard to other immune cell subsets the results are also conflicting [11, 14]. Since the latest meta-analysis of individual immune cell subsets from 2001, numerous studies on the relationship between depression and circulating immune cell subsets have been published – both regarding major leukocyte subsets [14,15,16,17,–18] and lymphocyte subsets [19,20,21,22,–23]. However, no systematic review and meta-analysis has recently reevaluated the amount of individual circulating immune cell subsets in patients diagnosed with depression. In addition, no meta-analysis has previously been conducted regarding circulating immune cells in CSF from patients with depression.
The objective of this systematic review and meta-analysis was to comprehensively investigate differences in the composition of circulating immune cells in blood and/or CSF from patients with depression compared to healthy controls. Furthermore, our aim was to explore whether there was an association between the amounts of immune cells and clinical features of the depression (e.g., severity of depression and antidepressant medication status).
Materials and methods
The study protocol was registered on PROSPERO (ID: CRD42021237884) and is available online. Meta-analysis of Observational Studies in Epidemiology (MOOSE) and The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines were followed (Supplementary Tables 1 and 2).
Study selection and search method
We included clinical studies which met the following criteria:
-
1.
Investigation of circulating immune cells in blood and/or CSF obtained from living, human study participants.
-
2.
Inclusion of patients with a primary diagnosis of depression according to a diagnostic system (Research Diagnostic Criteria (RDC), International Classification of Diseases (ICD), Diagnostic and Statistical Manual of Mental Disorders (DSM), or similar classifications), of both sexes and all races, aged 18 or older.
-
3.
Comparison to healthy controls.
-
4.
Publications in peer-reviewed journals.
-
5.
Publications in English.
We excluded studies on the following criteria:
-
1.
Contained previously published data. If study samples overlapped across studies, the smaller study group was excluded.
-
2.
Investigated depression in study participants with a diagnosis of psychotic or bipolar disorder (ICD-10: F20-F31.9).
-
3.
Investigated depression in participants with detected severe medical condition (including neurologic, vascular, autoimmune, malignant, endocrine, or infectious diseases with possible impact on immune cells which may disturb the interpretation of the immunological assessments).
-
4.
Investigated pregnant or lactating women.
-
5.
Had a control group admitted to hospital.
-
6.
Used other methods than direct measurements of immune cell composition.
We searched PubMed, EMBASE, Cochrane Library, Web of Science, Clinicaltrials.gov and PsycINFO from inception to the 12th of February 2021 using the following Medical Subject Headings (or similar headings) or text word terms:
(Depressive Disorder OR Depression OR Depressive) AND (Leukocyte* OR Leukocyte count OR White blood cell* OR WBC OR Complete blood count* OR CBC OR Peripheral blood mononuclear cell* OR PBMC OR Neutrophil* OR Basophil* OR Eosinophil* OR Monocyte* OR Lymphocyte* OR T-lymphocyte* OR T-cell* OR T helper OR T regulatory OR T cytotoxic OR Th1 OR Th2 OR Th17 OR B-lymphocyte* OR B-cell* OR Natural Killer OR CD3 OR CD4 OR CD8 OR CD14 OR CD16 OR CD19 OR CD20 OR CD25 OR CD56) AND (Blood OR Plasma OR Serum OR Spinal puncture OR Spinal tap OR Lumbar puncture OR Cerebrospinal fluid OR CSF).
Animal studies, case studies, review articles and conference abstracts were excluded. The search string was decided in cooperation with an information specialist from the National Library of Denmark. Reference lists of relevant reviews were searched for additional studies. One investigator (BHF) examined titles and abstracts, and two investigators (BHF and SOW) examined the remaining full text reports to determine the study’s fulfillment of the inclusion criteria. When the two investigators reached different decisions in the study selection, a third author (MEB) was consulted, and a consensus was reached.
Outcomes
The primary outcome was to investigate the amount (as counts or percentages) of circulating immune cells measured in blood and/or CSF of patients with depression compared to healthy controls.
Secondary outcomes included: associations between immune cell count and clinical features of the depression (e.g., severity of the depression and antidepressant medication status).
