Abstract
There is a need to improve the understanding of the neurobiological underpinnings of traumatic brain injury (TBI). Individuals with TBI experience comorbidities such as posttraumatic stress disorder (PTSD) with considerable symptom overlap including depression and hyperarousal, confounding the ability to identify specific TBI-related brain changes. The aims of the current study were to investigate hippocampal and amygdalar volumes in Veterans with TBI with (TBI + PTSD, n = 32) and without (TBI - PTSD, n = 25) PTSD. Shape analysis was employed to reveal any relationship between the hippocampus and depressive symptoms in TBI subgroups. 32 TBI + PTSD, 25 TBI - PTSD, and 25 age-matched healthy male Veterans underwent an MRI scan on a 3 Tesla scanner and a clinical evaluation. The TBI + PTSD and the TBI + hyperarousal (met criteria for the hyperarousal symptom cluster, regardless of PTSD diagnosis) subgroup had trend-level larger left amygdalar volume than the TBI - PTSD subgroup and the TBI - hyperarousal subgroup, respectively. However, there was no significant difference between the TBI group as a whole and healthy controls (HC). There was a significant negative correlation between the Hamilton Rating Scale for Depression score (HAM-D) and left hippocampal volume and a positive correlation between the HAM-D score and left amygdalar volume in the TBI group. Left hippocampal volume was correlated with the HAM-D score only in the TBI + PTSD and not in TBI - PTSD subgroup. Shape analyses revealed a significant correlation between the HAM-D score and the CA1 and subiculum regions of the left hippocampus. Our results suggest that the amygdala may be a neuroanatomical correlate in mediating PTSD-like symptoms in Veterans with TBI. The results of shape analysis suggest that alterations in the CA1 and subiculum subregions of hippocampus may have a role in depression and PTSD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability worldwide, with serious economic consequences. It has been estimated that over 10 million people are affected by TBI globally (Faul et al. 2010). Estimates suggest that 352,619 military personnel have sustained TBIs worldwide since 2000 with mild traumatic brain injury (mTBI) accounting for approximately 70–80% of all TBI among returning Veterans (Hoge et al. 2008) (Defense and Center 2012). Moreover, many Veterans with TBI also experience comorbid psychiatric disorders, including posttraumatic stress disorder (PTSD), creating challenges in diagnosis and treatment (Kaplan et al. 2018; Stein and McAllister 2009; Schneiderman et al. 2008). The prevalence of PTSD ranges from 33% to 39% in US military personnel with mTBI, which is twice as high as prevalence rates in Veterans without TBI (Carlson et al. 2011). Furthermore, there is significant overlap in symptoms of PTSD and TBI that include but are not limited to depression and hyperarousal (Stein and McAllister 2009). It has been hypothesized that TBI and PTSD result from a variety of pathophysiological mechanisms resulting in dysfunction in overlapping neural circuits leading to some common symptomology (Kaplan et al. 2018). However, it is possible that some neural underpinnings are unique to TBI and not observed in PTSD. Therefore, it is important to consider the comorbidity of PTSD in an imaging study of a TBI cohort, which may potentially advance our understanding of whether comorbid TBI and PTSD is a unique illness with a distinct etiology or neurobiological signature that differentiates it from TBI without PTSD.
Research has well established that individuals with TBI have widespread structural and functional brain alterations (Shenton et al. 2012; Ross 2011; McDonald et al. 2012; Van Boven et al. 2009). Subcortical regions including the hippocampus, amygdala, pallidum and thalamus have shown volumetric changes in individuals with TBI (Anderson et al. 1996; Bigler et al. 2010; Maller et al. 2014). Importantly, several studies have noted the influence of subcortically-mediated functions on subjective complaints. Prior research shows that the hippocampus plays a crucial role in learning and memory, spatial navigation, contextual fear-conditioning and neuroendocrine regulation and is vulnerable to TBI (Duvernoy 2005). Specifically, hippocampal volume reduction has been observed in TBI patients (Arciniegas et al. 2001; Bigler et al. 2002; Tomaiuolo et al. 2004; Ariza et al. 2006; Warner et al. 2010; Palacios et al. 2013). Further, hippocampal volume was associated with injury severity (Tate and Bigler 2000) and neuropsychological functions (Bigler et al. 1997; Tate and Bigler 2000). However, some studies did not report a significant relationship between hippocampal volumes and the clinical symptoms of TBI such as memory problems or TBI severity (Arciniegas et al. 2001; Ariza et al. 2006; Warner et al. 2010). Shape analysis, a method capable of detecting subtle volumetric changes that are often not revealed in traditional volumetric analyses (Wade et al. 2015; Kim et al. 2015), has previously shown deformations of the tail and head of the left hippocampus in TBI. However, the authors found no relationship between hippocampal morphology and clinical and cognitive variables (Palacios et al. 2013).
Another subcortical region that may influence emotional and cognitive symptoms related to TBI is the amygdala. The amygdala is essential for emotional processing and is associated with emotional memory, regulation and tone (Calder et al. 2001; LeDoux 2000; Davis and Whalen 2001). Amygdala volume reduction in adult patients with TBI has been previously reported (Warner et al. 2010). Two recent studies have shown amygdalar shape alterations in Veterans with TBI compared to Veterans with orthopedic injuries (Tate et al. 2016; Tate et al. 2018). Importantly, time since injury was positively correlated with radial distance of the right anterior amygdala, suggesting increased amygdala thickness in participants with TBI who were further removed from their injury (Tate et al. 2016). Surface features of the amygdala were significantly associated with verbal memory performance, suggesting that amygdalar shape alterations may influence the neurocognitive symptoms of TBI (Tate et al. 2018).
One of the major obstacles in understanding and effectively treating TBI in Veterans is the presence of co-morbidities such as PTSD. For example, in a large study of active duty Marine and Navy servicepersons who served in Operation Iraqi Freedom (OIF) or Operation Enduring Freedom (OEF), TBI doubled the likelihood of post-deployment PTSD symptoms (Yurgil et al. 2014). The comorbidity of TBI and PTSD has been associated with increased rates of neuropsychiatric complications that may prolong recovery, suggesting that the neurobiological mechanisms underlying the comorbid condition may be distinct or more severe than mechanisms underlying either disorder alone (Vanderploeg et al. 2009; Zatzick et al. 2010). Given the prevalence of comorbid TBI and PTSD, it is important to conduct TBI research in Veterans who have comorbid PTSD in order to better understand the underlying neural anatomical correlates, which may lead to better therapeutic targets for this devastating comorbid condition.
