Abstract
Objectives
To determine the incidence and risk factors of hearing loss (HL) in Brazilian neonates.
Study design
11,900 neonates were screened for hearing and congenital CMV (cCMV). Low and high-risk babies who did not pass their hearing screening and infants with cCMV were scheduled for a diagnostic audiologic evaluation.
Results
The incidence of HL was 2 per 1000 live-born infants (95% CI: 1–3). HL was higher in high-risk neonates than in low risk babies (18.6 vs. 0.3/1000 live births, respectively). Among infants exposed to isolated risk factors, association of HL with craniofacial abnormalities/syndromes (RR = 24.47; 95% CI: 5.9–100.9) and cCMV (RR = 9.54; 95% CI: 3.3–27.7) were observed. HL was 20 to 100-fold more likely in neonates exposed to ototoxic drugs in combination with cCMV or craniofacial/congenital anomalies.
Conclusions
Strategies for the prevention of cCMV and exposure to ototoxic drugs may decrease the incidence of HL in this population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Early-onset hearing loss beginning in infancy is one of the most common life-long disabilities with the prevalence ranging from one to four per thousand live births [1]. Universal hearing screening allows for the early identification of congenital or neonatal hearing losses and to investigate risk factors for hearing loss. Hearing screening programs are continually modified over time based on technologic improvements and new information on risk factors for hearing loss in different populations [2,3,4,5]. At the same time, studies that identify risk factors for hearing loss are essential for planning hearing screening and monitoring protocols applied to infants with risk factors.
Most of the population-based studies about the prevalence and risk factors for infant hearing loss have been conducted in developed countries [2, 5, 6]. In Brazil, the knowledge about the prevalence of hearing loss and its determinants are not well defined [7,8,9,10] making it difficult to develop early detection and intervention guidelines for public health agencies and providers.
The aims of this study were to determine the incidence of congenital and neonatal hearing loss based on the Universal Newborn Hearing Screening (UNHS) of a large cohort of Brazilian newborns. In addition, infants were also screened for congenital cytomegalovirus (cCMV) infection because of the importance of cCMV as a frequent cause of hearing loss (HL) [11].
Materials and methods
As part of the “Brazilian Cytomegalovirus Hearing and Maternal Secondary Infection Study” (BraCHS), a total of 11,900 neonates born from September 2013 to April 2017 were screened for hearing and cCMV infection [11]. The study was approved by the Research Ethics Committee of HCFMRP-USP (Process number 16.928/2013), and written informed consent was obtained from all participants. The cohort study was carried out at two public maternities: MATER and University Hospital at Ribeirão Preto Medical School (HCFMRP-USP). The first maternity hospital (MATER) provides care for low risk parturient. The second hospital, Clinical Hospital of Ribeirão Preto Medical School serves as a referral Center for high risk parturient but also provides care for low risk parturient.
Clinical and risk factors evaluation
Maternal and infant demographic and clinical data were obtained by means of applying standardized questionnaires to the mother and reviewing their medical records. As per standard of care, all mothers were screened for syphilis, human immunodeficiency virus, and toxoplasmosis using serological tests. A high maternal CMV seroprevalence in a representative age-stratified unselected pregnant women from age 12 to 46 years has been previously demonstrated in this population (97%; 95% confidence interval [CI], 95.8–98.0) [12]. All newborn infants underwent a complete physical examination.