Data extraction and bias assessment
One author (BHF) was responsible for data extraction, while SOW/NVS validated, and quality checked all extracted data. Data extraction included bibliographic data and characteristics of the participants including antidepressant medication status. In case of missing details, authors were contacted by email. In case of no response, a reminder was sent. Risk of bias was assessed by two authors (BHF and SOW/NVS) according to the Newcastle-Ottawa Scale for case-control studies [24]. The risk of publication bias was assessed with funnel plots for cell counts, percentages and ratios investigated in 10 or more studies. Furthermore, subgroup analyses comparing a total score of ≥4 vs <4 on the Newcastle-Ottawa Scale and publication before vs after the year 2000 were conducted. In addition, meta-regressions associating size of studies and publication year with standardized mean differences (SMDs) were performed for leukocyte, neutrophil, monocyte, lymphocyte, natural killer cell, T cell, T helper cell, T cytotoxic cell and B cell counts.
Statistical analysis
Meta-analyses were performed using standardized mean differences (SMDs). Results from the random-effects and fixed-effects models are presented. In random-effects meta analyses the DerSimonian–Laird estimator [25] was used to estimate the between study variance, τ, [26] with the inverse-variance weighting method. Bias-correction of SMD estimates were done by using Hedges’ g [27, 28]. χ² tests for heterogeneity were performed and I2 calculated to assess heterogeneity [29]. Possible publication bias was assessed by Egger’s test [30]. Medians, quartiles, and ranges were used for imputation of means and standard deviations, when necessary. To associate effect sizes with the average number of participants (harmonic mean), meta-regression was used. To investigate associations between depression severity and immune cell count, percentages, and ratios, subgroup analyses of inpatient versus outpatient status were performed, when information hereof was available in ten or more studies. Furthermore, meta-regression analyses were performed to investigate associations between Hamilton Depression Rating Scale (HAMD) score and SMDs for leukocyte, neutrophil, monocyte, lymphocyte, natural killer cell, T cell, T helper cell, T cytotoxic cell and B cell counts. All statistical analyses were conducted in R version 4.02 or later, using the meta-package [31]. All p-values were two-sided and p-values < 0.05 were considered significant.
Deviations from the protocol
Measurements on the function of immune cells were not included due to a too large variability in methods for assessing the amount of circulating immune cells in blood. Correlations between the immune cell count and prognosis, and associations between immune cell measures in CSF and in blood were not assessed due to lack of sufficient data.
Results
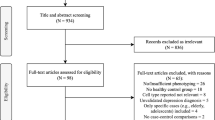
A total of 20.162 studies were identified. After removal of duplicates [32] and title/abstract screening, 659 studies remained for full text screening. A total of 104 studies had data required for a quantitative meta-analysis (Supplementary Fig. 1). Detailed study characteristics are presented in Supplementary Table 3 and a detailed overview of cell surface markers used for categorization of included blood immune cells is found in Fig. 1 and Supplementary Tables 4–8. Forest plots of significant results on immune cells counted in ≥2 studies are found in Figs. 2–5 and forest plots of immune cell ratios are found in Fig. 6. An overview of results for immune cell counts is provided in Supplementary Table 9 and for percentages in Supplementary Table 10. Forest plots presenting fixed effects models of significant results by random effects models are found in Supplementary Figs. 2–6. Forest plots of immune cell counts, percentages and ratios including cerebrospinal fluid counts are found in Supplementary Figs. 7–45 Results from the sensitivity analyses related to antidepressant treatment and depression severity are provided in Supplementary Figs. 46–76 and Supplementary Figs. 77–99, respectively. Bias assessment analyses are presented in Supplementary Tables 11 and 12 and Supplementary Figs. 100–165. Leave-one-out analyses are presented in Supplementary Figs. 166–174.
A An overview of immune cells counted in ≥2 studies included in the meta-analysis. B Immune cells counted in ≥2 studies and higher in depression compared to healthy controls. C Immune cells counted in ≥2 studies and lower in depression compared to healthy controls. D Immune cell ratios altered in depression compared to healthy controls.
Leukocyte count and major leukocyte subsets in blood
Leukocyte count
The leukocyte count was significantly higher among patients compared to healthy controls (35 studies [1436 patients]; SMD = 0.46; 95% CI: 0.31–0.60, I2 = 68%) (Fig. 2 and Supplementary Figs. 2 and 7).
Granulocytes
The granulocyte count (6 studies [150 patients]; SMD = 0.35; 95% CI: 0.12–0.58, I2 = 0%) were significantly higher in patients compared to healthy controls. Patients with depression also had a higher neutrophil count (24 studies [1442 patients]; SMD = 0.52; 95% CI: 0.33–0.71, I2 = 77%) and a higher neutrophil percentage (15 studies [568 patients]; SMD = 0.44; 95% CI: 0.27–0.60, I2 = 36%) compared to healthy controls. Basophil count (7 studies [547 patients]; SMD = 0.34; 95% CI: 0.17–0.52, I2 = 22%) and eosinophil count (7 studies [547 patients]; SMD = 0.17; 95% CI: 0.03–0.32, I2 = 0%) were higher in patients as well. However, the overall granulocyte, eosinophil and basophil percentages did not differ between groups. Results are shown in Fig. 3 and Supplementary Figs. 3 and 8–12.
