Abstract
The hippocampus and the amygdala play a central role in post-traumatic stress disorder (PTSD) pathogenesis. While alternations in volumes of both regions have been consistently observed in individuals with PTSD, it remains unknown whether these reflect pre-trauma vulnerability traits or acquired post-trauma consequences of the disorder. Here, we conducted a longitudinal panel study of adult civilian trauma survivors admitted to a general hospital emergency department (ED). One hundred eligible participants (mean age = 32.97 ± 10.97, n = 56 females) completed both clinical interviews and structural MRI scans at 1-, 6-, and 14-months after ED admission (alias T1, T2, and T3). While all participants met PTSD diagnosis at T1, only n = 29 still met PTSD diagnosis at T3 (a “non-Remission” Group), while n = 71 did not (a “Remission” Group). Bayesian multilevel modeling analysis showed robust evidence for smaller right hippocampus volume (P+ of ~0.014) and moderate evidence for larger left amygdala volume (P+ of ~0.870) at T1 in the “non-Remission” group, compared to the “Remission” group. Subregion analysis further demonstrated robust evidence for smaller volume in the subiculum and right CA1 hippocampal subregions (P+ of ~0.021–0.046) in the “non-Remission” group. No time-dependent volumetric changes (T1 to T2 to T3) were observed across all participants or between groups. Results support the “vulnerability trait” hypothesis, suggesting that lower initial volumes of specific hippocampus subregions are associated with non-remitting PTSD. The stable volume of all hippocampal and amygdala subregions does not support the idea of consequential, progressive, stress-related atrophy during the first critical year following trauma exposure.
Similar content being viewed by others
Introduction
Numerous studies have examined the relationship between post-traumatic stress disorder (PTSD) and the hippocampus and the amygdala, as both regions are implicated in the disorder’s pathogenesis and pathophysiology [1]. The hippocampus is involved in providing contextual memory for emotion-related processes, whereas the amygdala mediates fear learning, extinction, and regulation [2,3,4]. Prior literature typically reported smaller hippocampus volume in PTSD patients [5,6,7], while evidence of altered amygdala volume is more equivocal, with findings of no difference, smaller or larger amygdala volume in PTSD patients compared to controls [8, 9]. A major unresolved question is whether these altered volumes reflect a pre-trauma vulnerability trait [10, 11], an outcome of the disorder’s persistence associated distress [12, 13], or a combination of both [14].
Previous volumetric findings in PTSD populations did not provide a clear answer to the vulnerability vs. consequence debate for several reasons. First, most studies rely on cross-sectional designs and examine chronic PTSD samples [15], thus cannot disentangle predisposed from acquired volume abnormalities. The few longitudinal PTSD studies conducted to date [16,17,18] reported no change over time in either hippocampus or amygdala volumes, but those are limited by relatively low sample sizes (27 ≤ n ≤ 44) and follow-up durations (3- to 6-months post-trauma), that may not capture longer-term modifications of these regions [19]. Finally, studies typically examined only the whole hippocampus and/or amygdala, usually due to limited resolution and accuracy to segment their different subregions [20].
Yet, the hippocampus and amygdala consist of functionally and cytoarchitecturally distinct substructures that may play a different role in PTSD development and maintenance [21, 22]. Several animal studies described structural plasticity in specific subregions of these structures after stress exposure [23], but the translational value of these findings is limited by the lack of an adequate model system for PTSD. On the other hand, not many human studies investigated volumetric abnormalities of amygdala and hippocampus subregions in PTSD, and findings remain inconclusive [24,25,26]. To date, only two longitudinal studies examined subregion-specific volumetric changes in the hippocampus or the amygdala during the first year following trauma, a critical period in the etiology of PTSD [27]. Koch et al. [28] found that smaller hippocampal left dentate gyrus (DG) volumes at baseline predicted an increase in self-reported PTSD symptoms, and that the amount of trauma exposure between assessments was positively associated with an increase in left basal amygdala nucleus volume. On the other hand, Weis et al. [29] report that none of the subfield volumes at baseline were prospectively related to PTSD symptoms 6 months later. Finally, both studies did not find significant volumetric changes over time, or an association between volumetric changes and PTSD symptoms [28, 29].
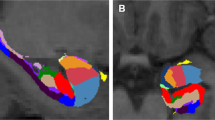
To address the above-mentioned knowledge gap, we conducted a longitudinal panel study of adult civilian trauma survivors admitted to a general hospital emergency department (ED) [30]. One hundred eligible participants (mean age = 32.97 ± 10.97 years, range = 18–64 years, n = 56 [56%] females) completed clinical interviews and MRI scans at 1-, 6-, and 14-months after ED admission (alias T1, T2, and T3). While all participants met PTSD diagnosis at T1, only n = 29 still met PTSD diagnosis at T3 (a “non-Remission” Group), while n = 71 did not (a “Remission” Group). At each time point, we obtained whole volumes of the right and left hippocampus and amygdala. Furthermore, we extracted volumes of specific hippocampal subregions previously implicated in PTSD literature [25, 31,32,33], namely the cornu ammonis 1 and 3 (CA1 and CA3), the DG, and the subiculum (see Fig. 1a). Similarly, we extracted volumes of specific amygdala subregions mentioned in PTSD literature [26, 34, 35], namely the lateral, central, and basal nuclei of the amygdala (LA, CeA, and BA, respectively; Fig. 1b).
Automatic segmentation of hippocampus and amygdala subregions, as generated with FreeSurfer 7.1.0 for one representative subject. Hippocampal subfields (a) and amygdala nuclei (b) are displayed in sagittal (left), coronal (middle), and axial (right) planes. Enlarged images are presented in a sagittal plane.
This design allowed us to address two critical aims: (1) to determine whether volumetric measures shortly after trauma can differentiate between those who recover from initial PTSD and those who develop the chronic disorder (“Remission” vs. “non-Remission” symptom trajectories); (2) to examine potential time-dependent volumetric changes over the first-year post-trauma (T1, T2, T3) across all individuals (n = 100) and between the two PTSD trajectories. While the first aim supports the role of the amygdala and hippocampus volumes as early (possibly predisposing) vulnerability factors for the disorder’s development, the second aim supports these alternations as acquired changes associated with the disorder’s persistence.
Methods and materials
Data informing this report were collected between 2015 and 2020 as part of the NIMH-funded “Neurobehavioral Moderators of Post-traumatic Disease Trajectories” study (MH103287). This research project was approved by the Tel-Aviv Sourasky Medical Center (TASMC) institutional review board (approval 0207/14) and was registered on ClinicalTrials.gov (NCT03756545). The study’s design and detailed methodologies have been previously published [30, 36,37,38] and those informing this work are summarized below.
