Abstract
Alcohol misuse and alcohol use disorder (AlUD) have neurobiological consequences. This meta-analysis of proton magnetic resonance spectroscopy (MRS) studies aimed to assess the differences in brain metabolite levels in alcohol misuse and AUD relative to controls (PROSPERO registration: CRD42020209890). Hedge’s g with random-effects modeling was used. Sub-group and meta-regression techniques explored potential sources of demographic and MRS parameter heterogeneity. A comprehensive literature review identified 43 studies, resulting in 69 models across gray and white matter (GM, WM). Lower N-acetylaspartate levels were found in frontal, anterior cingulate cortex (ACC), hippocampal, and cerebellar GM, and frontal and parietal WM, suggesting decreased neuronal and axonal viability. Lower choline-containing metabolite levels (all metabolites contributing to choline peak) were found in frontal, temporal, thalamic, and cerebellar GM, and frontal and parietal WM, suggesting membrane alterations related to alcohol misuse. Lower creatine-containing metabolite levels (Cr; all metabolites contributing to Cr peak) were found in temporal and occipital cortical GM, while higher levels were noted in midbrain/brainstem GM; this finding may have implications for using Cr as an internal reference. The lack of significant group differences in glutamate-related levels is possibly related to biological and methodological complexities. The few studies reporting on GABA found lower levels restricted to the ACC. Confounding variables were age, abstinence duration, treatment status, and MRS parameters (echo time, quantification type, data quality). This first meta-analysis of proton MRS studies consolidates the numerous individual studies to identify neurometabolite alterations within alcohol misuse and AUD. Future studies can leverage this new formalized information to investigate treatments that might effectively target the observed disturbances.
Similar content being viewed by others
Introduction
There is a clear connection between alcohol misuse, such as binge drinking (≥5 drinks for males, ≥4 drinks for females on an occasion) and heavy drinking (binge drinking ≥5 days/week), and an increased risk of developing alcohol use disorder (AUD) [1, 2]. In fact, ~40% of alcohol consumers endorse heavy episodic drinking [3], and one-third of adults in the US meet lifetime criteria for AUD [4, 5]. The personal and societal costs of alcohol misuse and AUD are high [3], yet efforts to prevent or treat these behaviors have only been modestly effective [5,6,7,8], necessitating improvement in current treatments and development of more efficacious treatments.
One step in the treatment development process is to investigate the neurobiological consequences of alcohol misuse and AUD. Chronic alcohol use is associated with complex, maladaptive neurobiological changes that contribute to the escalation and continuation of use [5, 9, 10]. The neural effects of alcohol use in humans can be measured through proton magnetic resonance spectroscopy (MRS) [11], a non-invasive in vivo imaging technique that quantifies the chemical composition of tissues (‘metabolites’) in localized regions. MRS can routinely detect relatively concentrated neurometabolites, including glutamate (Glu), glutamine (Gln), gamma-aminobutyric acid (GABA), N-acetylaspartate (NAA), choline-containing metabolites (Cho), creatine-containing metabolites (Cr), and myo-inositol (mI). Of note, the MRS signal is not able to be separated into distinct choline metabolites (phosphorylcholine, glycerophosphorylcholine, free choline, or acetylcholine) or creatine metabolites (creatine and phosophocreatine), thus we use the term “choline-containing metabolites” or “creatine-containing metabolites” to refer to any metabolite that will contribute to the choline or creatine peaks, respectively. See Supplementary Table 1 for an overview of these MRS metabolites and their relation to alcohol use ([11,12,13,14,15,16] for comprehensive reviews). Derived neurometabolite information can be leveraged when studying alcohol misuse and AUD to assist in characterizing neurometabolic alterations and informing development of treatment options that can specifically target detected abnormalities. Future work can then be dedicated to understanding if or how such changes in neurometabolite levels may correspond with treatment outcomes.
To our knowledge, this is the first published meta-analysis summarizing the extant literature on brain metabolite alterations associated with human alcohol use, relative to controls, measured with in vivo proton MRS. Broad inclusion criteria captured studies of alcohol use and AUD to comprehensively cover relevant populations (i.e., binge drinkers, treatment-seeking and treatment-naïve AUD individuals), brain regions, and neurometabolites. Sub-group analyses and meta-regressions explored potential sources of heterogeneity, including population demographics (abstinence duration, age, sex, and treatment-seeking status) and MRS parameters (echo time (TE), absolute versus relative quantification, and data quality metrics).
Methods
Search strategy and study eligibility
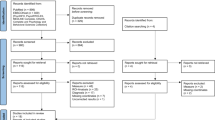
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines [17]. The protocol was pre-registered with PROSPERO (protocol number: CRD42020209890).
Relevant extant literature assessing the differences in brain metabolite levels between alcohol misuse/AUD groups relative to controls was identified by systematically searching PubMed, PsychINFO, Scopus, Google Scholar, CINHAL, and TRIP using the terms: “magnetic resonance spectroscopy” or “MRS” and “alcoholism”, “alcohol abuse”, “alcohol dependence”, “AUDs”, “polysubstance use” (See Supplementary Materials). Inclusion criteria were as follows: (1) use of in vivo proton MRS; (2) alcohol-using group defined by DSM, ICD, or other study-specific criteria; (3) healthy control group defined by study-specific criteria (note: some control groups did consume alcohol, but at a lower quantity/frequency with no history of AUD. See Supplementary Table 3a for more details); (4) direct comparison between the alcohol-using group and control group; (5) appropriate data to complete meta-analysis; and (6) published in English. Exclusion criteria were: (1) carbon or phosphorus MRS, preclinical NMR imaging; (2) other human neuroimaging technique (e.g., fMRI); (3) metabolite measures from body fluids (e.g., CSF, blood); and (4) comorbid psychiatric disorder or other illness (e.g., HIV, bipolar disorder, liver disease, Fetal Alcohol Syndrome) as the main population of interest. Studies were not excluded based on year published, age of participants, or metabolite quantification method (i.e., absolute metabolite concentration and metabolite resonance intensity relative to water or Cr). Systematic searches were conducted by one author (BDB) on May 17, 2021. All identified studies were then reviewed for potential inclusion by two reviewers (BDB and AEK) using Covidence Systematic Software (Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org; Supplementary Materials). Inter-rater reliability was high for title/abstracts (96% agreement, Cohen’s kappa k = 0.836) and full-text screening (92% agreement, k = 0.625).
Data extraction
Both study reviewers independently extracted data from all eligible studies using EXCEL. Spreadsheets were compared (97% agreement), and conflicting data entries were resolved via consultation between reviewers (BDB and AEK, with LMS as tiebreaker). See supplementary materials for complete list of variables extracted. To determine methodological quality of the studies, both reviewers independently completed the Appraisal Tool for Cross-sectional Studies (AXIS) [18] modified for proton MRS studies [19] within Covidence (Supplementary Table 2).
Meta-analysis statistical analyses
Comprehensive Meta-Analysis Version 3.0 [20] was used for all analyses. Significance was set at p < 0.05.