Monocytes
Overall monocyte count was significantly higher in patients with depression compared to healthy controls (27 studies [1049 patients]; SMD = 0.32; 95% CI: 0.11–0.53, I2 = 77%) (Fig. 4), however, with no significant difference in the monocyte percentages (17 studies [1559 patients]; SMD = −0.10; 95% CI: −0.29 to 0.08, I2 = 74%) (Supplementary Fig. 12). In the analysis of monocytes subsets, the intermediate monocyte count was higher in patients (3 studies [273 patients]; SMD = 0.39; 95% CI: 0.12–0.67, I2 = 25%) (Fig. 4), whereas there were no significant group differences regarding other monocyte subset amounts (Supplementary Figs. 4 and 13–16).
Lymphocytes
The lymphocyte count (44 studies [1857 patients]; SMD = 0.03; 95% CI: −0.11 to 0.16, I2 = 71%) did not differ significantly between groups, whereas the percentage of lymphocytes was lower among patients with depression (22 studies [821 patients]; SMD = −0.40; 95% CI: −0.56 to −0.23, I2 = 60%) (Supplementary Fig. 17).
Lymphocyte subsets in blood
Natural killer cells
The natural killer cell count was significantly higher in patients compared to healthy controls (20 studies [716 patients]; SMD = 0.45; 95% CI: 0.10–0.80, I2 = 88%), whereas the percentages did not differ between groups. The “Only CD16 + natural killer cell count” (3 studies [110 patients]; SMD = −0.72; 95% CI: −1.39 to −0.05, I2 = 79%) and CD16low natural killer cells percentages (3 studies [109 patients]; SMD = −0.31; 95% CI: −0.58 to −0.05, I2 = 0%) was significantly lower in patients compared to healthy controls. Results are found in Fig. 5, Supplementary Figs. 5 and 18–21.
T cells and T helper cells
No significant differences were found regarding the overall T cell count (28 studies [938 patients]; SMD = −0.04; 95% CI: −0.19 to 0.10, I2 = 52%) or overall T cell percentage (26 studies [1730 patients]; SMD = −0.06; 95% CI: −0.19 to 0.07, I2 = 52%) (Supplementary Fig. 22). Overall T helper cell count tended to be higher in patients with depression (31 studies [1265 patients]; SMD = 0.12; 95% CI: 0.00–0.24, I2 = 44%) compared to healthy controls, whereas there was no difference in T helper cell percentage (29 studies [997 patients]; SMD = −0.00; 95% CI: −0.16 to 0.15, I2 = 61%) (Supplementary Fig. 23).
T helper cell subsets
The memory T helper cell count (4 studies [172 patients]; SMD = 0.39; 95% CI: 0.11–0.67, I2 = 9%) (Fig. 5) as well as the percentage of memory T helper cells (5 studies [281 patients]; SMD = 0.40; 95% CI: 0.22–0.58, I2 = 0%) (Supplementary Fig. 29) were significantly higher in patients compared to healthy controls. There were no significant differences in the percentages of Th1, Th2 or Th17 cells when comparing patients to healthy controls (Supplementary Figs. 24–26), neither differed the percentage of T regulatory cells (Supplementary Fig. 27). No significant differences were found concerning the amount of naïve T helper cells or the percentage of activated T helper cells nor effector memory T helper cell percentages (Supplementary Figs. 28–31).
T cytotoxic cells and T cytotoxic cell subsets
No significant differences were found between patients with depression and healthy controls regarding the T cytotoxic cell count (31 studies [1265 patients]; SMD = −0.05; 95% CI: −0.18 to 0.07, I2 = 47%) or percentages of T cytotoxic cells and subsets (Supplementary Figs. 32–36).
Activated, naïve and memory T cells
A higher amount of several T cell subsets was found in patients, including a higher naïve T cell count (2 studies [77 patients]; SMD = 0.34; 95% CI: 0.03–0.64, I2 = 0%) and a higher amount of activated T cells: Both CD25 + activated T cell count (2 studies [77 patients]; SMD = 0.34; 95% CI: 0.04–0.64, I2 = 0%) (Fig. 5) and percentage of HLA-DR + activated T cells (4 studies [128 patients]; SMD = 0.50; 95% CI: 0.19–0.81, I2 = 0%) were higher in patients (Fig. 5 and Supplementary Figs. 37 and 38). No significant differences were found regarding memory T cells when comparing patients and healthy controls.