Infants were classified as low-risk or high-risk based on risk indicators for congenital/neonatal hearing loss according to The Joint Committee on Infant Hearing [13]. These included: family history of permanent childhood hearing loss; neonatal intensive care of more than 5 days or any of the following regardless of length of stay, such as assisted ventilation (either non-invasive or invasive ventilation), severe hyperbilirubinemia (requiring exchange transfusion), and exposure to ototoxic drugs (aminoglycosides, loop diuretics); infections diagnosed in fetus or newborn (congenital toxoplasmosis, cCMV or syphilis; perinatal herpesvirus infection); craniofacial anomalies, including those that involve the pinna, ear canal, ear tags, ear pits, and temporal bone anomalies, and congenital syndromes associated with hearing loss. In addition, maternal Zika virus (ZIKV) was also included because ZIKV has been added to the list of in-utero infections associated with hearing loss by the Joint Committee on Infant Hearing [14]. Screening and verification tests to confirm the risk factors was carried out as follows: Screening for cCMV infection was carried out using a PCR assay to detect CMV-DNA in newborn saliva specimens [15]. Confirmation of cCMV was done by testing a urine sample collected within the first three weeks of age using the PCR. The diagnosis of congenital toxoplasmosis was based on maternal seroconversion and detection of anti-T.gondii IgM in the neonate and/or the presence of clinical features related to congenital infection. Maternal Zika virus (ZIKV) infection was diagnosed in symptomatic mothers by detection of the ZIKV-RNA in blood and/or urine. Neonatal Herpes Simplex Virus (HSV) infection was identified in one symptomatic infant with a positive HSV-DNA PCR in surface samples.
Hearing screening procedures
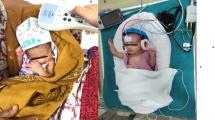
All babies were screened using a portable device (Madsen Accuscreen–GN Otometrics A/S, Denmark). Low-risk babies underwent transient otoacoustic emission (TOAE) testing and those who failed TOAE (one or both ears) underwent an automated auditory brainstem response testing (AABR-35dB Hearing Level-HL). A pass in both ears using TOAE, or a pass in both ears on AABR constituted an overall screen pass. High-risk babies underwent screening using a combined TOAE and AABR-35dBHL testing. AABR was used as referral decision for high-risk infants. Infants who fail OAE but have a “pass” in AABR in their re-screening were considered as having a “pass”. Babies who failed the AABR screening in one or both ears were considered to be screen referrals. For low and high-risk babies, if the pass criteria were not achieved, re-screening was performed with the same protocol and those who failed the re-screening in one or both ears were referred for diagnostic audiological assessment.
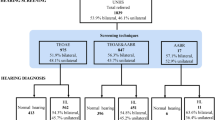
Most low-risk babies were screened within one month of life (n = 11,524; 96.8%) at a median age of 1 day (range 1–215 days). Infants who were admitted to the neonatal intensive care unit (NICU) were tested at a median age of 68 days (range 5–293 days). A flowchart of the hearing screening protocol and referral evaluations is shown in Fig. 1.
Diagnostic methods, hearing loss definitions and intervention
Diagnostic audiologic assessment included TOAE (Eclipse EP25; Interacoustics, Denmark), ABR, and acoustic immittance measures (Otoflex 100; Madsen). Air and bone-conduction ABR testing included click and frequency-specific tone-burst stimuli (Smart EP; Intelligent Hearing Systems. Miami, FL or Eclipse EP25; Interacoustics, Denmark). We considered air-conduction tone ABR normal levels as 35 dB nHL for 500 Hz and 1000 Hz, 30 dB nHL for 2000 Hz, and 25 dB nHL for 4000 Hz [16]. Children with craniofacial anomalies and abnormal immittance measures were tested with bone-conduction tone ABR. We considered bone-conduction tone ABR normal levels as 20 dB nHL for 500 Hz and 30 dB nHL for 2000 Hz. We used correction factors of −15, −10, −5, and 0 dB for estimating 500, 1000, 2000, and 4000 Hz pure-tone behavioral threshold (in dB HL) from tone-ABR thresholds [17]. Sensorineural hearing loss was determined by either the absence of an air-bone gap and/or by normal acoustic immittance results. Hearing loss was categorized in the better hearing ear [18] as mild (26–40 dB), moderate (41–60 dB), severe (61–80 dB), or profound (over 81 dB) on the basis of the decibel estimated hearing level (dB EHL) averaged over the ABR frequencies (500, 2000, and 4000 Hz). As complementary tests, a Behavior Observation Audiometry and a parental report of the infant’s response to sound stimuli were performed in infants aged 0 to 3 months.