We performed the current study to investigate volume alterations in the hippocampus and the amygdala in Veterans with TBI, further divided according to PTSD diagnosis and symptoms. To detect subtle volume changes, we employed shape analysis, a method that uses geometric modeling of the three-dimensional surfaces of the volumetric data to examine the expansions and contractions along the entire surface of a region of interest (ROI) (Bolzenius et al. 2018). Shape analysis was implemented to assess the relationships between clinical measures and the shape of subcortical structures including the hippocampus and amygdala. The aims of this study were (1) to compare the volume of the hippocampus and the amygdala between the HC and TBI groups, (2) to investigate whether there was any volumetric difference in the hippocampus and amygdala between the TBI with PTSD (TBI + PTSD) and TBI without PTSD (TBI - PTSD) subgroups; or between the TBI subgroups depending on presence of depressive and hyperarousal symptoms, (3) to identify whether clinical factors were related to shape and volume measures of the hippocampus and amygdala in the TBI group. In addition to overall PTSD diagnosis, we focused on the individual symptom cluster of hyperarousal given its presence in both TBI and PTSD in order to capture potentially important heterogeneity in neurobiological alterations related to this phenotype.
Methods
Participants
The Institutional Review Boards at the University of Utah and the George E. Wahlen Department of Veterans Affairs (VA) Medical Center approved this study. All participants provided written informed consent prior to participation in this study. Veterans with TBI and HCs were recruited from the George E. Wahlen VA Medical Center and the community via local advertisements and by word of mouth. Participants in the current study were between the ages of 21 and 54 years. Structural magnetic resonance imaging (MRI) data and clinical measurements were acquired from 25 Veterans with TBI - PTSD, 32 Veterans with TBI + PTSD, and 25 HCs. All participants completed the Ohio State University-TBI Identification Method (OSU-TBI) (Corrigan and Bogner 2007) to quantify the presence, number, and severity of lifetime TBI injuries, and the Structured Clinical Interview (SCID-P) (First et al. 2002) to establish DSM IV Axis I diagnoses including lifetime PTSD. Severity of TBI was assessed according to parameters described by Belanger and colleagues (Belanger et al. 2009). Veterans were classified as having mild TBI (mTBI) if they reported a head injury with an alteration of consciousness (AOC) up to 24 h or loss of consciousness (LOC) of 0 to 30 min. Moderate TBI was defined as an injury event with AOC between 24 h and 7 days or LOC between 30 min and 24 h. Severe TBI was defined as AOC greater than 7 days or LOC greater than 24 h. Exclusion criteria for all participants included major sensorimotor handicaps (e.g., deafness, blindness, paralysis); estimated full scale IQ < 80; history of claustrophobia, autism, schizophrenia, anorexia nervosa, or bulimia; active medical or neurological disease that would impact neurobiology or brain function; history of electroconvulsive therapy; and metal fragments or implants that would be contraindicated during the MRI scans. Measures of current clinical status were acquired prior to scanning, including the Hamilton Rating Scale for Depression (HAM-D) (Hamilton 1960) and the Hamilton Anxiety Rating Scale (HAM-A) (Hamilton 1959). Years of education and handedness were acquired from all participants in this study. The Wechsler Abbreviated Scale of Intelligence-Second Edition two-subtest version (Vocabulary and Matrix Reasoning) was administered to all participants to determine estimated IQ (Wechsler 2011).
We specifically focused some of our analyses on hyperarousal symptoms, as this cluster of symptoms has been found to be common to both PTSD and TBI (Stein and McAllister 2009). Of the Veterans with TBI (N = 57), 53 of them experienced a traumatic event (Criterion A Stressor) that would warrant further investigation into potential symptoms of PTSD. Of these 53 Veterans who were further assessed for presence of PTSD, 19 Veterans did not meet the criteria for having hyperarousal symptoms (TBI– hyperarousal), while 34 Veterans (TBI + hyperarousal), irrespective of having a PTSD diagnoses, endorsed enough hyperarousal symptoms to have met criteria for that specific symptom cluster within the PTSD diagnostic criteria.
Imaging acquisition
All brain MRI scans were performed in a 3 Tesla Siemens scanner (Erlangen, Germany) with a 12-channel head coil at the Utah Center for Advanced Imaging Research (UCAIR) located at the University of Utah. The structural protocol included a T1-weighted 3D MPRAGE GRAPPA sequence with echo time/repetition time/inversion time = 3.38 ms/2.0 s/1.1 s, 8° flip angle, 256 × 256 acquisition matrix, 256 × 256 mm2 field of view, 160 slices, 1.0 mm slice thickness. All structural MRI scans were read by a neuroradiologist to rule out gross pathology.
Imaging data processing procedures
DICOM image files were converted to NIFTI format using dcm2nii software (http://www.mccauslandcenter.sc.edu/mricro/mricron/) then all converted images were visually inspected to confirm whether they were appropriate for the analysis. One participant’s brain image appeared to have severe motion artifacts so it was removed from further analyses.
We conducted volumetric measurements and shape analyses in hippocampus and amygdala using the FMRIB’s Integrated Registration and Segmentation tool (FIRST) (Patenaude et al. 2011) in the FMRIB Software Library (FSL,version 4.1.6) (Smith et al. 2004). FIRST is an image analysis tool that conducts fully automatic segmentation of the whole brain and produces fifteen subcortical structures including the hippocampus and the amygdala. The models of shape and appearance in FIRST, based on multivariate Gaussian assumptions, are constructed from 336 manually-labeled brain images provided by the Center for Morphometric Analysis, Massachusetts General Hospital, Boston. FIRST processing consists of two linear registration stages. The first step is a robust registration of individual brain images to the Montreal Neurological Institute (MNI) 152 standard space by 12 degrees of freedom, and the second step is accurate registration using subcortical mask in the MNI space. Subcortical structures are then established by deformable meshes consisting of vertices and edges. FIRST performs automatic segmentation based on principle of a Bayesian framework and the shape of subcortical structures and intensities of imaging data are used for basis in this process. After these processes, we visually inspected both the outputs of the registration step and the segmented outputs of hippocampus and amygdala across each individual brain image. Images from 6 participants were removed because of poor registration quality or segmentation output, which did not improve even though we had performed these processes again with other options in FIRST.
To adjust whole brain volume differences between participants, the volumetric scaling factors were estimated by SIENAX (Structural Image Evaluation, using Normalisation, of Atrophy, version 2.6) in FSL software (Smith 2002). The first step of SIENAX is to remove non-brain tissue from each brain image. The next step is an estimation of the scaling between individual brain image and the standard template. Following, SIENAX completes brain tissue segmentation and estimates volumetric scaling factor from each brain image. We extracted the absolute volumes of the hippocampus and the amygdala from outputs of FIRST segmentation and calculated the normalized volumes of them by multiplying volumetric scaling factor of each image by the absolute volume. We removed one participant’s brain image because the left hippocampal volume deviated (above 3 SD) from the mean of all images. The final data includes analyses from 82 brain images.