Natural killer T cells and immature double positive T cells
The natural killer T cell count was lower in patients compared to healthy controls (2 studies [265 patients]; SMD = −0.38; 95% CI: −0.62 to –0.14, I2 = 0%) (Fig. 5). No difference was found regarding the percentage of natural killer T cells (Supplementary Fig. 39). There was a higher immature double positive T cell count in patients with depression compared to healthy controls (2 studies [74 patients]; SMD = 0.60; 95% CI: 0.18–1.01, I2 = 0%) (Fig. 5 and Supplementary Fig. 40).
B cells, unspecified antigen-presenting cells and activated lymphocytes
The amount of B cells, activated lymphocytes, and antigen-presenting cells were not significantly different when comparing patients with depression to healthy controls (Supplementary Figs. 41–43). However, interestingly the leave-one-out analysis of B cells revealed that exclusion of the study by Pavon et al. [33], altered the result to a significantly higher B cell count in patients compared to healthy controls (30 studies; SMD = 0.14; 95% CI: 0.01–0.26) (Supplementary Fig. 174).
Cell ratios
The CD4/CD8-ratio (T helper cell/T cytotoxic cell ratio) (26 studies [886 patients]; SMD = 0.14; 95% CI: 0.01–0.28, I2 = 42%), neutrophil/lymphocyte ratio (11 studies [1242 patients]; SMD = 0.24; 95% CI: 0.06–0.42, I2 = 73%), and T helper 17/T regulatory ratio (2 studies [93 patients]; SMD = 1.05; 95% CI: 0.15–1.95, I2 = 86%) were significantly higher in patients compared to healthy controls, whereas there was no difference in platelet/lymphocyte ratio (Fig. 6).
White cell count in cerebrospinal fluid
When including the CSF studies stating to have healthy controls, no significant difference in overall CSF white cell count (4 studies [290 patients]; SMD = 0.11; 95% CI: −0.05 to 0.28, I2 = 41%) was found (Supplementary Fig. 44A). However, in one of the studies the healthy controls had values clearly exceeding the cut-off for neuroinflammation [34], and when excluding this study and thus only including the studies with a healthy control group, the CSF white cell count was higher among the patients with depression than the healthy controls (3 studies [224 patients]; SMD = 0.20; 95% CI: 0.01–0.38, I2 = 0%) (Supplementary Fig. 44B). No other immune cell subsets in the CSF were investigated.
Sensitivity analyses on antidepressants
Studies on clearly defined antidepressant-naïve patients were sparse. However, there appeared to be a subgroup difference related to the length of discontinuation of antidepressants for leukocyte count (p < 0.01) and a near significant difference for lymphocyte count (p = 0.05) (Supplementary Figs. 46–58). Leukocyte counts were higher among patients ongoingly treated with antidepressants (6 studies [369 patients]; SMD = 0.53; 95% CI: 0.17–0.89, I2 = 74%) compared to antidepressant-naïve patients (1 study [15 patients]; SMD = −0.40; 95% CI: −0.96 to 0.16). The meta-regression analyses indicated the same association between duration of discontinuation of antidepressants for leukocytes (Supplementary Figs. 59–67). Meta-regression analyses on patients treated with antidepressants did not reveal any associations (Supplementary Figs. 68–76).
Sensitivity analyses on depression severity
The subgroup analysis of inpatient vs outpatient status revealed leukocyte counts to be higher in inpatients with depression, but not significant in outpatients or mixed groups with a significant subgroup difference (p = 0.04). Likewise, neutrophil counts were only higher in inpatients with significant subgroup differences (p < 0.01) (Supplementary Figs. 77–90). Meta-regression analyses revealed no significant associations to depression severity measured by HAM-D (Supplementary Figs. 91–99).
Bias assessment and related sensitivity analyses
The mean score according to the Newcastle-Ottawa Quality Assessment Scale across the 104 included studies was 3.89 points out of 8 possible points (Supplementary Tables 11 and 12). No studies received the maximum of eight stars. Most of the included studies had risk of bias regarding adequate case definition (82/104) and representativeness of cases (79/104). Moreover, approximately half of the studies had risk of bias concerning selection of controls (50/104), definition of controls (46/104) and comparability between cases and controls (38/104). Risk of bias was further assessed by subgroup analyses of Newcastle-Ottawa Scale score <4 versus ≥4, revealing significant subgroup differences in neutrophil percentage (p = 0.04) and T cell percentage (p = 0.03) (Supplementary Figs. 100–118). Subgroup analyses of studies published before year 2000 versus in year 2000 or later revealed several significant subgroup differences (Supplementary Figs. 119–137). Meta-regressions on size of studies and publication year showed significant associations between leukocyte and neutrophil count and publication year (p < 0.001), whereas there were no significant associations to size of studies (Supplementary Figs. 138–156). Funnel plots did not indicate publication bias (Supplementary Figs. 157–165). Leave-one-out-analyses did not alter results significantly despite as described for the overall B cell count (Supplementary Figs. 166–174).