Audiological diagnostic procedures were conducted by pediatric audiologist and when indicated, the infant was referred for early intervention and audiological habilitation.
Data analysis
Data were summarized using frequencies and percentages. To determine the association between hearing loss and the various risk factors, we initially examined the presence of one or more risk factors in infants with hearing loss. We then built a conditional inference tree, a non-parametric multivariate regression model that classifies the subjects according to the presence of hearing loss analyzing all of the factors simultaneously [19]. Then, these combinations were joined in three categories and tested as risk factors in the whole sample fitting log-binomial regression models to estimate relative risks and absolute risks difference with their 95% confidence intervals. A 5% significance level was set to all analysis. All statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and R 3.5.1 (The R Foundation for Statistical Computing).
Results
Hearing screening and referral for audiological assessment
Of the 12,366 infants whose mothers consented for the study period in both maternities, 11,900 (96.2%) were screened for hearing and congenital CMV (cCMV) infection, and among these, 11,724 (98.5%) were screened before discharge.
Of the 11,900 babies screened, 91 (0.8%) did not pass hearing screening and were referred for audiological assessment, and among these, 13 did not complete the study evaluation. Four of them died and the remaining 9 were missing from diagnostic hearing evaluation. Therefore, 11,887 neonates completed the study evaluations as planned, of whom, 1131 (9.5%) were high-risk babies and 10,756 (90.5%) were low-risk babies.
Hearing loss incidence and characteristics
Hearing loss was confirmed in 24/78 (30.8%) newborn infants who completed audiological evaluation at a median age of 115 days (22–361). The overall incidence rate of hearing loss was 2 per 1000 (24/11,887) live-born infants (95% CI: 1–3). The incidence of hearing loss was significantly higher in the high-risk infants, 18.6 per 1000 (21/1,131; 95% CI: 11.5–28.2) than in low-risk babies, 0.27 per 1000 (3/10,756; 95% CI: 0.06–0.81; RR = 66.57; 95% CI: 18.89, 222.84).
A detailed description of hearing loss characteristics and the presence of risk factors among the 24 infants with hearing loss is shown in Table 1. The type of hearing loss was sensorineural (SNHL) in 75% of infants and one infant had a permanent conductive hearing loss (4.2%), five infants (20.8%) were diagnosed with bilateral auditory neuropathy (AN) for an incidence of AN of 0.4 per 1000 (95% CI: 0.13–0.98) live births.
Hearing loss was bilateral in 18 (75%) and unilateral in 6 (25%) infants with an overall incidence of bilateral and unilateral hearing loss of 1.5 per 1000 (95% CI: 0.90–2.4), and 0.5 per 1000 (95% CI: 0.18–1.1), respectively. Most (19, 79.2%) infants had severe or profound hearing deficit and the remaining 5 infants had moderate hearing loss.
Of the 12 infants with bilateral SNHL, 10 were fitted with binaural hearing aids coupled with audiological habilitation and two underwent cochlear implantation. Among five infants with AN, one was fitted with hearing aid in one ear and had cochlear implant in the other ear. The remaining four infants are being assessed for hearing-aids. The infant with permanent conductive hearing loss uses a bone conductive hearing aid. Infants with unilateral HL are being monitored prospectively.
All infants with congenital CMV infection (cCMV) with and without hearing loss were followed with hearing monitorization every 6 months at median age of 36 months (18–36) [11]. Regarding the high-risk infants for hearing loss without cCMV, no audiologic follow up was done.