Main meta-analytic models
The main model required at least two distinct studies within a brain region per metabolite. For longitudinal studies, only baseline data were included to maintain independence of data [19]. If a study had more than 1 measurement within the same brain region, the data were treated as independent [19]. A remove-one analysis was performed as a sensitivity analysis to assess the impact of each study individually on the overall estimate; additionally, this was used to check for the influence of studies with multiple groups within the same brain region. Hedges’ g (unbiased standardized mean difference estimate) was used as the measure of effect size [21] with random-effects modeling [22]. Heterogeneity within studies was assessed through the Cochran’s Q, I2, tau, and tau2 statistics. Publication bias was assessed in models with >2 studies by: (1) funnel plots, (2) Duval and Tweedie trim and fill method [23]; and (3) Egger’s regression [24]. See Supplementary Materials for more details.
Sub-group analyses
The sub-group analyses required at least two studies within each group and ten studies between groups [22, 25]. Random-effects modeling with pooled estimates was used [22, 26]. Two sub-group analyses examined the potential effects of (1) treatment-seeking status (treatment-seeking v. naïve/non-treatment seeking) and (2) quantification type (absolute v. relative concentration) on effect sizes. Treatment-seeking status may be a proxy for severity of alcohol use [27,28,29,30], potentially affecting neurometabolic alterations [31,32,33]. Treatment-seeking status was applied to a study if it was explicitly described in the text, which ranged from stating the participants were seeking treatment to stating that participants were recruited from alcohol-specific programs, clinics, or hospitals. If it was not explicitly stated, studies were labeled as treatment-naïve (explicitly stated) or non-treatment seeking samples (if not explicitly stated as treatment-seeking or treatment-naïve).
MRS-derived metabolites can be quantified using absolute or relative (e.g., normalized to Cr) concentrations [34, 35]. Relative concentrations often use the Cr peak as an internal concentration reference in calculation of metabolite ratios, assuming Cr levels are stable and not affected by pathology. However, this assumption can be invalid, as Cr levels vary with brain activity, age, and different pathologic states [13, 36, 37]. (Note: Relative concentrations can also be calculated using water as the reference, but not many studies included used this method and thus is not included in the sub-group analysis).
Meta-regression
Meta-regressions required a minimum of ten studies for each model (Cochrane standard), and random-effects modeling was used. We identified five variables assumed to introduce major heterogeneity into our main models: duration of abstinence (days; alcohol group only), age (years), sex as a biological variable (SABV), MRS sequence-specific echo time (TE in milliseconds (ms) and log-transformed TE), and coefficient of variation (COV). Abstinence duration has been associated with variations in neurometabolite levels (for review see refs. [13, 16]), reflecting at least partial reversibility of metabolite level alterations with abstinence. Most studies included middle aged to older participants, yet a select few reported on young adult alcohol use; thus, given that metabolite levels can be age-dependent, age also introduces heterogeneity. Similarly, most studies included mostly male participants, so SABV (defined as percent of males included in the study) was assessed as clinical research has historically over-sampled males [38], which can have treatment ramifications [39, 40], and there have also been SABV differences in neurometabolite levels across clinical populations [41] and healthy control populations [42]. The TE was included as a variable with potential impact on ability to properly quantify metabolite signals, as its standard has changed over the years with improvements in technical developments [36, 37, 43]. COV is an MRS quality metric that has been shown to moderate metabolite quantification within clinical populations. In a recent meta-analysis, MRS studies with lower COV (considered to be of higher quality) had reduced heterogeneity and increased sensitivity within meta-analytic modeling [44]. COV was derived from the SD divided by the corresponding group mean, calculated [1] with alcohol and control groups combined for overall study data quality and [2] separately for alcohol and control groups.
Results
Study selection
Forty-three studies were included in the meta-analysis (Supplementary Fig. 1). The average sample size (alcohol and control groups combined) for each study was 53 (SD = 43). Eleven studies included only male participants (25.6%), with most studies having more male participants than female participants. The average age of participants included in each study was 42.20 (SD = 8.33; min average age = 21.6, max average age = 64.9). Race/ethnicity were rarely reported, thus was not able to be reported. Approximately half (n = 22, 51.2%) of the studies explicitly reported inclusion of participants who reported tobacco use, while other substance use was not consistently reported. The average standard alcoholic drinks per month for participants in the control group (reported in 37 studies) was 15 (SD = 12) and for the alcohol group (reported in all studies) was 318 (SD = 188). Abstinence duration ranged from active drinking to 1.7 years (median of sample); abstinence duration was not reported for control groups (Supplementary Table 3a).
For MRS, scanner types included Siemens (n = 27; 62.8%), GE (n = 8;18.6%), Philips (n = 4 ;9.3%), Bruker (n = 1; 2.3%), Varian Unity (n = 1; 2.3%), and the magnetic field strengths were 1.5 T (n = 21; 48.8%), 2 or 2.1 T (n = 3; 7.0%), 3 T (n = 16; 37.2%), and 4 T (n = 3; 7.0%). Twelve studies (27.9%) used magnetic resonance spectroscopic imaging (MRSI) to cover multiple brain VOIs, and 31 studies (72.1%) used single-voxel spectroscopy. Acquisition sequences were PRESS (n = 19), MEGA PRESS (n = 3), STEAM (n = 4), short echo (n = 3), J-editing sequence (n = 1), 17-slice turbo spin-echo (n = 1), 2D JD-PRESS (n = 3), and CT-PRESS (n = 1). Software used for analysis consisted of LC Model (n = 15), MATLAB (n = 4), MRUI (n = 2), SITOOLS (n = 3), LUISE (n = 1), manufacturer software (n = 2), automated spectral fitting program (n = 1), or in-house code (n = 18). For metabolite quantification, 25 studies (58.1%) used absolute concentration methods, 12 studies (27.9%) used ratio-to-creatine methods, 2 studies (4.7%) used both absolute concentration and ratio-to-creatine, 3 studies (7.0%) used ratio-to-Water, and 1 study (2.3%) used ratio-to-NAA. Cramér-Rao Lower Bounds (CRLB; data quality metric) cut-offs were reported in 14 studies (32.6%) and Full Width at Half Maximum or shimming values were reported in 2 studies (4.7%) (Supplementary Table 3b).
Overall results from main model
Data were organized by metabolite and volume-of-interest (VOI) (Supplementary Fig. 2), and the overall findings are presented in Fig. 1. Specifically, in alcohol-users compared to controls, lower NAA and Cho metabolite levels were found in many regions of the brain. Lower Cr levels were found in temporal and occipital GM regions, with higher levels only in midbrain/brainstem GM. Lower GABA levels were restricted to the anterior cingulate cortex (ACC). There were no significant differences for mI or glutamate-related (Glu, Gln, or their combination [Glx]) neurometabolite levels. Only significant findings are discussed within the metabolite-specific sections below, with full results detailed in Table 1.
N-acetylaspartate (NAA)
Cortical GM
Altered NAA levels were found within the ACC [32, 45,46,47,48,49,50,51,52,53] and frontal GM [33, 45, 47, 50, 54,55,56,57,58,59,60,61]. Both models indicated lower levels of NAA associated with the alcohol-using group (ACC: k = 14; g = −0.38; 95% CI −0.67 to −0.08; p = 0.02; I2 = 70.6%. Frontal GM: k = 15; g = −0.48; 95% CI −0.76 to −0.20; p = 0.001; I2 = 68.8%) (Fig. 2).