Discussion
This study is the largest and most comprehensive meta-analysis on the composition of circulating immune cells in unipolar depression to date. The meta-analysis is comprised of 104 studies in which 100 studies evaluated immune cells in blood, whereas only four studies evaluated immune cells in CSF. Our results indicate broad immune cell activation in unipolar depression. When evaluating absolute cell counts, a total of 12 subsets showed significant increases compared to healthy controls, whereas only CD16+ natural killer cells and natural killer T cell counts were lower in patients. Furthermore, several immune cell ratios were altered in patients with unipolar depression compared to healthy controls. These findings are consistent with previous published meta-analyses, showing leukocytosis, higher amounts of neutrophils, lower amounts of lymphocytes and subsequently an increased neutrophil/lymphocyte ratio [11,12,–13]. However, our updated meta-analysis includes considerably more studies than the previous meta-analyses (e.g., 14 more studies of leukocyte count as compared to the most recent of the previous meta-analyses [13]). This enables us, as the first, to explore and identify differences in more specialized leukocyte subsets, including our findings of higher intermediate monocyte count and higher memory T helper cell count among the patients with depression. Furthermore, we also explore the CSF white cell count, evaluate the individual studies’ risk of bias and the effect of antidepressant medication status on immune cell subsets.
Our results suggest a generalized increased immune activation in patients with unipolar depression, since the immune cell contrasts are not limited to one specific immune cell subset or lineage, but affect both the myeloid linage (e.g., monocytes and granulocytes) and the lymphoid linage (e.g., T cells and natural killer cells) indicating a generalized upregulation of the bone marrow activity. A recent study has shown that patients with unipolar depression can be differentiated into subgroups with either a myeloid-biased or lymphoid-biased immune cell profile [14]. Since unipolar depression has a heterogeneous appearance [35], it could be hypothesized that changes in specific cell linages might characterize specific subgroups of depression. Furthermore, previous studies have indicated a more pronounced inflammatory response to be related to more severe depression [36, 37]. This corroborates with the subgroup analyses of our study showing that higher levels of overall leukocytes, driven by higher levels of neutrophils, were associated with inpatient status and thus indicating that increased peripheral inflammation is associated with more severe depression. This could be explored further in new studies, as identification of subtypes of depression with specific immune cell profiles might pave the way for new immunotherapeutic strategies in the treatment of depression.
Experimental studies indicate that induced pro-inflammation increase depressive-like behavior in animals [38] and depressive-like symptoms in humans [39]. Childhood trauma [40] and environmental psychological stressors [41] have been associated with both increased peripheral inflammation and depression. Several mechanisms have been suggested to underlie this association, including alterations of the hypothalamic-pituitary-adrenal (HPA) axis through the impact on the kynurenine pathway and hereby the monoamine availability [42]. However, two recent meta-analyses evaluating blood and CSF monoamine metabolite levels found no significant differences on a group level, which question the current theory of lower brain serotonin levels as causative to depression in a majority of cases [9, 43]. Thus, the mechanisms underlying depression are not yet fully understood and likely heterogeneous, however, could perhaps be driven by brain alterations caused by systemic inflammation in subgroups. Interestingly, a recent animal study suggested that transplantation of bone marrow mononuclear cells can prevent depressive-like behavior in rats [44], indicating that more profound alterations in the peripheral immune system might be implicated in the pathogenesis of depression.