Participants
Potential participants for this study were 18 to 65 years old adult civilians consecutively admitted to a general hospital’s ED after one of the following events: motor-vehicle accident (MVA), bicycle accident, physical assault, robbery, hostilities, electric shock, fire, drowning, work accident, terror attack or a large-scale disaster.
Participants were included in the study if they met PTSD symptom criteria (specified below) within 1 month following trauma exposure (T1). Participants were excluded if they had an ED notation of severe head injury, coma upon ED admission, a medical condition that interfered with their ability to provide informed consent or apprehend the study’s procedures, a diagnosis of PTSD prior to ED admission, current substance abuse disorder, current suicidal ideations, lifetime psychotic illness, conditions precluding MRI scanning (e.g., pacemaker, metal implants, large tattoos, permanent makeup) or medical/psychological conditions that constituted treatment priority. All participants in this study provided oral consent to the study’s screening telephone interview and written informed consent upon attending a subsequent diagnostic and eligibility ascertainment clinical interview.
A final sample of n = 100 participants with valid clinical and structural brain data (at T1, T2, and T3) is included in the current report (i.e., “study completers”). While all participants (n = 100) met PTSD diagnostic criteria at T1, only n = 29 still met PTSD diagnosis at T3 (a “non-Remission” Group), while n = 71 did not (a “Remission” Group). For a comparison of demographic and clinical characteristics of the study groups (“Remission” vs. “non-Remission”), see Table 1.
Clinical assessments
A comprehensive clinical interview was conducted by trained clinicians, using the Clinician-Administered PTSD Scale (CAPS), to assess PTSD diagnosis and severity estimates at 1-, 6-, and 14-months after ED admission (T1, T2, and T3, respectively). In order to maintain continuity with decades of PTSD research based on DSM-IV definitions, and given the findings of nonoverlapping samples per definitional criteria used [39, 40], we administered a combined clinical interview assessing both CAPS-4 [41] and CAPS-5 [42] items at the same time. The usage of such hybrid PTSD diagnostic interviews is supported, particularly in longitudinal studies [43], and is in line with the recommendation to use broader cross-template definitions of PTSD for empirical research [44]. Here, a positive PTSD diagnosis was given to individuals who met either DSM-IV or DSM-5 diagnostic criteria or, in line with previous recommendations [45] endorsed a CAPS-4 total score of ≥40. Individuals who met either DSM-IV or DSM-5 diagnostic criteria but endorsed CAPS-4 total score lower than 40, were given a positive PTSD diagnosis.
MRI acquisition
Whole-brain anatomical images were conducted using a 3T MAGNETOM Prisma system (Siemens Medical Solutions, Erlangen, Germany) at the TASMC. These repeated structural MRI scans took place at three different time points following traumatic exposure (T1, T2, and T3). At each time point, a sagittal T1-weighted magnetization prepared rapid gradient echo sequence (TR/TE = 2400/2.29 ms, flip angle = 8°, voxel size = 0.7 mm3, field of view = 224 × 224 mm2, slice thickness = 0.7 mm) was used to acquire high-resolution structural images. Foam padding and earplugs were used to reduce head motion and scanner noise.
Hippocampus and amygdala segmentations
Hippocampal and amygdala segmentations were performed on structural T1-weighted images (voxel size = 0.7 mm3) using the longitudinal pipeline [46] of FreeSurfer image analysis suite [47] version 7.1.0. This longitudinal pipeline reduces the confounding effect of inter-individual variability, thus increasing the robustness of the method and yielding more sensitive brain volumes [48]. It further increases the reliability and statistical power by using an unbiased within-subject template space [46] (based on all available time-points of a subject), and allowed us to use all available data (i.e., also when one time-point out of three was missing).
Segmentations of hippocampus and amygdala subregions were obtained using a special-purpose module included in FreeSurfer version 7.1.0, an evolution of the previous hippocampal subfields’ module released with FreeSurfer 6.0. This tool uses a probabilistic atlas built with ultra-high-resolution ex-vivo MRI data (~0.1 mm isotropic) to produce an automated segmentation of the hippocampal substructures [49] and the nuclei of the amygdala [50]. Using this processing pipeline, hippocampal subfields and amygdala subnuclei were segmented simultaneously, ensuring that these two structures do not overlap or leave gaps in between [48,49,50]. Overall, this analysis provides volumes of 12 bilateral hippocampal subfields (subdivided in body and head when applicable) and volumes of 9 bilateral amygdala subnuclei.
As previous studies reported laterality/asymmetry effects (e.g., different volumes between the two hemispheres) in PTSD patients [51, 52], we have examined the left and right hippocampus and amygdala separately. Thus, for each participant at each time point (T1, T2, T3), volumes of the left and right amygdala and hippocampus were derived, as well as its intracranial volume. These measures were previously shown to have good agreement with manual volumetric assessment and other automatic methods [53,54,55,56]. We further extracted volumes of four specific hippocampal subregions previously implicated in PTSD literature [25, 31,32,33]—CA1, CA3, DG, and subiculum (Fig. 1a) These subregions showed excellent reliability between two consecutive days ~2-weeks after their traumatic injuries [29]. Similarly, we extracted volumes of specific subregions within the right and left amygdala previously implicated in PTSD literature [26, 34, 35]—LA, CeA, and BA (Fig. 1b). For more details regarding the a-priori selected subregions, see Supplementary Methods. Finally, all hippocampus and amygdala segmentations were visually inspected and checked according to standardized quality control procedures [25] (see Supplementary Methods and Fig. S1).
Procedure
The hospital’s ED computerized records
were available to the study team within 24 h of ED admission. Within these records, an ED “trauma” notation generated initial screening contacts from 3 days after the ED admission.
Initial telephone screening
was performed by trained study personnel within 10–14 days of ED admission, and only after individuals were discharged from the hospital. After explaining the purpose of the call and obtaining verbal consent, interviewers confirmed the occurrence of a psychologically traumatic event and associated distress, availability for the study, and salient exclusion criteria (i.e., 5–10 min “short interview”). Next, interviewers evaluated PTSD symptom severity using a modified dichotomous version of the PTSD checklist IV for civilians (PCL) [30, 57], study availability, and full exclusion criteria (i.e., 20–30 min “long interview”). Participants who met PTSD diagnostic criteria (except for the 1-month duration), and did not meet any of the exclusion criteria, received further verbal information about the study and were invited for a first clinical assessment.