A N-Acetylaspartate (NAA) findings across all volumes-of-interest (VOIs). B Choline-containing metabolites (Cho) across all VOIs. C Creatine-containing metabolites (Cr) across all VOIs. Circle = individual study effect. Diamond = overall effect size. # same study with >1 alcohol groups. ## same study with >1 VOI in brain region. *p < 0.05, **p < 0.01, ***p < 0.001.
White matter
Alcohol use also affected NAA levels within the frontal and parietal WM, with both models indicating significantly lower NAA levels within the alcohol-using group (Frontal WM: k = 17; g = −0.53; 95% CI −0.75 to −0.32; p = 0.000; I2 = 52.7%. Parietal WM: k = 7; g = −0.34; 95% CI −0.58 to −0.10; p = 0.005; I2 = 41.4%) (Fig. 2).
Subcortical GM
Altered levels of NAA were noted within the cerebellum (combined VOIs from cerebellar cortex [56, 57, 59, 62, 63], vermis [47, 54, 55, 57, 61, 64], and dentate nucleus [57]) and the hippocampus [65, 66]. Both models indicated lower NAA levels within the alcohol-using group (Cerebellum: k = 12; g = −0.36; 95% CI −0.65 to −0.07; p = 0.015; I2 = 66.6%. Hippocampus: k = 2; g = −1.79; 95% CI −3.28 to −0.30; p = 0.018; I2 = 80.4%) (Fig. 2). The model for the thalamus approached significance (k = 7; g = −0.25; 95% CI −0.53 to 0.02; p = 0.068) (Table 1). Removing the only study in treatment-naïve individuals [33] during the remove-one sensitivity analysis yielded a significant model for thalamic NAA.
Choline-containing metabolites (Cho)
Cortical GM
Cho levels were lower in alcohol-using groups compared to controls in the frontal [33, 45, 47, 50, 54,55,56,57,58,59,60, 67] and temporal GM [45, 55] (Frontal GM: k = 15; g = −0.26; 95% CI −0.46 to −0.05; p = 0.016; I2 = 44.6%. Temporal GM: k = 2; g = −0.49; 95% CI −0.83 to −0.11; p = 0.010; I2 = 0.0%). The effect of alcohol use on lower parietal GM Cho levels was marginal (k = 5; g = −0.27; 95% CI −0.04 to 0.59; p = 0.089; I2 = 45.7%) (Fig. 2).
White matter
Main models for frontal [33, 47, 51, 54, 55, 57, 60, 62, 68,69,70,71,72] and parietal WM [33, 54, 55, 70, 71, 73] were significant, indicating lower levels of Cho associated with the alcohol-using group (Frontal WM: k = 16; g = −0.30; 95% CI −0.52 to −0.07; p = 0.009; I2 = 51.6%. Parietal WM: k = 6; g = −0.28; 95% CI −0.52 to −0.04; p = 0.024; I2 = 28.0%) (Fig. 2). For parietal WM, the remove-one sensitivity analysis resulted in an insignificant model when four effects were individually removed from the model [54, 55, 70, 73]. Therefore, as the significance of this model is strongly influenced by individual studies, parietal WM Cho loss should be interpreted with caution.
Subcortical GM
The cerebellum (combined VOIs from cerebellar cortex [56, 57, 59, 62, 63], vermis [47, 54, 55, 57, 64], and dentate nucleus [57]) and thalamus models also indicated lower Cho in alcohol-using groups (Cerebellum: k = 12; g = −0.54; 95% CI −0.83 to −0.25; p = 0.000; I2 = 62.2%. Thalamus: k = 8; g = −0.29; 95% CI −0.53 to −0.05; p = 0.016; I2 = 15.7%) (Fig. 2). For the cerebellum, the trim and fill method indicated two additional effects would correct funnel asymmetry from publication bias (adjusted effect size g = −0.41). The model for the striatum approached significance (k = 5; g = −0.21; 95% CI −0.44 to 0.01; p = 0.061; I2 = 0.0%), becoming significant when one effect [54] was removed in a follow-up sensitivity analysis.
Creatine-containing metabolites (Cr)
Cortical GM
Both occipital [54, 74] and temporal GM [45, 55] models resulted in significanlty lower levels of Cr within alcohol-using groups than controls (Occipital GM: k = 2; g = −0.46; 95% CI −0.87 to −0.05; p = 0.027; I2 = 0.0%. Temporal GM: k = 2; g = −0.42; 95% CI −0.77 to −0.06; p = 0.022; I2 = 0.0%) (Fig. 2).
White matter
There were no significant alterations in Cr levels associated with alcohol use in white matter VOIs (Fig. 2).
Subcortical GM
Within the midbrain/brainstem VOIs [33, 54, 55, 75], the main model indicated significantly higher Cr levels in alcohol-using groups relative to controls (k = 4; g = 0.83; 95% CI 0.25 to 1.41; p = 0.005; I2 = 66.0%) (Fig. 2).
Myo-Inositol (mI)
There were no significant models with any VOI (Supplementary Fig. 3).
Glutamate, Glutamine, Glx (Glu, Gln, Glx)
The meta-analytic models for Glu, Gln, and Glx were all insignificant (Supplementary Fig. 4). Possible reasons for this are considered in the Discussion section.
GABA
The only significant model for GABA was from the ACC [31, 32, 45, 50, 76, 77]; GABA levels were lower in the alcohol-using group than controls (k = 6; g = −0.35; 95% CI −0.64 to −0.06; p = 0.016; I2 = 21.6%) (Fig. 3). The remove-one sensitivity analysis resulted in an insignificant model when three studies were individually removed [31, 32, 77]. Each of these studies investigated younger, treatment-naïve samples, suggesting that age and symptom severity affect GABA levels.
Sub-group analyses
Treatment v. treatment-naïve studies
There was one significant effect for treatment-seeking status, where studies including only treatment-seekers had significantly lower levels of NAA within frontal WM of alcohol groups compared to controls (treatment seeking k = 14, g = −0.63, p < 0.001, non-treatment seeking k = 3, g = −0.02, p = 0.953; Q = 4.73, p = 0.03; Table 2).
Absolute concentration v. relative concentration (Ratio-to-Cr)
Sub-group analysis found a significant difference for metabolite quantification type within frontal GM NAA. Only ratio-to-Cr studies showed significantly lower NAA levels in alcohol groups compared to controls, whereas the absolute concentration studies were only marginally different from controls (absolute concentration k = 11, g = −0.28, p = 0.06, ratio-to-Cr k = 4, g = −1.16, p < 0.001; Q = 7.92, p = 0.005; Table 2).
Corresponding exploratory sub-group analyses (when k < 10) were conducted for parietal GM NAA; occipital GM NAA, Cho, and mI; and cerebellar NAA and Cho. Analyses for occipital GM Cho and cerebellar NAA indicated that only ratio-to-Cr studies were significantly different from controls, while the corresponding absolute concentration studies were not. This is particularly consequential for occipital GM, where the main model resulted in significantly lower levels of Cr for alcohol-using groups, as compared to controls. The possibility that Cr levels in the brain are affected by alcohol use demands caution when interpreting reports of ratio-to-Cr group differences.