Risk factors for hearing loss
Overall, among the twenty-four infants with hearing loss, three were low-risk babies for hearing loss (Table 1). For the remaining 21 infants, one or more risk factors were identified, including 15/18 (83.3%) infants with SNHL. Table 2 shows relative risk for hearing loss in children with isolated risk factors and those with a combination of risk factors. A gradient of increasing relative risk for hearing loss with the increasing number of risk factors was observed.
Among infants with isolated risk factors, strong associations of HL with congenital infections (RR = 9.54; 95% CI: 3.29; 27.71) and craniofacial anomalies and/or congenital syndromes (RR = 24.47; 95% CI: 5.93;100.92) were observed. However, prolonged admission to the NICU, family history of hearing loss or exposure to ototoxic drugs were not associated with hearing loss likely secondary to small number of affected infants.
Hearing loss was 20 to 100-fold more likely in infants exposed to a combination of risk factors such as NICU admission plus exposure to ototoxic drugs; craniofacial anomalies or congenital syndromes plus NICU admission; and exposure to ototoxic drugs or NICU admission plus congenital infections than in infants without these exposures.
Of note, congenital CMV infection was diagnosed in seven of the 24 (29.2%) infants with hearing loss. In three infants (42%), cCMV was the only risk factor and the remaining 4 had additional risk factors. Detailed characteristics and outcome of infants with cCMV-related hearing loss in our cohort was previously described [11]. None of the children with Maternal ZIKV infection had hearing loss.
Similar to the results of univariate analyses, a multivariate analysis (Fig. 2) shows that the exposure to ototoxic drugs, the presence of congenital infection, and craniofacial/syndromes or anomalies significantly increased the probability of hearing loss. A higher risk for hearing loss seen in infants with congenital infection who were exposed to ototoxic drugs (20%) and in those with craniofacial anomalies who were also exposed to ototoxic drugs (18.8%).
Initially, the route node 1 (ototoxic drugs) is the one that better discriminate subjects with and without hearing loss. Afterwards, internal nodes are shown. Nodes 2 and 9 refer to congenital infection. Together with ototoxic drugs use and craniofacial anomalies (node 10), the better identification of subjects with hearing loss is made. Nodes 3 and 4 additionally classify subjects according to hearing loss and risk factors (NICU and family history of HL) for infants with negative results for ototoxic drugs and congenital infections.
Discussion
Based on the universal neonatal hearing screening in a large cohort of Brazilian neonates, we found an overall incidence of permanent congenital or neonatal hearing loss of 2 per 1000 live births infants. The major risk factors for hearing loss in this population were craniofacial anomalies and/or congenital syndromes and congenital CMV infection with the higher risk in infants who were also exposed to ototoxic medications.
As opposed to programs that only uses the OAE method, our strategy of screening high-risk infants with a combined OAE plus AABR and low-risk babies with OAE followed by AABR in those who failed OAE decreased the number of false-positive and referral rates and also identified infants with auditory neuropathy. The referral rate (0.8%) in our study was lower than that reported by previous studies (1.3–10.6%) using only OAE [8, 20, 21] thereby reducing the need for the second stage screening and the number of losses to follow-up. Although AABR is becoming an increasingly simple and quick procedure, it is more time-consuming and expensive compared to OAE and requires neonates be in a deeper sleeping state. Also, there is a concern that infants who fail OAE in one or both ears but pass AABR might have a mild loss that could be missed by this method [22, 23]. Considering that we have used the AABR equipment based on a click stimulus of 35 dB nHL, similar to the ones used in the universal hearing screening programs, it is likely that we were able to identify only those with moderate or greater hearing loss. The incidence of mild hearing loss in our study could be underestimated since the most weakness of UNHS programs around the world is that hearing loss between 20 and 35 dB is usually missed. Detection of milder losses would require the use of different stimulus levels equipment [23]. Therefore, we have emphasized that a “pass” does not eliminate the need to monitor language development, auditory skills and developmental milestones in all infants and children regardless of risk status.