Systemic inflammation has been shown to impair the blood-brain barrier [45], hereby potentially increasing the risk of peripheral immune cells entering the brain. When including the CSF studies stating to have healthy controls we did not find the CSF WCC to be significantly increased; however, one of the studies reported a range of CSF WCC up to 14 cells in one of their healthy control groups [34]. As this is markedly above the normal upper range of CSF WCC maximum of 5 cells, this is concerning since such high CSF WCC in the control group potentially masks group differences. When excluding this study and only including the CSF studies with healthy controls, CSF WCC was indeed increased among the patients with depression compared to healthy controls. Moreover, only one of the CSF studies had a primary aim of evaluating CSF WCC [37] and, thus, in the remaining three papers there are no details on information relevant to measurement of low-grade neuroinflammation by CSF WCC. This includes lack of information regarding correction for blood contamination and time from sample collection until laboratory analyses. The one study that corrected data for blood contamination found a CSF WCC to be higher among patients with depression and that the CSF WCC count was increased the most among patients with severe depression [37]. This dose-response relationship with severity of depression has only been evaluated in this one CSF WCC study [37]. Thus, more high-quality large-scale studies evaluating this direct marker of neuroinflammation is highly needed to explore inflammation in the brain related to depression.
Interestingly, we found a tendency towards long discontinuation of antidepressants (>14 days) to be associated with lower overall leukocyte count. As (some) antidepressants are thought to yield immunosuppressive effects [46] this result is somewhat surprising. However, as we also found inpatient-status to be associated with higher leukocyte count, the effect of antidepressants could be confounded by the most severely ill patients not having their medication discontinued. The tendency could also be due to different patterns in use of specific antidepressants prior to and after year 2000 as our sensitivity analysis hereof on leukocytes also revealed a subgroup difference.
Identification of subtypes of depression with specific immune cell profiles might pave the way for new immunotherapeutic strategies in the treatment of depression. Personalized immunotherapeutic treatment is widely used in cancer treatment - one example is adoptive cell therapy, where T-cells harvested from the patient are genetically modified ex-vivo to specifically target the tumor cells when reintroduced to the patient by infusion. Development of similar personalized treatment modalities for depression might help resolve the immune cell disruptions in this patient group.
Strengths and limitations
Despite being the most comprehensive meta-analysis on circulating immune cells in unipolar depression, several limitations should be considered. The laboratory methods used across the included studies were highly diverse and included (among others) microscopy, hematology analyzers and flow cytometric analyses. Especially the field of flow cytometric analysis and cell surface markers has evolved rapidly during the past forty years, thus compromising the comparability of studies on immune cells from the 1980–1990’s with more recent literature [22]. Furthermore, international consensus on cell surface markers for immunophenotyping is needed. Only peer-reviewed studies were included and therefore, conference abstracts and other types of gray literature were not eligible for this analysis. Additionally, most of the studies included in this meta-analysis are comprised of heterogenous patient groups regarding the characteristics of the depression (subtypes, severity, and duration), and therefore it was not possible to investigate associations between immune cells and different characteristics of the disease.
Most of the included studies showed bias regarding adequate definition and representativeness of cases. In addition, around half of the included studies did not match or adjust for important confounding factors such as age and sex and even fewer studies matched for body-mass index and smoking, which is known to influence the immune system [47,48,–49]. In addition, the degree of heterogeneity was high for many of the reported outcomes indicating a high variation among the included studies.
Conclusion and perspectives
This meta-analysis indicates immune activation in unipolar depression with alterations in both the myeloid and lymphoid immune cell linages. This activation appears to be most pronounced in inpatients, indicating a relation to depression severity. However, many of the included studies had risk of bias and did not match for important factors such as age, sex, body-mass index, and smoking. Future studies are needed to unravel whether the immune activation is restricted to a specific subtype of depression, while adjusting for important confounding factors. Moreover, further studies on immune cells in CSF are needed in order to explore whether cellular neuroinflammation is part of the pathogenesis of depression. Identification of the mechanism underlying the potential association between increased peripheral immune activity and depression would also enhance the understanding of the disorder and could lead to new immunotherapeutic treatment strategies in depression.
References
Spencer LJ, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858.
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17.
Gronemann FH, Jorgensen MB, Nordentoft M, Andersen PK, Osler M. Incidence of, risk factors for, and changes over time in treatment-resistant depression in Denmark: a register-based cohort study. J Clin Psychiatry. 2018;79:17m11845.
Reutfors J, Andersson TML, Brenner P, Brandt L, DiBernardo A, Li G, et al. Mortality in treatment-resistant unipolar depression: a register-based cohort study in Sweden. J Affect Disord. 2018;238:674–9.
Benros ME, Waltoft BL, Nordentoft M, Ostergaard SD, Eaton WW, Krogh J, et al. Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry. 2013;70:812–20.
Bonaccorso S, Marino V, Biondi M, Grimaldi F, Ippoliti F, Maes M. Depression induced by treatment with interferon-alpha in patients affected by hepatitis C virus. J Affect Disord. 2002;72:237–41.
Köhler OE, Benros M, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–91.
Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21:1696–709.