Meta-regression
Abstinence duration, age, and SABV (% Male)
Abstinence duration (reported only for alcohol groups; Table 3) showed a significant positive association (longer abstinence duration, greater positive study effect size) with frontal WM NAA levels (k = 17; β = 0.01, p = 0.045; R2 = 0.21). Conversely, abstinence duration showed a negative association (longer abstinence, greater negative study effect size) for ACC Glu levels (k = 15; β = −0.004, p = 0.036; R2 = 0.26). Both models became insignificant when an outlying long-term abstinent group was removed (mean time = 260 days from ref. [51]; mean time = 365 days from ref. [52], respectively; Supplementary Figs. 5 and 6).
Age (average across alcohol and control groups) was positively correlated with effect sizes for cerebellar NAA (k = 12; β = 0.13, p = 0.01; R2 = 0.38) and Cho levels (k = 11; β = 0.15, p = 0.000; R2 = 0.95) (Table 3; Supplementary Fig. 7).
SABV (defined as percent of males included in the study) was negatively associated with effect sizes for Cho levels in the ACC (k = 13; β = −0.02, p = 0.01; R2 = 0.68) and positively associated with effect sizes for Cho levels in the cerebellum (k = 12, β = 0.03, p < 0.001; R2 = 1.00) (Table 3; Supplementary Fig. 7).
MRS specific TE and data quality variables (COV)
The log-transformed TE was found to affect study effect sizes within GM (Table 4, Supplementary Fig. 8). Positive associations (largest positive study effect size at longer TE) were found between log-transformed TE and study effect sizes for ACC Glu (k = 12, β = 1.40, p = 0.002, R2 = 0.66) and Cr (k = 10, β = 1.14 p = 0.039, R2 = 0.42). Negative associations (largest negative study effect size at longer TE) were found between log-transformed TE and study effect sizes for frontal GM NAA (k = 14, β = −1.07, p = 0.004, R2 = 0.29) and Cho (k = 15, β = −0.71, p = 0.004, R2 = 0.77); and cerebellar Cho (k = 12, β = −0.90, p = 0.005, R2 = 0.68). Meta-regressions with the raw TE (ms) support these results, except for the meta-regression within ACC which became insignificant (Supplementary Table 4).
MRS data quality (COV; lower number reflects higher quality data) had no significant effects when the alcohol and control groups were combined (Table 3). When looking at COV within alcohol and control groups separately, the alcohol group had a negative association between COV and study effect sizes for Cr within the ACC (k = 11, β = −5.91, p = 0.005, R2 = 0.65). This suggests lower-quality data (higher COV) within the alcohol group was related to more negative effect sizes. For the control group, there as a positive association (higher-quality data related to more positive effect sizes) between COV and study effect sizes for NAA in the frontal GM (k = 15, β = 9.01, p = 0.019, R2 = 0.18). See Supplementary Materials for tables and figures (Supplementary Tables 4, 5; Supplementary Fig. 9).
Discussion
The objective of this meta-analysis was to assess the difference in MRS-determined brain metabolite levels in alcohol misuse and AUD groups relative to controls. Combining studies allowed for identification of significant neurometabolite alterations above and beyond the individual study level, providing better consolidated insights for future neuroscience-informed alcohol treatment development. The main findings reflect alcohol-related alterations in systems representing neuronal/axonal/mitochondrial function (NAA), membrane synthesis and structure (Cho), bioenergetics (Cr), and inhibitory neurotransmission (GABA). Neither brain regions nor metabolites were affected ubiquitously, supporting the idea that we are not capturing generalized toxic effects of alcohol misuse. NAA and Cho had the most alcohol-related aberrations throughout the brain. In alcohol users compared to controls, lower levels of NAA were observed in GM (ACC, frontal, temporal, hippocampal and cerebellar VOIS) and WM (frontal and parietal VOIs). Lower Cho levels were also observed in GM (frontal, temporal, and thalamic VOIs) and WM (frontal and parietal VOIs). Lower Cr levels were detected in temporal and occipital GM cortices, whereas Cr levels in midbrain/brainstem GM were higher. Alterations in GABA were restricted to the ACC, with (mostly younger, treatment naïve) alcohol users having lower levels of GABA than controls. There were no significant group differences for myo-inositol or Glu-related metabolite levels; the latter may be due to uncontrolled factors affecting Glu levels across studies. This meta-analysis is the first to provide formalized evidence that alcohol and AUD-related neurobiological alterations measured by MRS are present in many brain regions and tissues, affecting specific metabolites typically measured by MRS.
Importantly, some main models in this meta-analysis revealed significant neurometabolite alterations related to alcohol use above and beyond the individual study findings. This was particularly apparent for Cho (thalamus, frontal and parietal GM and WM), NAA (ACC), and Cr (occipital, temporal, and midbrain/brainstem GM), highlighting the importance of meta-analytic applications. Recognizing the influence of alcohol misuse on these metabolites may open new research lines into direct (e.g., pharmacotherapy) or indirect (e.g., efficacy markers) treatment targets.
While the lack of Glu-related metabolite findings may surprise at first glance, considering the well-studied relationship between alcohol use and glutamatergic neurotransmission (e.g., [78,79,80], many factors may explain the non-significant Glu-related models. Glutamate and Gln levels are intrinsically dynamic, related to highly regulated Glu homeostasis across different phases of consumption, withdrawal, recovery, and relapse [78,79,80]. In addition, there are known methodological factors influencing the ability to quantify Glu and low-intensity Gln multiplets reliably with MRS (e.g., TE, magnet strength) [81]. Together, they may account for the null findings. The detection of substantial heterogeneity across the Glu-related models was not surprising, and our exploration of potential sources of heterogeneity provided some insights for Glu within the ACC (the only region with enough data to complete analyses). In contrast with existing longitudinal studies measuring Glu in treatment-seekers after alcohol cessation [48, 50, 52], we did not find a strong association between abstinence duration and levels of Glu across studies. While the meta-regression with all studies indicated a negative association between study effect size in the ACC and abstinence duration (longer abstinence, lower Glu levels in the alcohol groups v. controls), this was driven by one study that included individuals abstinent for at least 365 days [52] and became insignificant during a sensitivity analysis. This finding is consistent with varying Glu levels across different phases of alcohol use and remission. Most studies assessing Glu levels were between 0 and 14 days of abstinence, so this relationship may be better understood with the inclusion of future cross-sectional and longitudinal studies across varying levels of abstinence. Significant meta-regressions were found for TE, suggesting an influence of echo time Glu metabolite levels. This finding speaks to the importance of using sequence parameters optimized for metabolites of interest, which are often updated through expert consensus recommendations (see refs. [36, 37, 82]). Taken together, the demographic and methodological heterogeneity may contribute to the difficultly to understand the relationship between alcohol misuse/AUD and Glu and its intermediates with the available cross-sectional MRS studies since the low number of effects limited further investigation (meta-regression or sub-group analysis) into such heterogeneity. Future conduct of longitudinal studies together with standardization of MRS parameters and quantification methods, or the adaptation of novel imaging techniques (i.e., GluCEST[83, 84]), may provide more meaningful insights on glutamatergic alterations in AUD.
Eight studies reported on GABA levels across four cortical brain regions, revealing significant differences between alcohol users and controls in the ACC only. As indicated by the remove-one analysis, younger adults with relatively short abstinence duration appear to drive the lower GABA levels, an observation that warrants follow-up. The measurement of GABA via MRS is relatively difficult given its low concentration and spectral overlap with other resonances [82]. All but two studies reporting GABA levels used an appropriate edited sequence (MEGA-PRESS or 2D J-PRESS) increasing the confidence in the findings [82, 85]. As with glutamatergic metabolites, the small number of studies (compared to NAA, Cho, Cr, and mI) and methodological complications may limit the current findings; thus, future standardized clinical MRS studies should further clarify alcohol effects on both Glu and GABA levels.