The overall incidence of confirmed hearing loss found in our population is consistent with that found in previous studies from developed and developing countries (1–4 cases per 1000 live births) [8,9,10, 21, 24].
The incidence of the hearing loss in low-risk babies in our study was 0.3/1000 live births, similar to that reported in previous studies in Brazilian infants without risk factors ranging from 0.1 to 0.4/1000 [8,9,10]. The prevalence of risk factors for hearing loss in the general population varies greatly depending on the risk classification that was used. For example, in a cohort of 1850 infants, White et al. [25] reported that 36.4% (4/11) of those with sensorineural hearing loss did not have any of the high-risk indicators. However, two of the four children had stayed for more than five days in the NICU. If the two children had been classified as high-risk in accordance with JCIH [13], only 18.2% would have been considered low-risk babies in their series. We found that most (83%) infants with SNHL had risk factors, with cCMV being the risk-factor for 29.2% of them, highlighting the importance of screening all infants for cCMV. Thus, neonates with asymptomatic cCMV, who could have been classified as low-risk babies were categorized as high-risk infants in our study. Since children with asymptomatic cCMV would not have been identified, the proportion of infants without risk factors in our study would have been 20.8% (5/24) without CMV screening at birth.
Similar to the findings in other populations [5, 24], the predominant (75%) type of hearing loss detected in our study was sensorineural. About a third of infants had unilateral sensorineural hearing loss (USHL), with a profound loss in about two thirds of them, as found in other studies [26, 27]. Among those with bilateral SNHL, most infants (83.3%) had severe or profound loss that could significantly interfere with the acquisition and development of speech and language. Auditory neuropathy spectrum disorder (ANSD), characterized by the presence of OAE and/or a cochlear microphonic potential with abnormal or absent ABR was detected in 20.8% of those with hearing loss or 0.4 per 1000 live births. Our results are similar to those described by Ngo et al. [28] from a newborn hearing screening program based upon AABR but higher than other studies which have reported incidence rates of ANSD ranging from 0.09 to 0.3/1000 live births [24, 29].
As expected, the incidence of hearing loss was considerably greater in neonates with risk factors than those without risk factors (18.6 vs. 0.3/1000 live births, respectively). The likelihood of permanent hearing loss in high-risk infants was nearly 70-fold higher than in low-risk babies highlighting the importance of completing a detailed audiological evaluation in these infants. Also, as reported by Ohl et al. [20], we demonstrated that as the number of risk factors increases, the probability of hearing loss increases with a maximum frequency of hearing loss in infants with three or more risk factors. Since about half of the infants with hearing loss had two or more predisposing factors, we used a univariate analysis for isolated and combined risk factors (Table 2). A multivariate inference tree analysis was performed to estimate the force of the presence of each factor (Fig. 2). In both univariate and multivariate analyses, craniofacial anomalies and/or congenital syndromes and congenital/perinatal infections were the major risk factors in this population and the additional exposure to ototoxic drugs increased the risk significantly. It has been reported that craniofacial anomalies [2, 4] and congenital syndromes, either due to inner ear malformation or to genetic causes are strongly associated with conductive and/or sensorineural hearing loss [3], mainly in those with a family history of childhood hearing loss [30, 31]. “Considering that the occurrence of hearing loss in the general population is low, relative risks can be large for patient groups in a high-risk category and be not so important concerning public health policies. We presented the risk measures in both relative risk, and absolute risk difference between groups for the accurate interpretation of the study results.” Although family history of childhood hearing loss has been frequently associated with hearing loss [3,4,5], we found that less than 1% of our cohort had a family history as a risk factor and only one infant with hearing loss (4.2%; 1/24) had this risk defined. However, studies have reported the occurrence of congenital hearing loss in Brazilian infants with a family history ranged from 12.5% to 17.6% [10, 32].