Mousten IV, Sørensen NV, Christensen RHB, Benros ME. Cerebrospinal fluid biomarkers in patients with unipolar depression compared with healthy control individuals: a systematic review and meta-analysis. JAMA Psychiatry. 2022. https://doi.org/10.1001/jamapsychiatry.2022.0645.
Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135:373–87.
Mazza MG, Lucchi S, Tringali AGM, Rossetti A, Botti ER, Clerici M. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in mood disorders: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84:229–36.
Herbert TB, Cohen S. Depression and immunity: a meta-analytic review. Psychol Bull. 1993;113:472–86.
Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199–226.
Lynall M-E, Turner L, Bhatti J, Cavanagh J, de Boer P, Mondelli V, et al. Peripheral blood cell-stratified subgroups of inflamed depression. Biol Psychiatry. 2020;88:185–96.
Hasselmann H, Gamradt S, Taenzer A, Nowacki J, Zain R, Patas K, et al. Pro-inflammatory monocyte phenotype and cell-specific steroid signaling alterations in unmedicated patients with major depressive disorder. Front Immunol. 2018;9:2693.
Cai L, Xu L, Wei L, Chen W. Relationship of mean platelet volume to MDD: a retrospective study. Shanghai Arch Psychiatry. 2017;29:21–29.
Euteneuer F, Dannehl K, del Rey A, Engler H, Schedlowski M, Rief W. Peripheral immune alterations in major depression: the role of subtypes and pathogenetic characteristics. Front Psychiatry. 2017;8:250.
Grosse L, Carvalho LA, Birkenhager TK, Hoogendijk WJ, Kushner SA, Drexhage HA, et al. Circulating cytotoxic T cells and natural killer cells as potential predictors for antidepressant response in melancholic depression. Restoration of T regulatory cell populations after antidepressant therapy. Psychopharmacology. 2016;;233:1679–88.
Noor Suryani MA, Siti Nor Fairus MS, Che Maraina CH, Wong KK, Mohd Nazri S, Mohd Azhar MY. Major depressive disorder patients on antidepressant treatments display higher number of regulatory T cells. Malays J Pathol. 2019;41:169–76.
Patas K, Willing A, Demiralay C, Engler JB, Lupu A, Ramien C, et al. T cell phenotype and T cell receptor repertoire in patients with major depressive disorder. Front Immunol. 2018;9:291.
Krause D, Stapf TM, Kirnich VB, Hennings A, Riemer S, Chrobok A, et al. Stability of cellular immune parameters over 12 weeks in patients with major depression or somatoform disorder and in healthy controls. Neuroimmunomodulation. 2018;25:7–17.
Becking K, Haarman BCM, Grosse L, Nolen WA, Claes S, Arolt V, et al. The circulating levels of CD4+ t helper cells are higher in bipolar disorder as compared to major depressive disorder. J Neuroimmunol. 2018;319:28–36.
Ahmetspahic D, Schwarte K, Ambrée O, Bürger C, Falcone V, Seiler K, et al. Altered B cell homeostasis in patients with major depressive disorder and normalization of CD5 surface expression on regulatory B cells in treatment responders. J Neuroimmune Pharm. 2018;13:90–99.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 2021.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Levine S, Rabinowitz J, Faries D, Lawson A, Ascher-Svanum H. Treatment response trajectories and antipsychotic medications: examination of up to 18 months of treatment in the CATIE chronic schizophrenia trial. Schizophr Res. 2012;137:141–6.
Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Behav Stat. 1981;6:107–28.
Hedges LV. Estimation of effect size from a series of independent experiments. Psychol Bull. 1982;92:490–9.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. 2nd ed. https://training.cochrane.org/: Cochrane Training; 2019.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ Br Med J. 1997;315:629.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–60.
Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104:240–3.
Pavon L, Sandoval-Lopez G, Eugenia Hernandez M, Loria F, Estrada I, Perez M, et al. Th2 cytokine response in Major Depressive Disorder patients before treatment. J Neuroimmunol. 2006;172:156–65.
Hattori K, Ota M, Sasayama D, Yoshida S, Matsumura R, Miyakawa T, et al. Increased cerebrospinal fluid fibrinogen in major depressive disorder. Sci Rep. 2015;5:11412.
Lynch CJ, Gunning FM, Liston C. Causes and consequences of diagnostic heterogeneity in depression: paths to discovering novel biological depression subtypes. Biol Psychiatry. 2020;88:83–94.
Köhler-Forsberg O, Buttenschøn HN, Tansey KE, Maier W, Hauser J, Dernovsek MZ, et al. Association between C-reactive protein (CRP) with depression symptom severity and specific depressive symptoms in major depression. Brain Behav Immun. 2017;62:344–50.