Heterogeneity is a common concern in meta-analyses. In the current work, 35.8% (24/69) of the main models showed moderate to substantial heterogeneity determined by I2 (Table 3). This type of data heterogeneity could be based in variations of alcohol- and control group inclusion criteria, demographics, MRS parameters, and MRS quality metrics. Heterogeneity is an important limitation to consider when interpreting findings within this meta-analysis, particularly within models with small number of effects (k < 10). Sub-group analyses and meta-regressions were included to probe at potential sources of demographic and MRS-specific heterogeneity across the models.
Most MRS studies included in this meta-analysis were within treatment-seeking individuals (74.4%), a group known to differ in demographic, clinical, and alcohol consumption variables from their treatment-naïve counterparts [27,28,29,30]. The sub-group analysis found an effect of treatment-seeking status on frontal WM NAA effects (lower levels in studies of treatment-seekers), and the remove-one sensitivity analysis also indicated a significant thalamic NAA model for studies in treatment-seekers only. Whereas most AUD research has been conducted with individuals seeking treatment [27, 28], the majority of individuals with problematic drinking do not seek treatment [86]. Relying on treatment-seeking status as a proxy for alcohol use severity is also limited, which is further complicated by the variety of healthcare systems, treatment options, and diagnosis criteria available in different countries. This creates a problem of generalizability of research findings to the much larger untreated AUD population and future studies might consider this disparity appropriately.
Meta-regressions were conducted to explore other potential sources demographic heterogeneity, including abstinence duration and age. Some longitudinal studies in treatment-seekers have noted corresponding changes in brain metabolites after variable lengths of abstinence [48, 50, 56, 57, 87]. Abstinence duration does not appear to be consistently related to differences in metabolite levels between alcohol and control groups based on the current meta-regressions, yet there were two significant models of note. First, as previously discussed, there was a negative association between abstinence duration and Glu in the ACC (longer abstinence, lower levels of Glu in alcohol groups). Second, there was a significant positive effect of abstinence duration on frontal WM NAA study effect sizes (longer abstinence, higher levels of NAA in alcohol groups v controls). Thus, the meta-regressions were able to highlight metabolites that are potentially sensitive to abstinence based on the current studies available. Studies providing increased granularity of abstinence duration will help to clarify this finding, as both models became insignificant when the study with the longest duration was removed. There was also an age effect on cerebellar NAA and Cho study effect sizes. While the age range was very narrow (average age 41–53), the age association might suggest specific cerebellar vulnerability in younger individuals who misuse alcohol. Future properly powered longitudinal studies will be able to provide a more specific timeframe of abstinence-related changes in different populations and better address the importance of alcohol-related alterations across the lifespan.
We also investigated the impact of MRS quantification type in a sub-group analysis and of MRS-specific TE and data quality (COV) in meta-regressions. MRS metabolite levels can be expressed as absolute concentrations [88,89,90] or relative concentrations (e.g, ratio-to-Cr or to-water); the latter assumes Cr or water levels as a stable internal concentration for reference [35]. Almost one-third of the included studies used ratio-to-Cr when reporting metabolite levels. The ratio-to-Cr approach, representing an internal calibration in attempts to reduce variance from uncontrolled sources of signal variability, works well when the Cr signals are invariant. If alcohol affects regional Cr levels, normalization to Cr may lead to erroneous interpretation of metabolite levels, calling into question this quantification method in this population. Indeed, we found that absolute Cr levels in alcohol-users were significantly altered in several brain regions investigated. These findings suggest that future studies should, if possible, avoid using ratio-to-Cr methods within alcohol misuse and AUD populations and be cautious of results using it may lead to erroneous conclusions.
Our results, and those of other [36, 44], show that TE influences effect size, making it a critical issue when designing new clinical studies. MRS-specific TE parameters specifically influenced GM study effects in the ACC (Cr and Glu), frontal GM (NAA and Cho), and cerebellum (Cho) (note: these models used log-transformed TE and all except ACC Cr meta-regressions were replicated with the raw TE). The effects of TE were consistently positive in the ACC (longer TE associated with higher levels of metabolites in alcohol groups) and consistently negative in the frontal GM and cerebellum (longer TE associated with lower levels of metabolites in alcohol groups). While the number of effects for some metabolites with relatively long TEs were limiting, the findings likely reflect the ability to measure peak areas in the presence of overlapping resonances. Imaging techniques and user expertize improve over time; therefore, it is important for future work to use the expert consensus recommendations for MRS sequence parameters, processing techniques, and data reporting to standardize the field and reduce heterogeneity among studies [36, 37, 82].
There are limitations to the current work. First, several variables of interest, such as additional data quality metrics (e.g., spectral linewidth, metabolite CRLB, or signal-to-noise ratio) and alcohol intake (better characterization of alcohol use severity on metabolite levels across the brain) were not able to be included in sub-group or meta-regressions due to the inconsistency in reporting across studies. Field strength was also not able to be assessed due to the majority of studies being either 1.5 T or 3 T, limiting the ability to run sub-group analyses or meta-regressions. Second, most of the studies available were comprised of older and overwhelmingly male populations, making both the percent male and the age meta-regressions limited in scope. Significant findings for both age and SABV indicates the need for future studies to be mindful of age and SABV when recruiting, as these factors could impact the generalizability of findings. Other important variables, such as race/ethnicity, or other substance use were rarely reported. Third, there were various processing pipelines and software used, which were not always described well enough to ensure reproducibility. As with other imaging techniques, the effects of software and various modeling choices is becoming increasingly more apparent within MRS data [91,92,93]. Fourth, there was not a “true” control for each model as the inclusion criteria varied from study to study; thus, some studies allowed alcohol consumption within the control group at a low frequency and quantity while others did not. Lastly, a limitation of MRS is that signals are derived and averaged over large voxels within the brain with varying tissue composition, precluding the ability to understand what is happening at a cellular level. The type of voxel (i.e., gray or white matter) was determined based on the description within the study, yet the actual percentage of various tissues were rarely reporting limiting the ability to cross check the VOI name with actual tissue composition. More studies are needed to better understand regionally specific mechanisms associated with alcohol specific MRS findings to optimize the translation value, as well as to investigate if “normalization” of metabolite levels corresponds with a significant treatment effect and improved AUD symptomology. These limitations highlight the need for standardization in data reporting; inclusion of participants across sex, age, and race/ethnicity to better generalize results; and further research to translate the MRS-derived findings to neurobiological mechanisms to support treatment development and assessment for AUD.