We performed shape analysis of the hippocampus and the amygdala using a tool in FSL software. We conducted vertex-by-vertex comparison to explore group differences and relationships with clinical measurements. The outcome of the vertex analysis was represented through the forms of meshes and these results were visualized by FSL View tool, a part of FSL software.
Statistics
Statistical analyses were performed in STATA software (Version 12.1; Stata Corporation, College Station, TX). Demographic and clinical variables were compared using two sample t-tests. Two sample t-tests were also used to compare brain volumes and clinical measurements between the following groups: HC vs. TBI, TBI - PTSD vs. TBI + PTSD, TBI - hyperarousal vs. TBI + hyperarousal. We used stepwise multiple linear regression analyses including age, years of education, HAM-D score, and number of TBI as independent variables and volume and shape of the hippocampus and the amygdala as dependent variables. Partial correlations, using age as a covariate, were employed to identify the relationships between the HAM-D score and volume and shape of the hippocampus and the amygdala.
Results
Demographics and clinical measures
All eighty-two participants in this study were men. There was no significant age difference between the HC and TBI groups (p = 0.41). HCs had more years of education than individuals in the TBI group (p < 0.001). The age of the TBI - PTSD subgroup was not significantly different from the TBI + PTSD subgroup (p = 0.20). The postinjury interval of the TBI - PTSD subgroup was longer than the TBI + PTSD subgroup (p = 0.04). The TBI + PTSD subgroup had fewer years of education (p = 0.02), higher number of TBI events (p = 0.05), higher HAM-D (p < 0.001), and higher HAM-A scores (p < 0.001) than the TBI - PTSD subgroup. With regard to group comparisons between the TBI - hyperarousal and TBI + hyperarousal subgroups, there were no differences in age (p = 0.32) and postinjury interval (p = 0.11) but significant differences were observed in years of education (P = 0.05), number of TBI events (p = 0.05), HAM-D scores (p < 0.001), and HAM-A scores (p < 0.001). All clinical and demographic information is shown in Tables 1, 2, and 3.
Volumetric scaling factors
The volumetric scaling factors derived from SIENAX did not differ between the HC and TBI groups (p = 0.21). In the TBI subgroups, there were no significant differences for these values between the TBI + PTSD and TBI - PTSD subgroups as well as between the TBI + hyperarousal and TBI - hyperarousal subgroups (Tables 4, 5, and 6).
Volumetric differences of Hippocampus and amygdala
There were no significant differences in hippocampal volume between the HC and TBI groups in the left or right hemisphere (p > 0.05). The volume of the left and right amygdala was also not significantly different between the groups (p > 0.05) (Table 4). Furthermore, the TBI + PTSD and TBI - PTSD as well as the TBI + hyperarousal and TBI - hyperarousal were not significantly different with regard to hippocampal volume (Tables 5 and 6). The TBI + PTSD subgroup showed a trend towards a significantly larger left amygdalar volume than the TBI - PTSD subgroup (p = 0.07) (Table 5). TBI + hyperarousal subgroup also exhibited a trend towards a significantly larger left amygdala volume than the TBI - hyperarousal subgroup (p = 0.06) (Table 6). However, there were no volumetric differences in the right amygdala between these subgroups (Tables 5 and 6). All hippocampal and amygdalar volumes were adjusted by the volumetric scaling factors prior to comparisons.
Stepwise multiple linear regression analyses
We conducted stepwise multiple linear regression analyses to examine whether clinical factors were associated with hippocampal and amygdalar volumes. Age, years of education, HAM-D scores, and number of TBI events were included as independent variables in all models because several previous studies have indicated that these factors are associated with hippocampal and amygdala morphology. Significant associations that were observed were those between HAM-D scores and the left hippocampus (t = − 2.13, p = 0.04) and HAM-D scores with the left amygdala (t = 2.01, p = 0.05); the other three variables: age, years of education and number of TBI events were excluded from all models because they did not reach significance.
Relationships between hippocampal/Amygdalar volumes and HAM-D scores
Since the result of the stepwise multiple linear regression indicated that the HAM-D score was associated with the volumes of hippocampus and amygdala in the TBI group, we conducted partial correlation analyses adjusted for the effect of age to further investigate the relationships between hippocampal/amygdalar volumes and the HAM-D scores. Consequently, we found that left hippocampal volume was negatively correlated with the HAM-D scores (r = − 0.27, p = 0.05) and left amygdalar volume showed a positive correlation with the HAM-D scores (r = 0.28, p = 0.04) in the TBI group (Table 7, Figs. 1 and 2).
Scatterplots between HAM-D score and hippocampal volume in veterans with TBI. Abbreviations: HAM-D, Hamilton Rating Scale for Depression; PTSD, Post-Traumatic Stress Disorder. Age was used as a covariate in all analyses. The solid lines represent the line of best fit for the data and the dotted lines indicate the 95% confidence interval
Scatterplots between HAM-D score and amygdala volume in veterans with TBI. Abbreviations: HAM-D, Hamilton Rating Scale for Depression; PTSD, Post-Traumatic Stress Disorder. Age was used as a covariate in all analyses. The solid lines represent the line of best fit for the data and the dotted lines indicate the 95% confidence interval
To investigate whether these results were dependent on the lifetime diagnosis of PTSD, we examined the relationships between hippocampal/amygdalar volumes and the HAM-D scores, adjusted for the effect of age in the TBI - PTSD and TBI + PTSD subgroups. There was a significant negative correlation between left hippocampal volume and the HAM-D score in the TBI + PTSD subgroup (r = − 0.39, p = 0.03). However, in the TBI - PTSD subgroup, the HAM-D scores were not related to left hippocampal volume (r = − 0.30, p = 0.15). The HAM-D scores were not significantly correlated with right hippocampus and bilateral amygdala in the TBI + PTSD and TBI - PTSD subgroups (Table 7, Figs. 1 and 2).
Relationship of HAM-D scores with the Hippocampus and amygdala
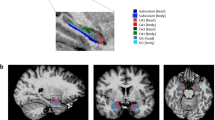
We further explored the relationship between the subregions of the hippocampus and the amygdala and HAM-D scores after adjusting for the effects of age in the TBI group using the outcome measures from the shape analyses (Fig. 3). The results from these analyses showed that the CA1 and the subiculum subregions of the left hippocampus and CA1 of the right hippocampus were significantly associated with the HAM-D scores. When we conducted the same analysis in TBI + PTSD Veterans, we obtained similar results in the left hippocampus, whereas we did not find any significant subregion in the right hippocampus associated with HAM-D scores. Shape analysis in the TBI - PTSD group indicated that there was no subregion was significantly associated with the HAM-D scores in the bilateral hippocampus. We could not detect any subregion within the amygdala that was significantly associated with HAM-D scores in the TBI group overall or within the TBI+ PTSD and TBI - PTSD subgroups.