Clinical interviews
took place within 1-month (23.9 ± 8.2 days) after ED admission at our lab. After signing informed consent, trained clinicians from the study team assessed PTSD diagnosis and severity using the CAPS (see “Clinical assessments” subsection). Additionally, the interviewers re-assessed (in-person) the presence of previously unnoticed study exclusion criteria. Identical follow-up clinical interviews took place at 6- and 14-months post-trauma (T2 and T3).
MRI scans
for eligible participants took place around 1-month (30.4 ± 9.5 days) after ED admission at our lab (after the clinical interview). Several participants were excluded at this stage since they were not eligible for MRI scans (e.g., pacemakers, non MRI-compatible implants, permanent make-up, large tattoos). Identical follow-up scans took place at T2 and T3.
Statistical analysis
The study’s primary outcome measures were right and left hippocampus and amygdala volumes (mm3) at T1, T2, and T3. Secondary outcome measures were volumes (mm3) of specific subregions within the hippocampus (CA1, CA3, DG, and subiculum) and within the amygdala (LA, CeA, and BA nuclei). Differences in both primary and secondary outcome measures were measured between the two study groups (Remission vs. non-Remission; see “Participants” subsection) both at T1 and across all time points (T1, T2, and T3).
Bayesian multilevel modeling (BML) analysis
Inspired by the recent work of Limbachia et al. [58], we leveraged the strengths of Bayesian multilevel modeling (BML) [59,60,61] to estimate volumetric changes over time and initial differences between the two study groups (Remission vs. non-Remission). One of the strengths of BML is that it allows the simultaneous estimation of multiple parameters within a single model, hence there is no need to apply a correction for multiple comparisons [62]. Here, we used two models for the two study aims: (1) to assess initial volumetric differences (at T1) between the study groups (Remission vs. non-Remission; main effect of Group); (2) to test volumetric changes across time (T1 to T2 to T3), across all individuals (main effect of Time) or between study groups (Group-by-Time interaction). Separate models were further conducted for the analysis of the total volumes (right and left amygdala and hippocampus) and for the analysis of the hippocampus and amygdala subregions (see “Hippocampus and amygdala segmentations” subsection). To account for differences in brain sizes, we included total intracranial volume as a covariate in the former, and whole and whole hippocampus/amygdala as covariates for the latter. All models included covariates of participants’ age and gender, “Remission” group as the baseline, a random slope for each region of interest, and “Subjects” as a random intercept. Model specifications also included 40 K iterations, a 99% acceptance rate (i.e., adaptive delta), and a maximum tree depth of 15. We evaluated collinearity and computed the variance inflation factor associated with all variables. All statistical assumptions were similar to those of Limbachia et al. [58] and are included in our code (see “Code availability” section). We report evidence in terms of P+, the probability of the presence of an effect of interest, based on the posterior distribution (ranging from 0 to 1) [58]. While values closer to P+ = 1 provide evidence that the effect of interest is greater than zero (e.g., larger volume in non-Remission vs. Remission group), values closer to P+ = 0 convey support for a reverse effect (e.g., larger volume in the non-Remission vs. Remission group). As values are closer to P+ = 0.5, they provide stronger evidence for no-effect (e.g., similar volumes between groups). We treat Bayesian probability values as providing a continuous amount of support for a given hypothesis (not dichotomously as in “significant” vs. “not significant”). Nevertheless, in line with previous work [58], we used cut-off points of P+ > 0.85 and P+ < 0.15 as evidence for differences between the groups.
Results
Screening, enrollment, and attrition
Figure 2 summarizes participants’ identification, screening, and consecutive evaluations. A total of 4058 consecutive ED-admitted trauma survivors were contacted by phone within 14 days following traumatic exposure, given information about the study, and provided informed assent. Of those, n = 3476 individuals underwent initial screening in which they confirmed the occurrence of a psychologically traumatic event and related symptoms (i.e., “short interview”), and n = 1351 underwent eligibility assessment further assessing acute stress symptoms, suggestive of indicative of chronic PTSD risk [63] (i.e., “long interview”). A total of 435 individuals who met the inclusion criteria for this study, and did not meet any of the exclusion criteria (see “Participants” subsection), were invited for an in-person clinical interview within 1-month post-trauma (T1), and n = 300 attended these interviews. Overall, 171 participants who completed both clinical interviews and MRI scans at T1 were formally enrolled. In this work, we focus on the n = 126 who met PTSD diagnosis at T1 (excluding a group of n = 45 enrolled participants that did not meet PTSD diagnosis at T1), to specifically examine the differences between individuals who recover from initial PTSD diagnosis and those who develop the chronic disorder. Out of these 126 individuals, n = 101 and n = 100 attended identical clinical assessments and MRI scans at T2 and T3, respectively.
Evaluating sampling bias
The demographic and clinical characteristics of the studied sample and the two study groups (i.e., “Remission” and “non-Remission”) are presented in Table 1 (see also “Participants” subsection). By design, and towards obtaining a large-enough number of PTSD patients at T3, participants with more severe early PTSD symptoms were selected to participate in clinical assessments and MRI scans at T1. To evaluate, however, eventual sampling bias as to age, gender, and trauma type, we performed a series of comparisons between individuals included and those not included at each step (see Fig. 2) on variables known to contribute to PTSD risk. Among participants who underwent initial screening (“short interview”; n = 3476), those included in the eligibility assessments (“long interview”; n = 1351) did not differ from those screened and not included (n = 2707) in age (35.51 ± 11.91 vs. 35.59 ± 11.88 years, respectively), gender distribution (55.3% vs. 56.2% females), or trauma type (92.8% MVA’s vs. 92.6%) (all p values > 0.05). Similarly, trauma survivors invited for clinical interviewers at T1 (n = 435) did not differ from those who were not invited (n = 916) in age (34.11 ± 11.16 vs. 34.82 ± 12.01 years), gender distribution (50.9% vs. 51.5% females) or trauma type (89.3% vs. 90.1% MVA’s) (all p values > 0.05). Among 126 participants with a positive PTSD diagnosis enrolled at T1, n = 100 “study completers” did not differ from those who did not complete the study (n = 26) in age (32.97 ± 10.97 vs. 32.23 ± 9.77 years), gender distribution (56.0% vs. 54.5% females), or trauma type (89.0% vs. 86.4% MVA’s) (all p values > 0.05). Finally, among the 100 “study completers”, n = 29 individuals with PTSD diagnosis at T3 (non-Remission group) did not differ from n = 71 participants without PTSD diagnosis at T3 (Remission group) in age, gender, and trauma type (all p values > 0.05; see Table 1). These two groups differed in PTSD symptom severity at all three time-points (T1, T2, and T3), with the non-Remission group showing more severe symptoms compared to the Remission group (all p values < 0.05; see Table 1).