Conclusion
Above and beyond individual studies, this meta-analysis provides formalized evidence that MRS can help identify the brain location(s) and types of metabolite(s) impacted by alcohol misuse and AUD for the first time. Future studies can leverage this new information to investigate specific pharmacological or other (e.g., behavioral, neurostimulation) treatments that might directly or indirectly effectively target the observed meta-neurometabolic disturbances. Region-specific alterations were detected for certain metabolites, with most significant models in cortical or subcortical GM. Specifically, lower levels of NAA and Cho were the most pervasive alcohol-related alterations throughout brain regions. Lower NAA levels likely indicate a decrease in neuronal (GM) and axonal (WM) viability or function, and changes in Cho metabolites may indicate altered cerebral cell synthesis and membrane turnover related to alcohol misuse. Thus, having been shown to be vulnerable to chronic alcohol use, these metabolites may constitute important targets for treatment or markers of treatment efficacy. Models for Glu/Gln/Glx were not significant, potentially reflecting the highly dynamic behavior of glutamatergic tone with treatment status and duration of abstinence and/or technical challenges in reliably measuring these metabolites [44, 81]. In addition, the few GABA studies conducted showed significantly lower levels of GABA within the ACC, suggesting that GABA-ergic treatment targets may continue to be of interest for future clinical trials. Overall, beyond surveying the extant literature, summarizing the chronic brain effects of AUD and highlighting specific needs for future studies, this work exemplifies the importance and potential translational value of using neuroscience-based approaches toward the development of new treatments for individuals with neuropsychiatric disorders.
References
Esser MB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC, Naimi TS. Prevalence of alcohol dependence among US adult drinkers, 2009–2011. Prev Chronic Dis. 2014;11:E206.
U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th Edition. December 2020. Available at DietaryGuidelines.gov.
Global status report on alcohol and health 2018. Geneva: World Health Organization; 2018. Licence: CC BY-NC-SA 3.0IGO.
Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757–66.
Witkiewitz K, Litten R, Leggio L. Advances in the science and treatment of alcohol use disorder. Sci Adv. 2019;5:eaax4043.
Jenson C, Cushing C, Aylward B, Craig J, Sorell D, Steele R. Effectiveness of motivational interviewing for adolescent substance use behavior change: a meta-analytic review. J Consulting Clin Psychol. 2011;79:433–40.
Blodgett JC, Del ReA, Maisel NC, Finney JW. A meta‐analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol: Clin Exp Res. 2014;38:1481–8.
Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta‐analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction 2013;108:275–93.
Gilpin NW, Koob GF. Neurobiology of alcohol dependence: focus on motivational mechanisms. Alcohol Res Health. 2008;31:185.
Robbins TW, Everitt B. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–36.
Hellem T, Shi X, Latendresse G, Renshaw PF. The utility of magnetic resonance spectroscopy for understanding substance use disorders: a systematic review of the literature. J Am Psychiatr Nurses Assoc. 2015;21:244–75.
Soares D, Law M. Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clin Radiol. 2009;64:12–21.
Meyerhoff DJ, Durazzo TC, Ende G. Chronic Alcohol Consumption, Abstinence and Relapse: Brain Proton Magnetic Resonance Spectroscopy Studies in Animals and Humans. In: Sommer W, Spanagel R, editors. Behavioral Neurobiology of Alcohol Addiction. Current Topics in Behavioral Neurosciences, vol 13. Berlin, Heidelberg: Springer; 2011. https://doi.org/10.1007/978-3-642-28720-6_131.
Meyerhoff DJ, Durazzo TC. Proton magnetic resonance spectroscopy in alcohol use disorders: a potential new endophenotype? Alcohol: Clin Exp Res. 2008;32:1146–58.
Rae CD. A guide to the metabolic pathways and function of metabolites observed in human brain 1 H magnetic resonance spectra. Neurochem Res. 2014;39:1–36.
Meyerhoff DJ. Brain proton magnetic resonance spectroscopy of alcohol use disorders. Handb Clin Neurol. 2014;125:313–37.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n160.
Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open. 2016;6:e011458.
Peek AL, Rebbeck T, Puts NA, Watson J, Aguila M-ER, Leaver AM. Brain GABA and glutamate levels across pain conditions: a systematic literature review and meta-analysis of 1H-MRS studies using the MRS-Q quality assessment tool. Neuroimage 2020;210:116532.
Borenstein M, Hedges L, Rothstein H. Comprehensive Meta-Analysis Version 3. 3 ed. Englewood, NJ: Biostat; 2013.
Hedges L, Olkin I. Statistical Methods for Meta-Analysis. San Diego, CA: Academic; 1985.
Borenstein M, Higgins JP. Meta-analysis and subgroups. Prev Sci. 2013;14:134–43.
Duval S, Tweedie R. Trim and fill: a simple funnel‐plot–based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56:455–63.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj 1997;315:629–34.
Richardson M, Garner P, Donegan S. Interpretation of subgroup analyses in systematic reviews: a tutorial. Clin Epidemiol Glob Health. 2019;7:192–8.
Borenstein M, Cooper H, Hedges L, Valentine J. Effect sizes for continuous data. Handb Res Synth Meta-Anal. 2009;2:221–35.
Fein G, Landman B. Treated and treatment-naive alcoholics come from different populations. Alcohol 2005;35:19–26.
Ray LA, Bujarski S, Yardley MM, Roche DJ, Hartwell EE. Differences between treatment-seeking and non-treatment-seeking participants in medication studies for alcoholism: do they matter? Am J Drug Alcohol Abus. 2017;43:703–10.
Haass‐Koffler CL, Piacentino D, Li X, Long VM, Lee MR, Swift RM, et al. Differences in Sociodemographic and Alcohol‐Related Clinical Characteristics Between Treatment Seekers and Nontreatment Seekers and Their Role in Predicting Outcomes in the COMBINE Study for Alcohol Use Disorder. Alcohol: Clin Exp Res. 2020;44:2097–108.
Rohn MC, Lee MR, Kleuter SB, Schwandt ML, Falk DE, Leggio L. Differences between treatment‐seeking and nontreatment‐seeking alcohol‐dependent research participants: an exploratory analysis. Alcohol: Clin Exp Res. 2017;41:414–20.
Prisciandaro JJ, Schacht JP, Prescot AP, Brenner HM, Renshaw PF, Brown TR, et al. Intraindividual changes in brain GABA, glutamate, and glutamine during monitored abstinence from alcohol in treatment‐naive individuals with alcohol use disorder. Addiction Biol. 2020;25:e12810.
Prisciandaro JJ, Schacht JP, Prescot AP, Renshaw PF, Brown TR, Anton RF. Brain glutamate, GABA, and glutamine levels and associations with recent drinking in treatment‐naive individuals with alcohol use disorder versus light drinkers. Alcohol: Clin Exp Res. 2019;43:221–6.
Meyerhoff D, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, et al. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcohol: Clin Exp Res. 2004;28:650–61.
Barantin L, Pape AL, Akoka S. A new method for absolute quantitation MRS metabolites. Magn Reson Med. 1997;38:179–82.
Li BS, Wang H, Gonen O. Metabolite ratios to assumed stable creatine level may confound the quantification of proton brain MR spectroscopy. Magn Reson imaging. 2003;21:923–8.
Öz G, Deelchand DK, Wijnen JP, Mlynárik V, Xin L, Mekle R, et al. Advanced single voxel 1H magnetic resonance spectroscopy techniques in humans: experts’ consensus recommendations. NMR Biomed. 2021;34:e4236.
Wilson M, Andronesi O, Barker PB, Bartha R, Bizzi A, Bolan PJ, et al. Methodological consensus on clinical proton MRS of the brain: Review and recommendations. Magn Reson Med. 2019;82:527–50.