Although the association between hearing loss and other factors including exposures to ototoxic drugs, and infants requiring intensive care remains controversial [33, 34], we demonstrate a strong interaction between ototoxic drugs and other factors. Therefore, we suggest that exposure to ototoxic drugs in this population should be minimized by treatments of shorter duration and careful monitoring of drug levels to less the impact on hearing.
The role of cCMV infection in hearing loss is well known [5, 20]. We have recently reported that cCMV is a major cause of hearing loss in this population and also described the incidence and characteristics of hearing in infants with cCMV [11]. As a single factor or in combination with exposure to ototoxic drugs, cCMV was involved in approximately one third of all permanent hearing loss, one third of infants with bilateral HL, and half of those with unilateral HL. These findings strongly argue for systematic screening for cCMV or integrating targeted cCMV screening in infants who fail UNHS to identify a significant proportion of unexplained SNHL in infants without known risk factors.
There were some limitations in this study. Although we have screened 11,900 infants, because hearing loss is a relatively infrequent event, our estimates resulted in wide confidence intervals. Our evaluation was able to detect early-onset moderate or severe HL but might have missed children with mild losses. A systematic evaluation for late-onset hearing loss was not carried out for infants with risk factors other than cCMV and other congenital infections. However, our study is the largest cohort of Brazilian newborn infants who were systematically screened for hearing using both OAE and AABR, and for cCMV. Our study represents the best estimates for hearing loss to date in a large population of infants, and shows that congenital malformations, congenital CMV infection, and ototoxic medications are the primary drivers of this condition. Moreover, screening infants with risk factors using concurrent rather than serial OAE and AABR increases specificity of the screening process and supports adopting this approach in clinical practice. Based on these data, public health strategies to prevent congenital infections, screen for cCMV, limit exposure to ototoxic drugs, and monitor those with risk factors may decrease the occurrence of hearing loss in children.
References
Nikolopoulos TP. Neonatal hearing screening: what we have achieved and what needs to be improved. Int J Pediatr Otorhinolaryngol. 2015;79:635–7.
Meyer C, Witte J, Hildmann A, Hennecke KH, Schunck KU, Maul K, et al. Neonatal screening for hearing disorders in infants at risk: incidence, risk factors, and follow-up. Pediatrics. 1999;104:900–4.
Bielecki I, Horbulewicz A, Wolan T. Risk factors associated with hearing loss in infants: an analysis of 5282 referred neonates. Int J Pediatr Otorhinolaryngol. 2011;75:925–30.
Wróbel MJ, Greczka G, Szyfter W. The risk factor profile of children covered by the Polish universal neonatal hearing screening program and its impact on hearing loss incidence. Int J Pediatr Otorhinolaryngol. 2014;78:209–13.
Wroblewska-Seniuk K, Dabrowski P, Greczka G, Szabatowska K, Glowacka A, Szyfter W, et al. Sensorineural and conductive hearing loss in infants diagnosed in the program of universal newborn hearing screening. Int J Pediatr Otorhinolaryngol. 2018;105:181–6.
Vashistha I, Aseri Y, Singh BK, Verma PC. Prevalence of hearing impairment in high risk infants. Indian J Otolaryngol Head Neck Surg. 2016;68:214–7.
Chapchap MJ, Segre CM. Universal newborn hearing screening and transient evoked otoacoustic emission: new concepts in Brazil. Scand Audio Suppl. 2001;1:33–6.
Bevilacqua MC, Alvarenga KF, Costa OA, Moret ALM. The universal newborn hearing screening in Brazil: from identification to intervention. Int J Pediatr Otorhinolaryngol. 2010;74:510–5.
Barboza ACS, Resende LM, Ferreira DBC, Lapertosa CZ, Carvalho SAS. Correlation between hearing loss and risk indicators in a neonatal hearing screening reference service. Audiol Commun Res. 2013;18:285–92. https://doi.org/10.1590/S2317-64312013000400009.
Pereira T, Costa KC, Pomilio MCA, Costa SMS, Rodrigues GRI, Sartorato EL. Etiological investigation of deaf in neonates screened in a universal newborn hearing screening program. Rev CEFAC. 2014;16:422–9.