Initial volumetric differences between study groups
At T1, we found robust evidence for smaller right hippocampus volume in the “non-Remission” group (P+ of ~0.014), relative to the “Remission” group (Fig. 3a). There was also moderate evidence for larger left amygdala volume (P+ of ~0.870) in the non-Remission group. Subregion analysis further demonstrated strong evidence for a smaller volume of the right subiculum (P+ of ~0.031), right CA1 (P+ of ~0.046), and left subiculum (P+ of ~0.021) of the hippocampus, in the non-Remission compared with the Remission group (Fig. 3b). There was no evidence for volumetric differences between groups in any of the other examined subregions (Main Effect of Group: P+ of ~0.184–0.715; Fig. 3b).
Posterior distributions are presented for the left and right hippocampus and amygdala volumes (a) and for their subregions (b). The left vertical axis depicts the different regions/subregions while the right vertical axis depicts the P+ values. Values closer to P+ = 0 provide evidence for greater volume in the Remission group (Remission > non-Remission, left side of the horizontal axis), while values closer to P+ = 1 convey support for greater volume in the non-Remission group (non-Remission > Remission, right side of the horizontal axis). As values are closer to P+ = 0.5, they provide stronger evidence for similar volumes between groups (e.g., no effect). Posterior distributions are colored according to their P+ values: dark blue to turquoise (0.00 < P+ > 0.15), gray (0.15 < P+ > 0.85), or light yellow to dark red (0.85 < P+ > 1.00). CA cornu ammonis, DG dentate gyrus, LA lateral amygdala, CeA central amygdala, BA basal amygdala.
Volumetric changes over time across all individuals and between groups
Results of the longitudinal analysis (from T1 to T3) showed no evidence for time-dependent changes in the amygdala or hippocampus across all participants (main effect of Time: P+ of ~0.457–0.573; Fig. 4a), or between the two study groups (Time*Group interaction: P+ of ~0.403–0.457; Fig. 4c). Consistent with these results, we found no significant associations between changes in PTSD severity (CAPS-5 scores, T3–T1) and changes in the left or right amygdala or hippocampal volumes (T3–T1), while controlling for subjects’ age, gender and total intracranial volume (see Table S1). Subregion analysis further demonstrated no evidence for time-dependent changes across all participants (main effect of Time: P+ of ~0.446–0.726; Fig. 4b) or between groups (Time*Group interaction: P+ of ~0.360–0.485; Fig. 4d). Similar results were found for volumetric changes from T1 to T2 (see Supplementary Results and Fig. S2). For individual volumetric trajectories of the hippocampus and amygdala, please refer to Fig. S3.
Posterior distributions are presented for the left and right hippocampus and amygdala volumes (a, b), and for their subregions (c, d). The left vertical axis depicts the different regions/subregions while the right vertical axis depicts the P+ values. For the main effect of time (T1 to T3) across all 100 participants (a, b), P+ values closer to 0 provide evidence for a general decrease in volume (left side of the horizontal axis), while P+ values closer to 1 convey support for a general increase in volume (right side of the horizontal axis). As values are closer to P+ = 0.5, they provide stronger evidence of no change in volume across all individuals (i.e., no effect). For the group-by-time interaction (T1–T3) (c, d), P+ values closer to 0 or 1 provide evidence for different volume changes over time between groups (Remission > non-Remission on the left side of the horizontal axis or Remission < non-Remission on the right side of the horizontal axis). As values are closer to P+ = 0.5, they provide stronger evidence for no-effect (i.e., similar volume changes between groups). Posterior distributions are colored according to their P+ values: dark blue to turquoise (0.00 < P+ > 0.15), gray (0.15 < P+ > 0.85), or light yellow to dark red (0.85 < P+ > 1.00). CA cornu ammonis, DG dentate gyrus, LA lateral amygdala, CeA central amygdala, BA basal amygdala.
Discussion
To the best of our knowledge, this is the first longitudinal study of recent trauma to date with repeated clinical and structural MTI assessments at three distinct time points during the first year following trauma exposure [30], a critical period for PTSD development or recovery [27]. This design provided a unique opportunity to evaluate associations between structural changes and PTSD symptom trajectories in a large sample of trauma-exposed individuals with an initial PTSD diagnosis. Specifically, we were interested in whether volumetric alternations of the hippocampus and the amygdala, previously observed in PTSD patients, represent early (possibly predisposing) risk factors for the disorder’s development or rather acquired changes in individuals who develop PTSD following traumatic exposure.
Early risk factors for PTSD development
We found robust evidence that smaller right hippocampus volume at T1 was associated with a “non-remitting PTSD” trajectory, compared with a “remission” trajectory. We further provide moderate evidence for a larger left amygdala volume in the non-Remission (vs. Remission) group. Together with previous evidence from twin studies [10, 11], these findings suggest that hippocampal and amygdala volumes might serve as risk factors for PTSD development, supporting the “vulnerability trait” hypothesis. These initial volumetric differences may arise from genetic factors [64], environmental factors (e.g., childhood trauma [65], early life stress [66]), and the interactions between them.
Furthermore, examining specific hippocampus and amygdala subregions that were previously implicated in PTSD literature, we demonstrate that initially reduced volumes of both left and right subiculum and right CA1 hippocampal subregions characterized individuals with non-remitting PTSD. The subiculum, a primary output region of the hippocampus, plays a role in contextual fear-conditioning [67, 68] and regulation of the stress response [69, 70], two central and impaired processes in PTSD. Future work should further investigate the structure and function of the subiculum in PTSD, given the importance of this region in the regulation of the HPA axis and dopaminergic responses to stress [71, 72]. With regard to the CA1 of the hippocampus, this subregion is critically involved in the formation, consolidation, and retrieval of context-dependent memories [73]. Accordingly, few cross-sectional studies reported smaller CA1 volumes in PTSD patients [25, 74]. Interestingly, recent work showed that a neural pathway starting from the CA1 to the dorsal subiculum to the entorhinal cortex is essential for memory retrieval [75]. Altogether, these results support the role of specific hippocampal subregions in the maladaptive processing of the traumatic event.
These results are in line with laterality effects previously reported in PTSD literature [51, 52]. While we found evidence for both smaller right and left hippocampus volume in the PTSD group, the effect of the right side was more robust (right: P+ of ~0.014; left: P+ of ~0.169; Fig. 3a), in line with a meta-analysis on hippocampal volume deficits in PTSD [51]. While both right and left amygdala volumes were larger in the PTSD group, the effect of the left side was more robust (right: P+ of ~0.669; left: P+ of ~0.870; Fig. 3a), consistent with two recent studies [28, 76]. Regarding the subregions, we found robust evidence for the effects of both left and right subiculum, but only for the right (but not left) CA1. Future studies should further investigate if the amygdala-hippocampal circuitry is indeed more apparent in one hemisphere over the other.