Discussion
The present study is the first study to assess volume and shape of the hippocampus and the amygdala in individuals with TBI by PTSD diagnosis and depressive and hyperarousal symptoms, all of which often overlap in Veterans with TBI and PTSD. The HC and TBI groups showed no volume differences in the hippocampus and the amygdala. However, we observed a trend towards an increased left amygdalar volume in the TBI + PTSD subgroup compared to the TBI - PTSD subgroup as well as the TBI + hyperarousal subgroup compared to the TBI - hyperarousal subgroup.
We further explored whether demographic and clinical measures were associated with the volume and shape of the hippocampus and the amygdala in the TBI group. Age and years of education showed no relationship with the volume and shape of the hippocampus and amygdala. Since number of TBI events was significantly different between groups, we analyzed the relationship between this variable and shape and volume measures of the hippocampus and amygdala, and these results showed no significant association. Thus, the number of TBI events does not significantly contribute to the between group difference in left amygdalar volume. The HAM-D scores were negatively correlated with the left hippocampus and were positively correlated with the left amygdala volumes. Furthermore, shape analyses revealed hippocampal subregions were associated with HAM-D scores in the TBI group. We also found that the relationship between HAM-D scores and the volume and shape of the hippocampus varied according to the diagnosis of PTSD in the TBI group.
No hippocampal volume difference between the HC and TBI groups
We did not observe any hippocampal volume difference between the HC and TBI groups. A number of prior studies have demonstrated reductions in hippocampal volume in TBI (Arciniegas et al. 2001; Ariza et al. 2006; Warner et al. 2010; Tomaiuolo et al. 2004; Palacios et al. 2013). The interval between time of brain injury and the MRI scan of participants in the current study was longer compared to previous publications, which may explain the discrepant findings. In the current study, the MRI scans were performed on average 15.2 years after first brain injury (SD, 10.5; range, 1–39). In the previous studies, the mean years from brain injury to MRI scan were 6.7 (range, 1.8–15.0) (Arciniegas et al. 2001), 1.7 (range, 0.3–9.4) (Tomaiuolo et al. 2004), 0.8 (range, 0.5–1.7) (Ariza et al. 2006), 0.7 (range, 0.5–1.2) (Warner et al. 2010), and 4.2 (range was not presented) (Palacios et al. 2013). Furthermore, the severity of the TBI also plays a critical role in determining the extent of neurobiological alterations (Dixon 2017). Though most of the TBI in this study were mild, we did not assess whether they were associated with blast exposure, which may account for the divergent findings (Dixon 2017). Finally, a number of Veterans in the study reported multiple TBIs (mean, 4; SD, 6.1; range, 1–33). Thus, heterogeneity in time since injury, severity of injury, and number of TBIs are important factors to consider when interpreting neuroimaging findings in TBI. It is possible that the decrease in hippocampal volume recovers over time.
Hippocampal and Amygdalar volume difference between the TBI + PTSD and TBI - PTSD subgroups
We expected that the TBI + PTSD subgroup would demonstrate smaller hippocampal volume than the TBI - PTSD subgroup given prior evidence of the association between PTSD and reduced hippocampal volume (Bremner et al. 1995). However, we failed to observe a significant difference in the volume of the bilateral hippocampus between these groups. Exposure to trauma irrespective of PTSD diagnosis has been associated with alterations in the hippocampal volume. For example, a meta-analysis study reported that hippocampal volume reduction was found in both trauma-exposed group with PTSD and trauma-exposed group without PTSD when compared to the HC group (Woon and Hedges 2008). Another meta-analysis study showed left hippocampal volume reduction in a trauma-exposed group without PTSD compared to a trauma-exposed group with PTSD, but right hippocampal volumes demonstrated no difference between groups (Woon et al. 2010). Thus, it is possible that the TBI – PTSD subgroup also was exposed to trauma potentially due to the TBI, which could have contributed to the lack of significant differences between the TBI + PTSD and TBI – PTSD groups with regard to hippocampal volume. We did not record whether the TBI and PTSD were associated events and hence we are unable to test the above hypothesis.
We did observe a trend towards a higher left amygdalar volume in the TBI + PTSD (compared to TBI - PTSD) and in the TBI + hyperarousal (compared to the TBI – hyperarousal). PTSD has been previously associated with altered volume of the amygdala, although the results have been equivocal (Morey et al. 2012; Depue et al. 2014). Two meta-analyses that included amygdala volumetry showed inconsistent differences between trauma-exposed participants with and without PTSD. The first meta-analysis found lower volumes with small effect sizes in both the left (effect size = −.22) and right amygdala (−.18) but only after restricting analysis to the subset of studies that produced a homogeneous sample (Karl et al. 2006). The second meta-analysis demonstrated only a trend association (p = .06), with a small effect size (Hedges’ g = −.29), between smaller left amygdala volume and PTSD patients as compared to trauma-unexposed healthy control (Woon and Hedges 2009). To the best of our knowledge, this is the first study to compare amygdalar volumes in a comorbid diagnostic group (TBI + PTSD) to a TBI only group (TBI-PTSD). A previous study in Veterans reported reduced amygdalar volume in comorbid TBI and PTSD as compared to Veterans who did not have either diagnosis (Depue et al. 2014). Furthermore, a trend towards increase in left amygdalar volume in the TBI + hyperarousal group compared to the TBI – hyperarousal group suggests that the presence of hyperarousal symptoms may drive the difference in amygdalar volume between TBI + PTSD and TBI – PTSD groups. This is in agreement with a previous study that posited that the amygdala dysregulation in PTSD is specifically linked to the hyperarousal symptom cluster (Suvak and Barrett 2011). Our finding of increased amygdala volume will have to be replicated in future studies, especially since our findings were significant at a trend-level.
Implications of the relationship between hippocampal morphology and depressive symptoms
We found a significant negative correlation between left hippocampal volume and the HAM-D scores in the TBI group. Moreover, shape analyses revealed that the CA1 subregion and the subiculum of the left hippocampus and the CA1 subregion of the right hippocampus were significantly correlated with the HAM-D scores. Our findings strongly indicate that depressive symptoms in individuals with TBI are related to alterations in hippocampal morphology. The association between depressive symptoms and hippocampal morphology in TBI in the current study was dependent on PTSD diagnosis. We found a significant relationship between hippocampal morphology and the HAM-D scores in the TBI + PTSD subgroup, but not in the TBI - PTSD subgroup. These findings suggest that pathophysiology of PTSD may mediate the association between hippocampal morphology and depressive symptoms in TBI, underscoring the importance of considering the diagnosis of PTSD when investigating brain alterations in a TBI cohort.