Yamamoto AY, Anastasio ART, Tanaka EM, Isaac ML, Manfredi AKS, Cavalcante JMS, et al. Contribution of congenital cytomegalovirus (cCMV) to permanent hearing loss in a highly seropositive population: the BraCHS study. Clin Infect Dis. 2020;70:1379–84.
Yamamoto AY, Castellucci RA, Aragon DC, Mussi-Pinhata MM. Early high CMV seroprevalence in pregnant women from a population with a high rate of congenital infection. Epidemiol Infect. 2013;141:2187–91.
American Academy of Pediatrics, Joint Committee on Infant Hearing. Position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120:898–921.
American Academy of Pediatrics, Joint Committee on Infant Hearing. Position statement: principles and guidelines for early hearing detection and intervention programs. J Early Hear Detection Intervention. 2019;4:1–43.
Yamamoto AY, Mussi-Pinhata MM, Marin LJ, Brito RM, Oliveira PF, Coelho TB. Is saliva as reliable as urine for detection of cytomegalovirus DNA for neonatal screening of congenital CMV infection? J Clin Virol. 2006;36:228–30.
British Columbia Early Hearing Program (BCEHP). A service of BC Children´s Hospital and the Provincial Health Services Authority- Audiology Assessment Protocol. 2012. Version 4.1. http://www.phsa.ca/Documents/bcehpaudiologyassessmentprotocol.pdf (Accessed 25 Feb 2019).
Stapells DR, Gravel JS, Martin BA. Thresholds for auditory brain stem responses to tones in notched noise from infants and young children with normal hearing or sensorineural hearing loss. Ear Hear. 1995;16:361–71.
WHO. World Health Organization. Prevention of blindness and deafness. Grades of hearing impairment. http://who.int/pbd/deafness/hearing_impairment_grades/en (Accessed 25 Feb 2019).
Speybroeck N. Classification and regression trees. Int J Public Health. 2012;57:243–6.
Ohl C, Dornier L, Czajka C, Chobault JC, Tavernier L. Newborn hearing screening on infants at risk. Int J Pediatr Otorhinolaryngol. 2009;73:1691–5.
Wroblewska-Seniuk K, Greczka G, Dabrowski P, Szyfter W, Mazela J. The results of newborn hearing screening by means of transient otoacoustic emissions - has anything changed over 10 years? Int J Pediatr Otorhinolaryngol. 2017;96:4–10.
Johnson JL, White KR, Widen JE, Gravel JS, James M, Kennalley T, et al. A multicenter evaluation of how many infants with permanent hearing loss pass a two-stage otoacoustic emissions/automated auditory brainstem response newborn hearing screening protocol. Pediatrics. 2005;116:663–72.
Levit Y, Himmelfarb M, Dollber S. Sensitivity of the automated auditory brainstem response in neonatal hearing screening. Pediatrics. 2015;136:641–7.
Saki N, Bayat A, Hoseinabadi R, Nikakhlagh S, Karimi M, Dashti R. Universal newborn hearing screening in southwestern Iran. Int J Pediatr Otorhinolaryngol. 2017;97:89–92.
White KR, Vohr BR, Maxon AB. Screening all newborns for hearing loss using transient evoked otoacoustic emissions. Int J Pediatr Otorhinolaryngol. 1994;29:203–17.
Barsky-Firkser L, Sun S. Universal newborn hearing screenings: a three-year experience. Pediatrics. 1997;99:E4.
Finitzo T, Albright K, O’Neal J. The newborn with hearing loss: detection in the nursery. Pediatrics. 1998;102:1452–60.
Ngo RY, Tan HK, Balakrishnan A, Lim SB, Lazaroo DT. Auditory neuropathy/auditory dys-synchrony detected by universal newborn hearing screening. Int J Pediatr Otorhinolaryngol. 2006;70:1299–306.