Acquired changes during the first year following trauma exposure
Our results provide strong evidence for the stability of the hippocampus and amygdala volumes during the first 14 months following trauma exposure (T1 to T2 to T3) across all individuals (n = 100). Furthermore, we found no significant change in any of the hippocampal or amygdala subregions, regardless of individuals’ clinical trajectory (e.g., whether they developed PTSD or showed remission from initial symptoms). While recent work showed that playing Tetris video game was associated with an increased hippocampal volume of PTSD patients within a 6-week period [77], other longitudinal PTSD studies report no volumetric change for longer time periods, in the amygdala and hippocampus [16,17,18], or in their subregions [28, 29].
Interestingly, a study of earthquake trauma survivors found no change in hippocampal gray matter density from pre-trauma to 3–4 months post-trauma in survivors who did not develop PTSD [78]; However, a significant decrease in hippocampal gray matter density appeared in these survivors 1-year post-trauma [79], suggesting that volumetric changes in the hippocampus may develop slowly and gradually over the year following trauma. Together with our results, it is possible that persistent stress symptoms in PTSD cause gradual hippocampal volume reduction over longer time periods (e.g., more than a year following trauma) [17].
Strengths and limitations
First, as this study did not obtain volumetric measurements before the traumatic event, we used 1-month hippocampus and amygdala volumes as a proxy of pre-trauma volumes. Nevertheless, most studies to date which include pre-trauma MRI measures examined specific sub-populations at high risk for trauma exposure (e.g., soldiers [13], police recruits [28]). As these often involve resilient and predominantly male samples, most subjects show only sub-clinical PTSD symptoms [13, 28], limiting their generalizability to clinical populations (i.e., PTSD patients). Second, longer follow-up durations may reveal further differences between chronic PTSD and recovered trauma survivors. Nevertheless, 14 months post-trauma is the longest follow-up duration reported to date, and a clinically stable time point by which over 90% of recovery from PTSD is expected [27]. Another limitation concerns the inclusion of only participants with initial PTSD diagnosis (at T1). This was dictated by the need to secure a large enough number of PTSD patients at T3 (n = 29), and the understanding that those with initial symptoms are the focus of clinical attention and indicated interventions. Our sample was also limited by trauma type, including mostly MVA’s (89% at T1; see Table 1). The results presented here require replications in less selective samples, including individuals with subthreshold PTSD and varying traumatic events [80, 81] (e.g., terror attacks, sexual assaults). Finally, we did not collect data on participants’ previous trauma exposures and have only partial data on individuals’ treatments between study visits.
Within these limitations, however, both sampling and retention biases in this work were reasonably good. Therefore, individuals included in this work fairly well represent the general population admitted to the ED following exposure in terms of age, gender, and trauma type. Furthermore, individuals enrolled in this study and lost to follow-up (n = 26) did not significantly differ from those who remained and completed the study (n = 100). Participants were screened for early PTSD symptoms to enrich the sample and increase the likelihood of endpoint PTSD. Finally, given that PTSD diagnosis varies upon DSM taxonomic classification and is frequently criticized [82, 83], here we optimized participants’ clinical characterization by combining two “gold standard” structured instruments capturing both DSM-IV and DSM-5 PTSD definitions. Together with the long follow-up of 14-months post-trauma, indicative of the clinical course of PTSD development [27, 84], our results are likely to be generalized to adult civilians admitted to the ED following trauma exposure, and can safely guide further explorations. Methodological strengths of this work include higher-resolution structural T1-weighted images (0.7 mm3 compared to the most common 1 mm3 resolution [85]) and the use of the longitudinal analysis pipeline (reducing the confounding effect of inter-individual variability) [48]. Importantly, Wisse et al. [85] cautioned against using hippocampal subfield volumetry from 1 mm3 resolution images, and recommended the use of higher-resolution MRI scans.
Conclusion
This work supports the role of volumetric abnormalities in specific hippocampal subregions as early vulnerability traits associated with non-recovery from acute PTSD. It further demonstrates stable volumes of hippocampus and amygdala subregions during the first 14 months following trauma exposure, thus not supporting the idea of progressive, stress-related atrophy of these regions in the critical first-year post-trauma.
Data availability
The dataset and materials used in this work is available by contacting the study PIs on a reasonable request.
Code availability
Scripts to reproduce data analysis associated with the current manuscript can be found at: https://github.com/KoremNSN/longitudinal_anatomy.
References
Liberzon I, Abelson JL. Context processing and the neurobiology of post-traumatic stress disorder. Neuron. 2016;92:14–30. https://www.sciencedirect.com/science/article/pii/S0896627316306407.
Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35.
LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7:54–64. https://www.nature.com/articles/nrn1825.
van Rooij SJH, Ravi M, Ely TD, Michopoulos V, Winters SJ, Shin J, et al. Hippocampal activation during contextual fear inhibition related to resilience in the early aftermath of trauma. Behav Brain Res. 2021;408:113282.
O’Doherty DCM, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res Neuroimaging. 2015;232:1–33.
Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–87. http://www.nature.com/articles/nrn3339.
Ben-Zion Z, Artzi M, Niry D, Keynan NJ, Zeevi Y, Admon R, et al. Neuroanatomical risk factors for posttraumatic stress disorder in recent trauma survivors. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:311–9. http://biorxiv.org/content/early/2019/08/01/721134.abstract.
Kuo JR, Kaloupek DG, Woodward SH. Amygdala volume in combat-exposed veterans with and without posttraumatic stress disorder: a cross-sectional study. Arch Gen Psychiatry. 2012;69:1080–6. https://jamanetwork.com/journals/jamapsychiatry/fullarticle/1370685.
Bromis K, Calem M, Reinders AATS, Williams SCR, Kempton MJ. Meta-analysis of 89 structural MRI studies in posttraumatic stress disorder and comparison with major depressive disorder. Am J Psychiatry. 2018;175:989–98. http://ajp.psychiatryonline.org/doi/10.1176/appi.ajp.2018.17111199.
Kremen WS, Koenen KC, Afari N, Lyons MJ. Twin studies of posttraumatic stress disorder: differentiating vulnerability factors from sequelae. Neuropharmacology. 2012;62:647–53.
Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–7. https://www.nature.com/articles/nn958.
Bremner JD, Hoffman M, Afzal N, Cheema FA, Novik O, Ashraf A, et al. The environment contributes more than genetics to smaller hippocampal volume in Posttraumatic Stress Disorder (PTSD). J Psychiatr Res. 2021;137:579–88.