Zucker I, Prendergast BJ. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol Sex Differ. 2020;11:1–14.
Agabio R, Pani PP, Preti A, Gessa GL, Franconi F. Efficacy of medications approved for the treatment of alcohol dependence and alcohol withdrawal syndrome in female patients: a descriptive review. Eur Addict Res. 2016;22:1–16.
Agabio R, Pisanu C, Luigi Gessa G, Franconi F. Sex differences in alcohol use disorder. Curr Med Chem. 2017;24:2661–70.
Endres D, Tebartz van Elst L, Maier SJ, Feige B, Goll P, Meyer SA, et al. Neurochemical sex differences in adult ADHD patients: an MRS study. Biol Sex Differ. 2019;10:1–11.
Jung RE, Haier RJ, Yeo RA, Rowland LM, Petropoulos H, Levine AS, et al. Sex differences in N-acetylaspartate correlates of general intelligence: an 1H-MRS study of normal human brain. Neuroimage 2005;26:965–72.
Prisciandaro JJ, Mikkelsen M, Saleh MG, Edden RA. An evaluation of the reproducibility of 1H-MRS GABA and GSH levels acquired in healthy volunteers with J-difference editing sequences at varying echo times. Magn Reson Imaging. 2020;65:109–13.
Smucny J, Carter CS, Maddock RJ. Medial Prefrontal Cortex Glutamate is Reduced in Schizophrenia and Moderated by Measurement Quality: a Meta-analysis of 1H-MRS Studies. Biol Psychiatry. 2021;90:643–51.
Abé C, Mon A, Durazzo TC, Pennington DL, Schmidt TP, Meyerhoff DJ. Polysubstance and alcohol dependence: unique abnormalities of magnetic resonance-derived brain metabolite levels. Drug Alcohol Depend. 2013;130:30–7.
Bauer J, Pedersen A, Scherbaum N, Bening J, Patschke J, Kugel H, et al. Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology 2013;38:1401–8.
Durazzo TC, Pathak V, Gazdzinski S, Mon A, Meyerhoff DJ. Metabolite levels in the brain reward pathway discriminate those who remain abstinent from those who resume hazardous alcohol consumption after treatment for alcohol dependence. J Stud Alcohol Drugs. 2010;71:278–89.
Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, Tunc-Skarka N, et al. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol Psychiatry. 2012;71:1015–21.
Lee E, Jang D-P, Kim J-J, An SK, Park S, Kim I-Y, et al. Alteration of brain metabolites in young alcoholics without structural changes. Neuroreport 2007;18:1511–4.
Mon A, Durazzo TC, Meyerhoff DJ. Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend. 2012;125:27–36.
Schweinsburg BC, Taylor MJ, Videen JS, Alhassoon OM, Patterson TL, Grant I. Elevated myo‐inositol in gray matter of recently detoxified but not long‐term abstinent alcoholics: a preliminary MR spectroscopy study. Alcohol: Clin Exp Res. 2000;24:699–705.
Thoma R, Mullins P, Ruhl D, Monnig M, Yeo RA, Caprihan A, et al. Perturbation of the glutamate–glutamine system in alcohol dependence and remission. Neuropsychopharmacology 2011;36:1359–65.
Yeo RA, Thoma RJ, Gasparovic C, Monnig M, Harlaar N, Calhoun VD, et al. Neurometabolite concentration and clinical features of chronic alcohol use: a proton magnetic resonance spectroscopy study. Psychiatry Res: Neuroimaging. 2013;211:141–7.
Abé C, Mon A, Hoefer ME, Durazzo TC, Pennington DL, Schmidt TP, et al. Metabolic abnormalities in lobar and subcortical brain regions of abstinent polysubstance users: magnetic resonance spectroscopic imaging. Alcohol Alcohol. 2013;48:543–51.
Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcohol‐induced brain damage: a preliminary metabolite imaging study. Alcohol: Clin Exp Res. 2004;28:1849–60.
Bendszus M, Weijers H-G, Wiesbeck G, Warmuth-Metz M, Bartsch AJ, Engels S, et al. Sequential MR imaging and proton MR spectroscopy in patients who underwent recent detoxification for chronic alcoholism: correlation with clinical and neuropsychological data. Am J Neuroradiol. 2001;22:1926–32.
Ende G, Welzel H, Walter S, Weber-Fahr W, Diehl A, Hermann D, et al. Monitoring the effects of chronic alcohol consumption and abstinence on brain metabolism: a longitudinal proton magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58:974–80.
Fein G, Meyerhoff D, Di Sclafani V, Ezekiel F, Poole N, MacKay S, et al. 1H magnetic resonance spectroscopic imaging separates neuronal from glial changes in alcohol-related brain atrophy. Chapter NIAAA Res Monogr No. 1994;27:227–41.
Jagannathan N, Desai N, Raghunathan P. Brain metabolite changes in alcoholism: an in vivo proton magnetic resonance spectroscopy (MRS) study. Magn Reson Imaging. 1996;14:553–7.
Schweinsburg BC, Alhassoon OM, Taylor MJ, Gonzalez R, Videen JS, Brown GG, et al. Effects of alcoholism and gender on brain metabolism. Am J Psychiatry. 2003;160:1180–3.
Xia Y, Ma D, Hu J, Tang C, Wu Z, Liu L, et al. Effect of metabotropic glutamate receptor 3 genotype on N-acetylaspartate levels and neurocognition in non-smoking, active alcoholics. Behav Brain Funct. 2012;8:1–11.
Parks MH, Dawant BM, Riddle WR, Hartmann SL, Dietrich MS, Nickel MK, et al. Longitudinal brain metabolic characterization of chronic alcoholics with proton magnetic resonance spectroscopy. Alcohol: Clin Exp Res. 2002;26:1368–80.
Seitz D, Widmann U, Seeger U, Nägele T, Klose U, Mann K, et al. Localized proton magnetic resonance spectroscopy of the cerebellum in detoxifying alcoholics. Alcohol: Clin Exp Res. 1999;23:158–63.
Martin PR, Gibbs SJ, Nimmerrichter AA, Riddle WR, Welch LW, Willcott MR. Brain proton magnetic resonance spectroscopy studies in recently abstinent alcoholics. Alcohol: Clin Exp Res. 1995;19:1078–82.
Frischknecht U, Hermann D, Tunc‐Skarka N, Wang GY, Sack M, van Eijk J, et al. Negative association between MR‐spectroscopic glutamate markers and gray matter volume after alcohol withdrawal in the hippocampus: a translational study in humans and rats. Alcohol: Clin Exp Res. 2017;41:323–33.
Gazdzinski S, Durazzo TC, Yeh P-H, Hardin D, Banys P, Meyerhoff DJ. Chronic cigarette smoking modulates injury and short-term recovery of the medial temporal lobe in alcoholics. Psychiatry Res: Neuroimaging. 2008;162:133–45.
de Souza RSM, Rosa M Jr, Rodrigues TM, Escobar TDC, Gasparetto EL, Nakamura-Palacios EM. Lower choline rate in the left prefrontal cortex is associated with higher amount of alcohol use in alcohol use disorder. Front Psychiatry. 2018;9:563.
Wang JJ, Durazzo TC, Gazdzinski S, Yeh PH, Mon A, Meyerhoff DJ. MRSI and DTI: a multimodal approach for improved detection of white matter abnormalities in alcohol and nicotine dependence. NMR Biomed: Int J Devoted Dev Appl Magn Resonance In vivo. 2009;22:516–22.