Sørensen NV, Orlovska-Waast S, Jeppesen R, Klein-Petersen AW, Christensen RHB, Benros ME. Neuroinflammatory biomarkers in cerebrospinal fluid from 106 patients with recent-onset depression compared with 106 individually matched healthy control subjects. Biol Psychiatry. 2022. https://doi.org/10.1016/j.biopsych.2022.04.002.
Cattane N, Vernon AC, Borsini A, Scassellati C, Endres D, Capuron L, et al. Preclinical animal models of mental illnesses to translate findings from the bench to the bedside: Molecular brain mechanisms and peripheral biomarkers associated to early life stress or immune challenges. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol. 2022;58:55–79.
Bollen J, Trick L, Llewellyn D, Dickens C. The effects of acute inflammation on cognitive functioning and emotional processing in humans: a systematic review of experimental studies. J Psychosom Res. 2017;94:47–55.
Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry. 2016;21:642–9.
Häfner S, Emeny RT, Lacruz ME, Baumert J, Herder C, Koenig W, et al. Association between social isolation and inflammatory markers in depressed and non-depressed individuals: results from the MONICA/KORA study. Brain Behav Immun. 2011;25:1701–7.
Brown SJ, Huang X-F, Newell KA. The kynurenine pathway in major depression: what we know and where to next. Neurosci Biobehav Rev. 2021;127:917–27.
Moncrieff J, Cooper RE, Stockmann T, Amendola S, Hengartner MP, Horowitz MA. The serotonin theory of depression: a systematic umbrella review of the evidence. Mol Psychiatry. 2022. https://doi.org/10.1038/s41380-022-01661-0. Online ahead of print.
Costa-Ferro ZSM, do Prado-Lima PAS, Onsten GA, Oliveira GN, Brito GC, Ghilardi IM, et al. Bone marrow mononuclear cell transplant prevents rat depression and modulates inflammatory and neurogenic molecules. Prog Neuropsychopharmacol Biol Psychiatry. 2022;113:110455.
Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun. 2017;60:1–12.
Eyre HA, Lavretsky H, Kartika J, Qassim A, Baune BT. Modulatory effects of antidepressant classes on the innate and adaptive immune system in depression. Pharmacopsychiatry. 2016;49:85–96.
Bauer ME. Stress, glucocorticoids and ageing of the immune system. Stress. 2005;8:69–83.
Johannsen NM, Priest EL, Dixit VD, Earnest CP, Blair SN, Church TS. Association of white blood cell subfraction concentration with fitness and fatness. Br J Sports Med. 2010;44:588–93.
Mehta H, Nazzal K, Sadikot RT. Cigarette smoking and innate immunity. Inflamm Res J Eur Histamine Res Soc. 2008;57:497–503.
Funding
This study was funded by grants from The Lundbeck Foundation (grant number R268–2016–3925 and R277–2018–1411). The funding agencies had no impact on the study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Contributions
MEB, SOW and BHF designed the study and systematic literature search. BHF conducted the screening of titles and abstracts. BHF and SOW did full text screening independently. BHF extracted all data. Data was validated by either NVS or SOW. TBB and NØ helped categorize immune cells. RHC made the statistical analysis plan and conducted the statistical analyses. All authors contributed to data interpretation. RHC, NVS and BHF conducted figures and tables. NVS drafted the manuscript including preparation of the supplementary. All authors contributed to the editing of the manuscript and all authors have read and accepted the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sørensen, N.V., Frandsen, B.H., Orlovska-Waast, S. et al. Immune cell composition in unipolar depression: a comprehensive systematic review and meta-analysis. Mol Psychiatry 28, 391–401 (2023). https://doi.org/10.1038/s41380-022-01905-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01905-z
- Springer Nature Limited
This article is cited by
-
Acute COVID-19 severity markers predict post-COVID new-onset psychiatric disorders: A 2-year cohort study of 34,489 patients
Molecular Psychiatry (2024)
-
Peripheral blood cellular immunophenotype in suicidal ideation, suicide attempt, and suicide: a systematic review and meta-analysis
Molecular Psychiatry (2024)
-
Bayesian network-based Mendelian randomization for variant prioritization and phenotypic causal inference
Human Genetics (2024)
-
Identification of mitophagy-related biomarkers and immune infiltration in major depressive disorder
BMC Genomics (2023)
-
Natur und Immunmechanismen psychischer Erkrankungen
Der Nervenarzt (2023)