Admon R, Leykin D, Lubin G, Engert V, Andrews J, Pruessner J, et al. Stress-induced reduction in hippocampal volume and connectivity with the ventromedial prefrontal cortex are related to maladaptive responses to stressful military service. Hum Brain Mapp. 2013;34:2808–16. http://doi.wiley.com/10.1002/hbm.22100.
Admon R, Milad MR, Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci. 2013;17:337–47. https://www.sciencedirect.com/science/article/pii/S1364661313001046.
Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, et al. Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: Subcortical Volumetry Results from Posttraumatic Stress Disorder Consortia. Biol Psychiatry. 2018;83:244–53. https://pubmed.ncbi.nlm.nih.gov/29217296/.
Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, et al. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry. 2001;158:1248–51. http://psychiatryonline.org/doi/abs/10.1176/appi.ajp.158.8.1248.
Xie H, Claycomb Erwin M, Elhai JD, Wall JT, Tamburrino MB, Brickman KR, et al. Relationship of hippocampal volumes and posttraumatic stress disorder symptoms over early posttrauma periods. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:968–75.
Quidé Y, Andersson F, Dufour-Rainfray D, Descriaud C, Brizard B, Gissot V, et al. Smaller hippocampal volume following sexual assault in women is associated with post-traumatic stress disorder. Acta Psychiatr Scand. 2018;138:312–24.
Mulder ER, de Jong RA, Knol DL, van Schijndel RA, Cover KS, Visser PJ, et al. Hippocampal volume change measurement: quantitative assessment of the reproducibility of expert manual outlining and the automated methods FreeSurfer and FIRST. Neuroimage. 2014;92:169–81.
Wisse LEM, Biessels GJ, Geerlings MI. A critical appraisal of the hippocampal subfield segmentation package in FreeSurfer. Front Aging Neurosci. 2014;6:261.
Amaral OB, Luft T, Cammarota M, Izquierdo I, Roesler R. Temporary inactivation of the dorsal hippocampus induces a transient impairment in retrieval of aversive memory. Behavioural Brain Res. 2007;180:113–8.
Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44:581–4. https://pubmed.ncbi.nlm.nih.gov/15541306/.
Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–33. https://www.nature.com/articles/nrn2651.
Zhang L, Lu L, Bu X, Li H, Tang S, Gao Y, et al. Alterations in hippocampal subfield and amygdala subregion volumes in posttraumatic subjects with and without posttraumatic stress disorder. Hum Brain Mapp. 2021;42:2147–58. https://onlinelibrary.wiley.com/doi/full/10.1002/hbm.25356.
Chen LW, Sun D, Davis SL, Haswell CC, Dennis EL, Swanson CA, et al. Smaller hippocampal CA1 subfield volume in posttraumatic stress disorder. Depress Anxiety. 2018;35:1018–29. https://onlinelibrary.wiley.com/doi/abs/10.1002/da.22833?casa_token=iaYLPypZkKoAAAAA:eU9NURQY7wo6wogd9ZhQ6KMw7fwWPOIhlT2OTgTAHewFUuX6uMYtepmQcmIMUly5ZmoMYua8wiXgfw.
Morey RA, Clarke EK, Haswell CC, Phillips RD, Clausen AN, Mufford MS, et al. Amygdala nuclei volume and shape in military veterans with posttraumatic stress disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:281–90. https://pubmed.ncbi.nlm.nih.gov/32029420/.
Shalev AY, Freedman S. PTSD following terrorist attacks: a prospective evaluation. Am J Psychiatry. 2005;162:1188–91. http://www.ncbi.nlm.nih.gov/pubmed/15930068.
Koch SBJ, van Ast VA, Kaldewaij R, Hashemi MM, Zhang W, Klumpers F, et al. Larger dentate gyrus volume as predisposing resilience factor for the development of trauma-related symptoms. Neuropsychopharmacology. 2021;46:1283–92. https://www.nature.com/articles/s41386-020-00947-7.
Weis CN, Webb EK, Huggins AA, Kallenbach M, Miskovich TA, Fitzgerald JM, et al. Stability of hippocampal subfield volumes after trauma and relationship to development of PTSD symptoms. Neuroimage. 2021;236:118076.
Ben-Zion Z, Fine NB, Keynan NJ, Admon R, Halpern P, Liberzon I, et al. Neurobehavioral moderators of post-traumatic stress disorder (PTSD) trajectories: study protocol of a prospective MRI study of recent trauma survivors. Eur J Psychotraumatol. 2019;10:1683941. https://doi.org/10.1080/20008198.2019.1683941.
Hayes JP, Hayes S, Miller DR, Lafleche G, Logue MW, Verfaellie M. Automated measurement of hippocampal subfields in PTSD: Evidence for smaller dentate gyrus volume. J Psychiatr Res Pergamon. 2017;95:247–52.
Szeszko PR, Bierer LM, Bader HN, Chu K-W, Tang CY, Murphy KM, et al. Cingulate and hippocampal subregion abnormalities in combat-exposed veterans with PTSD. J Affect Disord. 2022;311:432–9.
Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry. 2010;67:296–303. https://jamanetwork.com/journals/jamapsychiatry/fullarticle/210641.
Veer IM, Oei NYL, van Buchem MA, Spinhoven P, Elzinga BM, Rombouts SARB. Evidence for smaller right amygdala volumes in posttraumatic stress disorder following childhood trauma. Psychiatry Res Neuroimaging. 2015;233:436–42.
Akiki TJ, Averill CL, Wrocklage KM, Schweinsburg B, Scott JC, Martini B, et al. The association of PTSD symptom severity with localized hippocampus and amygdala abnormalities. Chronic Stress. 2017;1. https://pubmed.ncbi.nlm.nih.gov/28825050/.
Ben-Zion Z, Shany O, Admon R, Keynan NJ, Avisdris N, Balter SR, et al. Neural responsivity to reward versus punishment shortly after trauma predicts long-term development of posttraumatic stress symptoms. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022;7:150–61. https://pubmed.ncbi.nlm.nih.gov/34534702/.
Schultebraucks K, Ben-Zion Z, Admon R, Keynan JN, Liberzon I, Hendler T, et al. Assessment of early neurocognitive functioning increases the accuracy of predicting chronic PTSD risk. Mol Psychiatry. 2022. https://www.nature.com/articles/s41380-022-01445-6.
Ben-Zion Z, Zeevi Y, Keynan NJ, Admon R, Kozlovski T, Sharon H, et al. Multi-domain potential biomarkers for post-traumatic stress disorder (PTSD) severity in recent trauma survivors. Transl Psychiatry. 2020;10:1–11. https://doi.org/10.1038/s41398-020-00898-z.