Ende G, Hermann D, Demirakca T, Hoerst M, Tunc‐Skarka N, Weber‐Fahr W, et al. Loss of control of alcohol use and severity of alcohol dependence in non‐treatment‐seeking heavy drinkers are related to lower glutamate in frontal white matter. Alcohol: Clin Exp Res. 2013;37:1643–9.
Gazdzinski S, Durazzo TC, Mon A, Yeh P-H, Meyerhoff DJ. Cerebral white matter recovery in abstinent alcoholics—a multimodality magnetic resonance study. Brain 2010;133:1043–53.
Schweinsburg BC, Taylor MJ, Alhassoon OM, Videen JS, Brown GG, Patterson TL, et al. Chemical pathology in brain white matter of recently detoxified alcoholics: a 1H magnetic resonance spectroscopy investigation of alcohol‐associated frontal lobe injury. Alcohol: Clin Exp Res. 2001;25:924–34.
Zahr NM, Carr RA, Rohlfing T, Mayer D, Sullivan EV, Colrain IM, et al. Brain metabolite levels in recently sober individuals with alcohol use disorder: Relation to drinking variables and relapse. Psychiatry Res: Neuroimaging. 2016;250:42–9.
Durazzo TC, Gazdzinski S, Rothlind JC, Banys P, Meyerhoff DJ. Brain metabolite concentrations and neurocognition during short‐term recovery from alcohol dependence: preliminary evidence of the effects of concurrent chronic cigarette smoking. Alcohol: Clin Exp Res. 2006;30:539–51.
Mason GF, Petrakis IL, de Graaf RA, Gueorguieva R, Guidone E, Coric V, et al. Cortical gamma-aminobutyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biol Psychiatry. 2006;59:85–93.
Bloomer CW, Langleben DD, Meyerhoff DJ. Magnetic resonance detects brainstem changes in chronic, active heavy drinkers. Psychiatry Res: Neuroimaging. 2004;132:209–18.
Prisciandaro J, Tolliver B, Prescot A, Brenner H, Renshaw P, Brown T, et al. Unique prefrontal GABA and glutamate disturbances in co-occurring bipolar disorder and alcohol dependence. Transl Psychiatry 2017;7:e1163–e.
Silveri MM, Cohen‐Gilbert J, Crowley DJ, Rosso IM, Jensen JE, Sneider JT. Altered anterior cingulate neurochemistry in emerging adult binge drinkers with a history of alcohol‐induced blackouts. Alcohol: Clin Exp Res. 2014;38:969–79.
Gonzales RA, Jaworski JN. Alcohol and glutamate. Alcohol Health Res World. 1997;21:120.
Brousse G, Arnaud B, Vorspan F, Richard D, Dissard A, Dubois M, et al. Alteration of glutamate/GABA balance during acute alcohol withdrawal in emergency department: a prospective analysis. Alcohol Alcohol. 2012;47:501–8.
Spanagel R. The role of the glutamatergic system in alcohol addiction. Fortschr der Neurologie-Psychiatr. 2003;71:S33–5.
Ramadan S, Lin A, Stanwell P. Glutamate and glutamine: a review of in vivo MRS in the human brain. NMR Biomed. 2013;26:1630–46.
Choi IY, Andronesi OC, Barker P, Bogner W, Edden RA, Kaiser LG, et al. Spectral editing in 1H magnetic resonance spectroscopy: Experts’ consensus recommendations. NMR Biomed. 2021;34:e4411.
Cai K, Haris M, Singh A, Kogan F, Greenberg JH, Hariharan H, et al. Magnetic resonance imaging of glutamate. Nat Med. 2012;18:302–6.
Cai K, Singh A, Roalf DR, Nanga RPR, Haris M, Hariharan H, et al. Mapping glutamate in subcortical brain structures using high‐resolution GluCEST MRI. NMR Biomed. 2013;26:1278–84.
Harris AD, Saleh MG, Edden RA. Edited 1H magnetic resonance spectroscopy in vivo: Methods and metabolites. Magn Reson Med. 2017;77:1377–89.
Tuithof M, Ten Have M, Van Den Brink W, Vollebergh W, De Graaf R. Treatment seeking for alcohol use disorders: treatment gap or adequate self-selection? Eur Addict Res. 2016;22:277–85.
Bartsch AJ, Homola G, Biller A, Smith SM, Weijers H-G, Wiesbeck GA, et al. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain 2007;130:36–47.
Jansen JF, Backes WH, Nicolay K, Kooi ME. 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology 2006;240:318–32.
Ernst T, Kreis R, Ross B. Absolute quantitation of water and metabolites in the human brain. I. Compartments and water. J Magn Reson Ser B. 1993;102:1–8.
Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, et al. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magnetic Resonance in Medicine: An Official. J Int Soc Magn Reson Med. 2006;55:1219–26.
Zöllner HJ, Považan M, Hui SC, Tapper S, Edden RA, Oeltzschner G. Comparison of different linear‐combination modeling algorithms for short‐TE proton spectra. NMR Biomed. 2021;34:e4482.
Zöllner HJ, Tapper S, Hui SC, Barker PB, Edden RA, Oeltzschner G. Comparison of linear combination modeling strategies for edited magnetic resonance spectroscopy at 3 T. NMR Biomed. 2022;35:e4618.
Craven AR, Bhattacharyya PK, Clarke WT, Dydak U, Edden RA, Ersland L, et al. Comparison of seven modelling algorithms for γ‐aminobutyric acid–edited proton magnetic resonance spectroscopy. NMR Biomed. 2022:e4702. https://doi.org/10.1002/nbm.4702. Online ahead of print.
Acknowledgements
We would like to acknowledge Teri Lynn Herbert (MUSC) for her invaluable help with setting up the literature searches for this meta-analysis. Dr Anna Kirkland is currently funded through the NIDA T32 at MUSC (DA007288-30), Brittney Browning is funded through the NIAAA T32 (AA007474-34), and Dr Lindsay Squeglia is funded through an NIAAA K23 (AA025399) and R01 (AA027399).
Author information
Authors and Affiliations
Contributions
Conceptualization: AEK, BDB, LMS. Methodology: AEK, BDB, LL, DJM, LMS. Software: AEK, BDB. Formal Analysis: AEK. Data Curation: AEK, BDB. Writing—Original Draft: AEK, BDB, LMS. Writing—Review & Editing: All authors. Visualization: AEK, BDB, RG. Supervision: LMS.
Corresponding author
Ethics declarations
Competing interests
LL is a U.S. federal employee at the National Institutes of Health, and his work is funded by the NIDA and NIAAA intramural research program. Outside his federal employment, he receives an honorarium from the UK Medical Council on Alcoholism (Editor-in-Chief for Alcohol and Alcoholism) and royalties from Routledge for a textbook. All other authors report no biomedical financial interests or potential competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kirkland, A.E., Browning, B.D., Green, R. et al. Brain metabolite alterations related to alcohol use: a meta-analysis of proton magnetic resonance spectroscopy studies. Mol Psychiatry 27, 3223–3236 (2022). https://doi.org/10.1038/s41380-022-01594-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01594-8
- Springer Nature Limited