The human hippocampus can be segmented into subregions based on anatomical features: the CA1, through CA4 regions, the subiculum, and the dentate gyrus (DG) with each of the subregions exhibiting distinct structural, cellular, and functional properties (Duvernoy 2005). The hippocampus is one of the most extensively studied subcortical structures in patients with psychiatric disorders. Numerous but not all studies have demonstrated that major depression is associated with a reduction in total hippocampal volume, as confirmed by a recent meta-analysis (Videbech and Ravnkilde 2004; Geuze et al. 2005; McKinnon et al. 2009). Relatively fewer studies have examined hippocampal subfields in relation to depression. A cross-sectional study in 37 drug-naive patients with major depressive disorder (MDD) showed deformations in the CA1, CA2, CA3 subregions, and subiculum of the bilateral hippocampus as compared to HCs (Cole et al. 2010). The authors, however, did not find any relationship between hippocampal volume and depressive symptoms. Another study in 21 female patients with unremitting MDD reported that the CA3 and the subiculum in the left hippocampus and the subiculum/the end of the tail in the right hippocampus were associated with depressive symptoms (Tae et al. 2011). Furthermore, the left hippocampus exhibited more significant contraction than the right hippocampus in depression (Tae et al. 2011). The results of 5-year longitudinal study employing shape analysis revealed changes in the CA1-CA3 subfield and subiculum in the MDD group at the 5-year timepoint but not at baseline when compared to HCs (Isikli et al. 2013). Furthermore, a significant negative correlation was found with the number of days in depression without antidepressant treatment in the CA1 region in the head and tail of the hippocampus bilaterally, in the absence of volume differences in the hippocampus in the depressed group when compared to HCs at baseline and the 5-year follow-up time point (Isikli et al. 2013). Recent studies of hippocampal subfields in MDD have found negative correlation between depression and the DG, subiculum, and the CA subregions (Travis et al. 2015; Huang et al. 2013; Lindqvist et al. 2014). Finally, a recent study by Averill and colleagues reported a negative correlation between depression severity and volume of several hippocampal subregions including the DG, CA1, CA2/3 and CA4 in a cohort of Veterans with a subset diagnosed with PTSD (Averill et al. 2017). With regard to the association between HAM-D scores and CA1 and subiculum regions in the TBI + PTSD group, it is interesting that the CA1 has been implicated in context-dependent fear extinction and retrieval of contextual memory (Ji and Maren 2008). The subiculum, which is a part of the limbic memory system plays a critical role in fear conditioning and regulates the stress response as well (Maren 1999; O'Mara 2006). Together these studies underscore the importance of investigating focused relationships of hippocampal subfields (rather than total hippocampus) and symptomology given the implication of specialization in these subfields across the hippocampus.
Although we reported that amygdala volume was positively correlated with the HAM-D scores, shape analyses failed to reveal any amygdala subregion related to these scores. It is possible that volume enlargement had occurred in the entire amygdala so that we could not detect specific regions associated with the HAM-D scores. Secondly, since amygdala is a relatively small structure compared to the hippocampus, we may have insufficient power to observe subtle changes of amygdala with the current MRI resolution.
Strengths and limitations
Our study has three primary strengths. The first is a large sample size consisting of twenty-five healthy controls and fifty-seven Veterans with TBI who completed neuroimaging. A large sample size in the TBI group allowed us to compare volume and shape alterations based on PTSD diagnosis. Second, we investigated hippocampus and amygdala using a well-validated tool, FIRST. FIRST, as noted previously, has been used in numerous neuroimaging studies to conduct shape analysis of subcortical structures including the hippocampus and the amygdala. The outputs of FIRST were validated by Nugent and colleagues (Nugent et al. 2013), who reported that the test-retest reliability was excellent in the hippocampus (ICC: left side, 0.901; right side, 0.872) and acceptable in the amygdala (ICC: left side, 0.790; right side, 0.710). Furthermore, the absolute agreement between FSL and manual segmentation was acceptable in the hippocampus (ICC: left side, 0.711; right side, 0.724). Third, we considered the effect of PTSD symptoms and diagnosis in the hippocampus and amygdala analyses in this cohort. TBI and PTSD are highly comorbid and our sample reflects the overlap of these two diagnoses. Additionally, all of our structured diagnostic interviews were completed by licensed psychologists and postdoctoral fellows, giving us confidence in the PTSD diagnoses and also in interpreting the relationship between PTSD symptoms and imaging variables.
Several limitations must be considered when interpreting our findings. First, the postinjury intervals of Veterans in the TBI cohort are relatively long. This is both a strength and a limitation, as it provides a more clear picture of neuroimaging differences after a longer post-TBI interval compared to some other studies of TBI. Second, we did not correct for multiple comparisons for results of the shape analyses. Third, most of the participants in this study were identified as having mild TBI. Mild TBI is the most common type of TBI, making these results generalizable to a larger number of Veterans (Hoge et al. 2008). However, it is hard to generalize our findings Veterans with moderate or severe TBI. Fourth, the current study comprised of only male Veterans and hence the results are not generalizable to female Veterans. Given the increase in the proportion of female Veterans in all branches of U.S. Armed Forces and previous studies showing sex differences in Veterans with TBI (McGlade et al. 2015), it is critical that future studies include female participants. Finally, due to ethical reasons, we did not stop the participants from taking their medication, which may have potentially confounded the findings. Future studies should further delineate the effects of medication on neurobiological changes associated with PTSD and TBI.
Conclusion
In summary, this study investigated the volume and the shape of the hippocampus and the amygdala in Veterans with TBI. Further, we also analyzed volume and shape analyses according to PTSD diagnosis in Veterans with TBI. We found a trend towards larger left amygdalar volume in the TBI + PTSD subgroup than the TBI - PTSD subgroup. HAM-D scores were associated with hippocampal and amygdalar volumes in the TBI group. Results from the shape analyses showed that the HAM-D scores were correlated with hippocampal subfield alterations in the TBI group, which was related to the diagnosis of PTSD. Our findings provide evidence that PTSD comorbidity is critical to consider in Veterans with TBI and that the neurobiological alterations in Veterans with TBI and PTSD may be distinct and more severe than those in Veterans with TBI alone. Shape analyses may be an important technique to reveal biological underpinnings that may underlie the clinical symptoms associated with TBI.
References
Anderson, C. V., Wood, D. M., Bigler, E. D., & Blatter, D. D. (1996). Lesion volume, injury severity, and thalamic integrity following head injury. Journal of Neurotrauma, 13(2), 59–65. https://doi.org/10.1089/neu.1996.13.59.