Hoge CW, Yehuda R, Castro CA, McFarlane AC, Vermetten E, Jetly R, et al. Unintended consequences of changing the definition of posttraumatic stress disorder INDSM-5 critique and call for action. JAMA Psychiatry. 2016;73:750–2. https://jamanetwork.com/journals/jamapsychiatry/article-abstract/2524846.
Hoge CW, Riviere LA, Wilk JE, Herrell RK, Weathers FW. The prevalence of post-traumatic stress disorder (PTSD) in US combat soldiers: a head-to-head comparison of DSM-5 versus DSM-IV-TR symptom criteria with the PTSD checklist. Lancet Psychiatry. 2014;1:269–77. https://www.sciencedirect.com/science/article/pii/S2215036614702354.
Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. http://www.springerlink.com/index/10.1007/BF02105408.
Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, et al. The Clinician-Administered PTSD Scale for DSM–5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30:383–95.
Jackson CE, Currao A, Fonda JR, Kenna A, Milberg WP, McGlinchey RE, et al. Research utility of a CAPS-IV and CAPS-5 hybrid interview: posttraumatic stress symptom and diagnostic concordance in recent-era U.S. veterans. J Trauma Stress. 2021. https://pubmed.ncbi.nlm.nih.gov/34973042/.
Stein DJ, McLaughlin KA, Koenen KC, Atwoli L, Friedman MJ, Hill ED, et al. DSM-5 and ICD-11 definitions of posttraumatic stress disorder: Investigating “narrow” and “broad” approaches. Depress Anxiety. 2014;31:494–505. http://www.ncbi.nlm.nih.gov/pubmed/24894802.
Weathers FW, Ruscio AM, Keane TM. Psychometric properties of nine scoring rules for the clinician- administered posttraumatic stress disorder scale. Psychol Assess. 1999;11:124–33.
Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–18.
Fischl B. FreeSurfer. Neuroimage. 2012;62:774–81.
Iglesias JE, van Leemput K, Augustinack J, Insausti R, Fischl B, Reuter M. Bayesian longitudinal segmentation of hippocampal substructures in brain MRI using subject-specific atlases. Neuroimage. 2016;141:542–55.
Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–37.
Saygin ZM, Kliemann D, Iglesias JE, van der Kouwe AJW, Boyd E, Reuter M, et al. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. Neuroimage. 2017;155:370–82.
Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1181–8. https://pubmed.ncbi.nlm.nih.gov/20600466/.
Nelson MD, Tumpap AM. Posttraumatic stress disorder symptom severity is associated with left hippocampal volume reduction: a meta-analytic study. CNS Spectr. 2017;22:363–72. https://pubmed.ncbi.nlm.nih.gov/27989265/.
Tae WS, Kim SS, Lee KU, Nam EC, Kim KW. Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology. 2008;50:569–81. http://springerlink.bibliotecabuap.elogim.com/10.1007/s00234-008-0383-9.
Sánchez-Benavides G, Gómez-Ansón B, Sainz A, Vives Y, Delfino M, Peña-Casanova J. Manual validation of FreeSurfer’s automated hippocampal segmentation in normal aging, mild cognitive impairment, and Alzheimer Disease subjects. Psychiatry Res Neuroimaging. 2010;181:219–25. https://www.sciencedirect.com/science/article/pii/S0925492709002443.
Wenger E, Mårtensson J, Noack H, Bodammer NC, Kühn S, Schaefer S, et al. Comparing manual and automatic segmentation of hippocampal volumes: reliability and validity issues in younger and older brains. Hum Brain Mapp. 2014;35:4236–48. https://onlinelibrary.wiley.com/doi/abs/10.1002/hbm.22473.
Schoemaker D, Buss C, Head K, Sandman CA, Davis EP, Chakravarty MM, et al. Hippocampus and amygdala volumes from magnetic resonance images in children: assessing accuracy of FreeSurfer and FSL against manual segmentation. Neuroimage. 2016;129:1–14.
Weathers FW, Huska JA, Keane TM. The PTSD Checklist-Civilian Version (PCL-C). Boston, MA: National Center for PTSD; 1991, 1994.
Limbachia C, Morrow K, Khibovska A, Meyer C, Padmala S, Pessoa L. Controllability over stressor decreases responses in key threat-related brain areas. Commun Biol. 2021;4:1–11.
Chen G, Xiao Y, Taylor PA, Rajendra JK, Riggins T, Geng F, et al. Handling multiplicity in neuroimaging through Bayesian lenses with multilevel modeling. Neuroinformatics. 2019;17:515–45.
McElreath R. Statistical rethinking: a Bayesian course with examples in R and Stan. New York: Chapman and Hall/CRC; 2018. p. 1–469.
Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. Cambridge: Cambridge University Press; 2006.
Gelman A, Hill J, Yajima M. Why we (usually) don’t have to worry about multiple comparisons. J Res Educ Eff. 2012;5:189–211. https://www.tandfonline.com/doi/abs/10.1080/19345747.2011.618213?casa_token=Me9Li5bNQ0cAAAAA:1MjfztU3T9JEWjFQRtlbCEG75yeLOsdH0SidrcOuhinuipX2HjM7vnuPhnJTR7VtaQvU9S_jt62_9w.
Shalev AY, Ankri Y, Israeli-Shalev Y, Peleg T, Adessky R, Freedman S. Prevention of posttraumatic stress disorder by early treatment: results from the Jerusalem trauma outreach and prevention study. Arch Gen Psychiatry. 2012;69:166–76. http://www.ncbi.nlm.nih.gov/pubmed/21969418.
Szeszko PR, Lipsky R, Mentschel C, Robinson D, Gunduz-Bruce H, Sevy S, et al. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry. 2005;10:631–6.
Samplin E, Ikuta T, Malhotra AK, Szeszko PR, DeRosse P. Sex differences in resilience to childhood maltreatment: effects of trauma history on hippocampal volume, general cognition and subclinical psychosis in healthy adults. J Psychiatr Res. 2013;47:1174–9. https://pubmed.ncbi.nlm.nih.gov/23726669/.
Humphreys KL, King LS, Sacchet MD, Camacho MC, Colich NL, Ordaz SJ, et al. Evidence for a sensitive period in the effects of early life stress on hippocampal volume. Dev Sci. 2019;22:e12775. https://onlinelibrary.wiley.com/doi/full/10.1111/desc.12775.
de Melo MB, Favaro VM, Oliveira MGM. The dorsal subiculum is required for contextual fear conditioning consolidation in rats. Behav Brain Res. 2020;390:112661.