Boudewyns A, Declau F, Van Den Ende J, Dirckx S, Van de Heyning P. Auditory neuropathy spectrum disorder (ANSD) in referrals from neonatal hearing screening at a well-baby clinic. Eur J Pediatr. 2016;175:993–1000.
Cone-Wesson B, Vohr BR, Sininger YS, Widen JE, Folsom RC, Gorga MP, et al. Identification of neonatal hearing impairment: infants with hearing loss. Ear Hear. 2000;21:488–507.
Dumanch KA, Holte L, O’Hollearn T, Walker E, Clark J, Oleson J. High risk factors associated with early childhood hearing loss: a 3-year review. Am J Audio. 2017;26:129–42.
Onoda RM, Azevedo MF, Santos AM. Neonatal hearing screening: failures, hearing loss and risk indicators. Braz J Otorhinolaryngol. 2011;77:775–83.
Fuchs A, Zimmermann L, Graz MB, Cherpillod J, Tolsa JF, Buclin T, et al. Gentamicin exposure and sensorineural hearing loss in preterm infants. PLoS ONE. 2016;8:1–11.
Puia-Dumitrescu M, Bretzius OM, Brown N, Fitz-Henley JA, Ssengonzi R, Wechsler CS, et al. Evaluation of gentamicin exposure in the neonatal intensive care unit and hearing function at discharge. J Pediatr. 2018;203:131–6.
Funding
All phases of this study were supported by FAPESP, Brazil, (Grant 2013/06579-0 to MMMP); and Eunice Kennedy Shriver NICHD, USA (Grant 2R01HD061959-07A2 to WJB).
Author information
Authors and Affiliations
Contributions
ARTA conceptualized and designed the study, drafted the initial manuscript, revised critically and approved the final version of the manuscript. She also supervised the application of the hearing screening and audiological tests. AYY conceptualized and designed the study, supervised the CMV screening and the follow up of CMV-infected infants, was in charge of data management, revised critically and approved the final version of the manuscript. ETM conceptualized and designed the study, provided supervision and care for infants with suspected and confirmed hearing loss, helped with data acquisition, revised critically and approved the final version of the manuscript. MM M-P conceptualized the BRACHS cohort study, obtained funds, supervised activities for data collection, took part of the manuscript preparation, and definition of data analysis, revised critically and approved the final version of the manuscript. AKSM and JMSC participated in the application of the hearing screening and audiological tests, being in charge of the completeness of the audiological protocol activities and data acquisition, revised critically and approved the final version of the manuscript. BCPL participated in the care of the children who underwent electrophysiological examinations under anesthesia, helped with data acquisition, revised critically and approved the final version of the manuscript. DCA carried out the statistical analyses and interpretation of data, revised critically and approved the final version of the manuscript. SB conceptualized the BRACHS cohort study, and reviewed the manuscript for important intellectual content and approved the final version of the manuscript. KBF participated in the conceptualization and data managing planning for the BRACHS cohort study, reviewed and approved the final version of the manuscript. WJB conceptualized the BRACHS cohort study, and reviewed the manuscript for important intellectual content, and approved the final version of the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects related to the accuracy or integrity of any part of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anastasio, A.R.T., Yamamoto, A.Y., Massuda, E.T. et al. Comprehensive evaluation of risk factors for neonatal hearing loss in a large Brazilian cohort. J Perinatol 41, 315–323 (2021). https://doi.org/10.1038/s41372-020-00807-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-020-00807-8
- Springer Nature America, Inc.
This article is cited by
-
Risk factors for infant hearing loss: a meta-analysis
European Journal of Pediatrics (2024)
-
Sheep as a large animal model for hearing research: comparison to common laboratory animals and humans
Laboratory Animal Research (2023)
-
Genetic etiology of non-syndromic hearing loss in Latin America
Human Genetics (2022)