Arciniegas, D. B., Topkoff, J. L., Rojas, D. C., Sheeder, J., Teale, P., Young, D. A., Sandberg, E., Reite, M. L., & Adler, L. E. (2001). Reduced hippocampal volume in association with p50 nonsuppression following traumatic brain injury. The Journal of Neuropsychiatry and Clinical Neurosciences, 13(2), 213–221. https://doi.org/10.1176/jnp.13.2.213.
Ariza, M., Serra-Grabulosa, J. M., Junque, C., Ramirez, B., Mataro, M., Poca, A., et al. (2006). Hippocampal head atrophy after traumatic brain injury. Neuropsychologia, 44(10), 1956–1961. https://doi.org/10.1016/j.neuropsychologia.2005.11.007.
Averill, C. L., Satodiya, R. M., Scott, J. C., Wrocklage, K. M., Schweinsburg, B., Averill, L. A., Akiki T. J., Amoroso T., Southwick S. M., Krystal J. H., Abdallah C. G. (2017). Posttraumatic stress disorder and depression symptom severities are differentially associated with hippocampal subfield volume loss in combat veterans. Chronic Stress (Thousand Oaks), 1, https://doi.org/10.1177/2470547017744538.
Belanger, H. G., Uomoto, J. M., & Vanderploeg, R. D. (2009). The veterans health Administration's (VHA's) Polytrauma system of care for mild traumatic brain injury: Costs, benefits, and controversies. The Journal of Head Trauma Rehabilitation, 24(1), 4–13. https://doi.org/10.1097/HTR.0b013e3181957032.
Bigler, E. D., Blatter, D. D., Anderson, C. V., Johnson, S. C., Gale, S. D., Hopkins, R. O., & Burnett, B. (1997). Hippocampal volume in normal aging and traumatic brain injury. AJNR. American Journal of Neuroradiology, 18(1), 11–23.
Bigler, E. D., Anderson, C. V., & Blatter, D. D. (2002). Temporal lobe morphology in normal aging and traumatic brain injury. AJNR. American Journal of Neuroradiology, 23(2), 255–266.
Bigler, E. D., Abildskov, T. J., Wilde, E. A., McCauley, S. R., Li, X., Merkley, T. L., Fearing, M. A., Newsome, M. R., Scheibel, R. S., Hunter, J. V., Chu, Z., & Levin, H. S. (2010). Diffuse damage in pediatric traumatic brain injury: A comparison of automated versus operator-controlled quantification methods. Neuroimage, 50(3), 1017–1026. https://doi.org/10.1016/j.neuroimage.2010.01.003.
Bolzenius, J. D., Wade, B. S. C., Velez, C. S., Drennon, A. M., Cooper, D. B., Kennedy, J. E., Reid, M. W., Bowles, A. O., Thompson, P. M., Gutman, B., Lewis, J. D., Ritter, J. L., York, G. E., Bigler, E. D., & Tate, D. F. (2018). Relationships between subcortical shape measures and subjective symptom reporting in US Service members with mild traumatic brain injury. The Journal of Head Trauma Rehabilitation, 33(2), 113–122. https://doi.org/10.1097/htr.0000000000000379.
Bremner, J. D., Randall, P., Scott, T. M., Bronen, R. A., Seibyl, J. P., Southwick, S. M., Delaney, R. C., McCarthy, G., Charney, D. S., & Innis, R. B. (1995). MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. The American Journal of Psychiatry, 152(7), 973–981. https://doi.org/10.1176/ajp.152.7.973.
Calder, A. J., Lawrence, A. D., & Young, A. W. (2001). Neuropsychology of fear and loathing. Nature Reviews. Neuroscience, 2(5), 352–363. https://doi.org/10.1038/35072584.
Carlson, K. F., Kehle, S. M., Meis, L. A., Greer, N., Macdonald, R., Rutks, I., et al. (2011). Prevalence, assessment, and treatment of mild traumatic brain injury and posttraumatic stress disorder: A systematic review of the evidence. The Journal of Head Trauma Rehabilitation, 26(2), 103–115. https://doi.org/10.1097/HTR.0b013e3181e50ef1.
Cole, J., Toga, A. W., Hojatkashani, C., Thompson, P., Costafreda, S. G., Cleare, A. J., Williams, S. C., Bullmore, E. T., Scott, J. L., Mitterschiffthaler, M. T., Walsh, N. D., Donaldson, C., Mirza, M., Marquand, A., Nosarti, C., McGuffin, P., & Fu, C. H. (2010). Subregional hippocampal deformations in major depressive disorder. Journal of Affective Disorders, 126(1–2), 272–277. https://doi.org/10.1016/j.jad.2010.03.004.
Corrigan, J. D., & Bogner, J. (2007). Initial reliability and validity of the Ohio State University TBI identification method. The Journal of Head Trauma Rehabilitation, 22(6), 318–329. https://doi.org/10.1097/01.htr.0000300227.67748.77.
Davis, M., & Whalen, P. J. (2001). The amygdala: Vigilance and emotion. Molecular Psychiatry, 6(1), 13–34.
Defense, & Center, V. B. I. (2012). DoD worldwide numbers for TBI. Defense and Veterans Brain Injury Center, Silver Spring Maryland.
Depue, B. E., Olson-Madden, J. H., Smolker, H. R., Rajamani, M., Brenner, L. A., & Banich, M. T. (2014). Reduced amygdala volume is associated with deficits in inhibitory control: A voxel- and surface-based morphometric analysis of comorbid PTSD/mild TBI. BioMed Research International, 2014, 691505. https://doi.org/10.1155/2014/691505.
Dixon, K. J. (2017). Pathophysiology of traumatic brain injury. Physical Medicine and Rehabilitation Clinics of North America, 28(2), 215–225. https://doi.org/10.1016/j.pmr.2016.12.001.
Duvernoy, H. M. (2005). The human Hippocampus: Functional anatomy, vascularization and serial sections with MRI 3rd edition. American Journal of Neuroradiology, 26(10), 2702.
Faul, M., Xu, L., Wald, M., & Coronado, V. (2010). Traumatic brain injury in the United States: Emergency department visits, hospitalizations and deaths.
First, M. B., Spitzer, Robert L, Gibbon Miriam, and Williams, Janet B W (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. New York: Biometrics Research, New York State Psychiatric Institute.
Geuze, E., Vermetten, E., & Bremner, J. D. (2005). MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Molecular Psychiatry, 10(2), 160–184. https://doi.org/10.1038/sj.mp.4001579.
Hamilton, M. (1959). The assessment of anxiety states by rating. The British Journal of Medical Psychology, 32(1), 50–55.
Hamilton, M. (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23, 56–62.