Maren S. Neurotoxic or electrolytic lesions of the ventral subiculum produce deficits in the acquisition and expression of Pavlovian fear conditioning in rats. Behav Neurosci. 1999;113:283–90. https://pubmed.ncbi.nlm.nih.gov/10357453/.
O’Mara S. Controlling hippocampal output: the central role of subiculum in hippocampal information processing. Behav Brain Res. 2006;174:304–12.
Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–24.
Radley JJ, Sawchenko PE. A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J Neurosci. 2011;31:9683–95. https://pubmed.ncbi.nlm.nih.gov/21715634/.
Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus,and subiculum. Proc Natl Acad Sci USA. 2012;109. www.pnas.org/cgi/doi/10.1073/pnas.1115396109.
Bartsch T, Döhring J, Rohr A, Jansen O, Deuschl G. CA1 neurons in the human hippocampus are critical for autobiographical memory, mental time travel, and autonoetic consciousness. Proc Natl Acad Sci USA. 2011;108:17562–7.
Postel C, Mary A, Dayan J, Fraisse F, Vallée T, Guillery-Girard B, et al. Variations in response to trauma and hippocampal subfield changes. Neurobiol Stress. 2021;15:100346.
Roy DS, Kitamura T, Okuyama T, Ogawa SK, Sun C, Obata Y, et al. Distinct neural circuits for the formation and retrieval of episodic memories. Cell. 2017;170:1000–12.e19. http://www.cell.com/article/S0092867417308206/fulltext.
van Rooij SJH, Smith RD, Stenson AF, Ely TD, Yang X, Tottenham N, et al. Increased activation of the fear neurocircuitry in children exposed to violence. Depress Anxiety. 2020;37:303.
Butler O, Herr K, Willmund G, Gallinat J, Kuhn S, Zimmermann P. Trauma, treatment and Tetris: video gaming increases hippocampal volume in male patients with combat-related posttraumatic stress disorder. J Psychiatry Neurosci. 2020;45:279–87. https://www.jpn.ca/content/45/4/279.
Sekiguchi A, Sugiura M, Taki Y, Kotozaki Y, Nouchi R, Takeuchi H, et al. Brain structural changes as vulnerability factors and acquired signs of post-earthquake stress. Mol Psychiatry. 2013;18:618–23. https://pubmed.ncbi.nlm.nih.gov/22614289/.
Sekiguchi A, Kotozaki Y, Sugiura M, Nouchi R, Takeuchi H, Hanawa S, et al. Resilience after 3/11: structural brain changes 1 year after the Japanese earthquake. Mol Psychiatry. 2014;20:553–4. https://www.nature.com/articles/mp201428.
Liu H, Petukhova MV, Sampson NA, Aguilar-Gaxiola S, Alonso J, Andrade LH, et al. Association of DSM-IV posttraumatic stress disorder with traumatic experience type and history in the World Health Organization World Mental Health Surveys. JAMA Psychiatry. 2017;74:270–81. https://jamanetwork.com/journals/jamapsychiatry/fullarticle/2595039.
Hinojosa CA. Does hippocampal volume in patients with posttraumatic stress disorder vary by trauma type? Harv Rev Psychiatry. 2022;30:118. https://journals.lww.com/hrpjournal/Fulltext/2022/03000/Does_Hippocampal_Volume_in_Patients_with.3.aspx.
Galatzer-Levy IR, Bryant RA. 636,120 ways to have posttraumatic stress disorder. Perspect Psychol Sci. 2013;8:651–62.
Zoellner LA, Bedard-Gilligan MA, Jun JJ, Marks LH, Garcia NM. The evolving construct of posttraumatic stress disorder (PTSD): DSM-5 criteria changes and legal implications. Psychol Inj Law. 2013;6:277–89. http://springerlink.bibliotecabuap.elogim.com/10.1007/s12207-013-9175-6.
Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry. 1995;52:1048–60. http://archpsyc.jamanetwork.com/article.aspx?doi=10.1001/archpsyc.1995.03950240066012.
Wisse LEM, Chételat G, Daugherty AM, de Flores R, la Joie R, Mueller SG, et al. Hippocampal subfield volumetry from structural isotropic 1 mm3 MRI scans: a note of caution. Hum Brain Mapp. 2021;42:539–50. https://onlinelibrary.wiley.com/doi/full/10.1002/hbm.25234.
Acknowledgements
The authors would like to thank our wonderful research team at Tel-Aviv Sourasky Medical Center—including Naomi Fine, Nili Green, Mor Halevi, Sheli Luvton, Yael Shavit, Olga Nevenchannaya, Iris Rashap, Efrat Routledge, and Ophir Leshets—for their significant contributions to participants, screening, enrollment, assessments, and follow-up. We extend our gratitude to all the participants of this study, who completed all the assessments at three different time points after experiencing a traumatic event, thus contributing to scientific research on post-traumatic psychopathology.
Funding
This work was supported by award number R01-MH-103287 from the National Institute of Mental Health (NIMH) given to AYS (PI), IL, and TH (co-Investigators, subcontractors), and had undergone critical review by the NIMH Adult Psychopathology and Disorders of Aging study section.
Author information
Authors and Affiliations
Contributions
TH, IL, and AYS designed, obtained funding, supervised, and oversaw the implementation of the study. ZB-Z, AYS, and TH had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. ZB-Z, JNK, and RA carried out the procedural aspects of the study, the research assistants’ training, guidance and monitoring, management of participants, and QA of data. ZB-Z, NK, OD, and TRS analyzed the results and drafted the initial manuscript together with IH-R, AYS, and TH. Critical revisions of the manuscript were conducted by NK, OD, TRS, JNK, RA, IH-R, IL, AYS, and TH, until finalizing it. All authors have read and approved the final manuscript and its submission to “Molecular Psychiatry”.
Corresponding author
Ethics declarations
Competing interests
TH is the chief medical officer of “GrayMatters Health Co” (Haifa, Israel). All other authors report no potential conflicts of interest to declare.
Ethical approval
The research study met all ethical regulations as required by the ethics committee at Tel-Aviv Sourasky Medical Center (Reference number 0207/14). All subjects gave written informed consent in accordance with the Declaration of Helsinki and received financial remuneration at the end of each time-point (1-, 6-, and 14-months post-trauma). The study’s ClinicalTrials.gov registration ID is NCT03756545 (clinicaltrials.gov/ct2/show/NCT03756545).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ben-Zion, Z., Korem, N., Spiller, T.R. et al. Longitudinal volumetric evaluation of hippocampus and amygdala subregions in recent trauma survivors. Mol Psychiatry 28, 657–667 (2023). https://doi.org/10.1038/s41380-022-01842-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01842-x
- Springer Nature Limited