Hoge, C. W., McGurk, D., Thomas, J. L., Cox, A. L., Engel, C. C., & Castro, C. A. (2008). Mild traumatic brain injury in U.S. soldiers returning from Iraq. The New England Journal of Medicine, 358(5), 453–463. https://doi.org/10.1056/NEJMoa072972.
Huang, Y., Coupland, N. J., Lebel, R. M., Carter, R., Seres, P., Wilman, A. H., & Malykhin, N. V. (2013). Structural changes in hippocampal subfields in major depressive disorder: A high-field magnetic resonance imaging study. Biological Psychiatry, 74(1), 62–68. https://doi.org/10.1016/j.biopsych.2013.01.005.
Isikli, S., Ugurlu, O., Durmusoglu, E., Kizilates, G., Kitis, O., Ozan, E., et al. (2013). Altered hippocampal formation shape in first-episode depressed patients at 5-year follow-up. Journal of Psychiatric Research, 47(1), 50–55. https://doi.org/10.1016/j.jpsychires.2012.08.022.
Ji, J., & Maren, S. (2008). Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learning & Memory, 15(4), 244–251. https://doi.org/10.1101/lm.794808.
Kaplan, G. B., Leite-Morris, K. A., Wang, L., Rumbika, K. K., Heinrichs, S. C., Zeng, X., Wu, L., Arena, D. T., & Teng, Y. D. (2018). Pathophysiological bases of comorbidity: Traumatic brain injury and post-traumatic stress disorder. Journal of Neurotrauma, 35(2), 210–225. https://doi.org/10.1089/neu.2016.4953.
Karl, A., Schaefer, M., Malta, L. S., Dorfel, D., Rohleder, N., & Werner, A. (2006). A meta-analysis of structural brain abnormalities in PTSD. Neuroscience and Biobehavioral Reviews, 30(7), 1004–1031. https://doi.org/10.1016/j.neubiorev.2006.03.004.
Kim, G. H., Lee, J. H., Seo, S. W., Kim, J. H., Seong, J. K., Ye, B. S., Cho, H., Noh, Y., Kim, H. J., Yoon, C. W., Oh, S. J., Kim, J. S., Choe, Y. S., Lee, K. H., Kim, S. T., Hwang, J. W., Jeong, J. H., & Na, D. L. (2015). Hippocampal volume and shape in pure subcortical vascular dementia. Neurobiology of Aging, 36(1), 485–491. https://doi.org/10.1016/j.neurobiolaging.2014.08.009.
LeDoux, J. E. (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23, 155–184. https://doi.org/10.1146/annurev.neuro.23.1.155.
Lindqvist, D., Mueller, S., Mellon, S. H., Su, Y., Epel, E. S., Reus, V. I., Rosser, R., Mahan, L., Mackin, R. S., Yang, T. T., & Wolkowitz, O. M. (2014). Peripheral antioxidant markers are associated with total hippocampal and CA3/dentate gyrus volume in MDD and healthy controls-preliminary findings. Psychiatry Research, 224(3), 168–174. https://doi.org/10.1016/j.pscychresns.2014.09.002.
Maller, J. J., Thomson, R. H., Pannek, K., Bailey, N., Lewis, P. M., & Fitzgerald, P. B. (2014). Volumetrics relate to the development of depression after traumatic brain injury. Behavioural Brain Research, 271, 147–153. https://doi.org/10.1016/j.bbr.2014.05.047.
Maren, S. (1999). Neurotoxic or electrolytic lesions of the ventral subiculum produce deficits in the acquisition and expression of Pavlovian fear conditioning in rats. Behavioral Neuroscience, 113(2), 283–290.
McDonald, B. C., Saykin, A. J., & McAllister, T. W. (2012). Functional MRI of mild traumatic brain injury (mTBI): Progress and perspectives from the first decade of studies. Brain Imaging and Behavior, 6(2), 193–207. https://doi.org/10.1007/s11682-012-9173-4.
McGlade, E., Rogowska, J., & Yurgelun-Todd, D. (2015). Sex differences in orbitofrontal connectivity in male and female veterans with TBI. Brain Imaging and Behavior, 9(3), 535–549. https://doi.org/10.1007/s11682-015-9379-3.
McKinnon, M. C., Yucel, K., Nazarov, A., & MacQueen, G. M. (2009). A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. Journal of Psychiatry & Neuroscience, 34(1), 41–54.
Morey, R. A., Gold, A. L., LaBar, K. S., Beall, S. K., Brown, V. M., Haswell, C. C., Nasser, J. D., Wagner, H. R., McCarthy, G., & Mid-Atlantic MIRECC Workgroup. (2012). Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Archives of General Psychiatry, 69(11), 1169–1178. https://doi.org/10.1001/archgenpsychiatry.2012.50.
Nugent, A. C., Luckenbaugh, D. A., Wood, S. E., Bogers, W., Zarate, C. A., Jr., & Drevets, W. C. (2013). Automated subcortical segmentation using FIRST: Test-retest reliability, interscanner reliability, and comparison to manual segmentation. Human Brain Mapping, 34(9), 2313–2329. https://doi.org/10.1002/hbm.22068.
O'Mara, S. (2006). Controlling hippocampal output: The central role of subiculum in hippocampal information processing. Behavioural Brain Research, 174(2), 304–312. https://doi.org/10.1016/j.bbr.2006.08.018.
Palacios, E. M., Sala-Llonch, R., Junque, C., Fernandez-Espejo, D., Roig, T., Tormos, J. M., Bargallo, N., & Vendrell, P. (2013). Long-term declarative memory deficits in diffuse TBI: Correlations with cortical thickness, white matter integrity and hippocampal volume. Cortex, 49(3), 646–657. https://doi.org/10.1016/j.cortex.2012.02.011.
Patenaude, B., Smith, S. M., Kennedy, D. N., & Jenkinson, M. (2011). A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage, 56(3), 907–922. https://doi.org/10.1016/j.neuroimage.2011.02.046.
Ross, D. E. (2011). Review of longitudinal studies of MRI brain volumetry in patients with traumatic brain injury. Brain Injury, 25(13–14), 1271–1278. https://doi.org/10.3109/02699052.2011.624568.
Schneiderman, A. I., Braver, E. R., & Kang, H. K. (2008). Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: Persistent postconcussive symptoms and posttraumatic stress disorder. American Journal of Epidemiology, 167(12), 1446–1452. https://doi.org/10.1093/aje/kwn068.
Shenton, M. E., Hamoda, H. M., Schneiderman, J. S., Bouix, S., Pasternak, O., Rathi, Y., Vu, M. A., Purohit, M. P., Helmer, K., Koerte, I., Lin, A. P., Westin, C. F., Kikinis, R., Kubicki, M., Stern, R. A., & Zafonte, R. (2012). A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging and Behavior, 6(2), 137–192. https://doi.org/10.1007/s11682-012-9156-5.
Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. https://doi.org/10.1002/hbm.10062.
Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E., Johansen-Berg, H., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23(Suppl 1), S208–S219. https://doi.org/10.1016/j.neuroimage.2004.07.051.
Stein, M. B., & McAllister, T. W. (2009). Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. The American Journal of Psychiatry, 166(7), 768–776. https://doi.org/10.1176/appi.ajp.2009.08101604.
Suvak, M. K., & Barrett, L. F. (2011). Considering PTSD from the perspective of brain processes: A psychological construction approach. Journal of Traumatic Stress, 24(1), 3–24. https://doi.org/10.1002/jts.20618.
Tae, W. S., Kim, S. S., Lee, K. U., Nam, E. C., Choi, J. W., & Park, J. I. (2011). Hippocampal shape deformation in female patients with unremitting major depressive disorder. AJNR. American Journal of Neuroradiology, 32(4), 671–676. https://doi.org/10.3174/ajnr.A2367.
Tate, D. F., & Bigler, E. D. (2000). Fornix and hippocampal atrophy in traumatic brain injury. Learning & Memory, 7(6), 442–446.
Tate, D. F., Wade, B. S., Velez, C. S., Drennon, A. M., Bolzenius, J., Gutman, B. A., et al. (2016). Volumetric and shape analyses of subcortical structures in United States service members with mild traumatic brain injury. Journal of Neurology, 263(10), 2065–2079. https://doi.org/10.1007/s00415-016-8236-7.
Tate, D. F., Wade, B. S. C., Velez, C. S., Drennon, A. M., Bolzenius, J. D., Cooper, D. B., Kennedy, J. E., Reid, M. W., Bowles, A. O., Thompson, P. M., Gutman, B. A., Lewis, J. D., Ritter, J. L., York, G. E., & Bigler, E. D. (2018). Subcortical shape and neuropsychological function among U.S. service members with mild traumatic brain injury. Brain Imaging and Behavior, 13, 377–388. https://doi.org/10.1007/s11682-018-9854-8.
Tomaiuolo, F., Carlesimo, G. A., Di Paola, M., Petrides, M., Fera, F., Bonanni, R., et al. (2004). Gross morphology and morphometric sequelae in the hippocampus, fornix, and corpus callosum of patients with severe non-missile traumatic brain injury without macroscopically detectable lesions: A T1 weighted MRI study. Journal of Neurology, Neurosurgery, and Psychiatry, 75(9), 1314–1322. https://doi.org/10.1136/jnnp.2003.017046.
Travis, S., Coupland, N. J., Silversone, P. H., Huang, Y., Fujiwara, E., Carter, R., Seres, P., & Malykhin, N. V. (2015). Dentate gyrus volume and memory performance in major depressive disorder. Journal of Affective Disorders, 172, 159–164. https://doi.org/10.1016/j.jad.2014.09.048.
Van Boven, R. W., Harrington, G. S., Hackney, D. B., Ebel, A., Gauger, G., Bremner, J. D., et al. (2009). Advances in neuroimaging of traumatic brain injury and posttraumatic stress disorder. Journal of Rehabilitation Research and Development, 46(6), 717–757.
Vanderploeg, R. D., Belanger, H. G., & Curtiss, G. (2009). Mild traumatic brain injury and posttraumatic stress disorder and their associations with health symptoms. Archives of Physical Medicine and Rehabilitation, 90(7), 1084–1093. https://doi.org/10.1016/j.apmr.2009.01.023.
Videbech, P., & Ravnkilde, B. (2004). Hippocampal volume and depression: A meta-analysis of MRI studies. The American Journal of Psychiatry, 161(11), 1957–1966. https://doi.org/10.1176/appi.ajp.161.11.1957.
Wade, B. S., Valcour, V. G., Wendelken-Riegelhaupt, L., Esmaeili-Firidouni, P., Joshi, S. H., Gutman, B. A., et al. (2015). Mapping abnormal subcortical brain morphometry in an elderly HIV+ cohort. Neuroimage Clin, 9, 564–573. https://doi.org/10.1016/j.nicl.2015.10.006.
Warner, M. A., Youn, T. S., Davis, T., Chandra, A., Marquez de la Plata, C., Moore, C., Harper, C., Madden, C. J., Spence, J., McColl, R., Devous, M., King, R. D., & Diaz-Arrastia, R. (2010). Regionally selective atrophy after traumatic axonal injury. Archives of Neurology, 67(11), 1336–1344. https://doi.org/10.1001/archneurol.2010.149.
Wechsler, D. (2011). Wechsler Abbreviated Scale of Intelligence-Second Edition (WASI-II). San Antonio: NCS Pearson.
Woon, F. L., & Hedges, D. W. (2008). Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: A meta-analysis. Hippocampus, 18(8), 729–736. https://doi.org/10.1002/hipo.20437.
Woon, F. L., & Hedges, D. W. (2009). Amygdala volume in adults with posttraumatic stress disorder: A meta-analysis. The Journal of Neuropsychiatry and Clinical Neurosciences, 21(1), 5–12. https://doi.org/10.1176/jnp.2009.21.1.5.
Woon, F. L., Sood, S., & Hedges, D. W. (2010). Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: A meta-analysis. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 34(7), 1181–1188. https://doi.org/10.1016/j.pnpbp.2010.06.016.
Yurgil, K. A., Barkauskas, D. A., Vasterling, J. J., Nievergelt, C. M., Larson, G. E., Schork, N. J., Litz, B. T., Nash, W. P., Baker, D. G., & Marine Resiliency Study Team. (2014). Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty marines. JAMA Psychiatry, 71(2), 149–157. https://doi.org/10.1001/jamapsychiatry.2013.3080.
Zatzick, D. F., Rivara, F. P., Jurkovich, G. J., Hoge, C. W., Wang, J., Fan, M. Y., Russo, J., Trusz, S. G., Nathens, A., & Mackenzie, E. J. (2010). Multisite investigation of traumatic brain injuries, posttraumatic stress disorder, and self-reported health and cognitive impairments. Archives of General Psychiatry, 67(12), 1291–1300. https://doi.org/10.1001/archgenpsychiatry.2010.158.
Acknowledgements
This study was supported by funds from Merit Review 5101 CX000253-02 and VISN 19 MIRECC (The Mental Illness Research, Education and Clinical Centers), Salt Lake City, UT, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there are no financial and personal relationships with other people or organizations that could inappropriately influence (bias) this work.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bae, S., Sheth, C., Legarreta, M. et al. Volume and shape analysis of the Hippocampus and amygdala in veterans with traumatic brain injury and posttraumatic stress disorder. Brain Imaging and Behavior 14, 1850–1864 (2020). https://doi.org/10.1007/s11682-019-00127-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-019-00127-2