Abstract
Auditory neuropathy spectrum disorder (ANSD) is a particular kind of hearing disorder characterised by normal outer hair cell function and abnormal or absent auditory brain stem responses. Little data are available regarding the prevalence of this condition in healthy newborns. We performed a retrospective medical records review of 791 referrals from universal neonatal hearing screening (UNHS) at a well-baby clinic to investigate the prevalence of ANSD. Hearing screening was performed by automated auditory brain stem response (ABR) testing. A diagnosis of ANSD was established when ABR tracings were absent in the presence of otoacoustic emissions and/or a cochlear microphonic. Amongst 201 infants with confirmed congenital hearing loss, 13 infants were diagnosed with ANSD. The condition was unilateral in six and bilateral in seven infants. A risk factor for hearing loss could be identified in three infants. Abnormalities on magnetic resonance imaging were found in six infants; five of them had cochlear nerve deficiency.
Conclusion: The prevalence of ANSD was 6.5 % amongst well babies with confirmed congenital hearing loss identified through UNHS. The estimated incidence of ANSD in our population of newborns at the well-baby clinic was 0.09/1000 live births. Magnetic resonance revealed an underlying anatomical abnormality in about half of the patients.
What is known: • Auditory neuropathy dyssynchrony spectrum disorder (ANSD) is a particular form of hearing loss, mostly encountered in neonatal intensive care unit (NICU) graduates. • Little data are available on the prevalence and risk factors for ANSD in healthy newborns. |
What is new: • The estimated prevalence of ANSD in healthy newborns is 0.09/1000 live births. • In about half of the healthy newborns with ANSD, a structural abnormality was detected on magnetic resonance imaging of the posterior fossa/brain. |
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The term auditory neuropathy spectrum disorder (ANSD) encompasses a spectrum of conditions characterised by normal outer hair cell function (present otoacoustic emissions and/or cochlear microphonic) and abnormal or absent auditory brain stem responses [4]. The prevalence of ANSD may vary between 1 and 40 % depending upon the study population [4]. The condition is related to a disruption of the temporal coding of acoustic signals in the auditory nerve fibres, resulting in the impairment of auditory perceptions that rely on temporal cues [10]. Children with ANSD present with some or all of the following features: behavioural thresholds may range from normal to profound hearing loss and may vary in time between test situations and speech perception scores may be consistent with or much poorer than would be predicted from the audiogram. Behavioural thresholds do not correlate with objective measures such as auditory brain stem responses or auditory steady state responses (ASSR); speech perception in noise may be poorer than expected from the behavioural audiogram. These features pose particular challenges for the management of these children, and treatment should be individually tailored [12]. Early detection of these children through newborn hearing screening and subsequent referral for a comprehensive audiological and etiological workup followed by appropriate rehabilitation is advocated.
We performed a retrospective analysis to investigate the prevalence, risk factors, underlying cause and subsequent treatment for all infants with ANSD diagnosed following referral from neonatal hearing screening in a well-baby clinic.
Methods
Organisation of universal neonatal hearing screening in Flanders
In Flanders, a community-based screening programme has been implemented by the federal health care agency (Kind and Gezin). Newborns are screened by dedicated nurses, between the age of 3 and 4 weeks by means of an automatic auditory brain stem response (AABR) at the well-baby clinic. A PASS from screening indicates hearing thresholds at 35 dB hearing loss (HL) or lower. Infants with a REFER from screening on one or both ears are referred to one of the 23 certified referral centres in Flanders. Since the start of this screening programme in 1998, the Ear-Nose-Throat (ENT) Department of the Antwerp University Hospital serves as referral centre and performs a comprehensive audiometric and etiological workup in all referred newborns as described previously [9].
Audiological workup after referral from universal neonatal hearing screening screening
The audiological assessment is performed by an audiologist and consists of click evoked ABR testing, measurement of ASSR, high-frequency (1000 Hz) tympanometry and recording of transient evoked otoacoustic emissions (TEOAEs). Insert phones are used for testing with rarefaction and condensation polarity clicks at moderate-high stimulation levels (70–80 dB normal hearing level (nHL)). In case of absent or grossly abnormal ABR tracings, an additional analysis is performed to address the presence of a cochlear microphonic. The presence of a cochlear microphonic (CM) is an essential diagnostic criterion for ANSD. The interested reader may find a more detailed description and illustration of the cochlear microphonic in the addendum. A CM is considered present when a waveform appears in the first few milliseconds of the tracing and is the only part of the waveform that reverses polarity from rarefaction to condensation. A control trial is then performed with the sound tube of the insert phone clamped to rule out transducer artefacts. Testing is performed during natural sleep or under sedation (chloral hydrate) or general anaesthesia according to the infant’s condition. A diagnosis of ANSD is established when the following findings are unilaterally or bilaterally present: presence of TOAEs (at least 70 % reproducibility and signal/noise ratio ≥6 for at least three frequencies) and/or CM and absent ABR tracing or abnormal ABR (typical ABR waveforms cannot be recognised on visual inspection of the ABR tracings).
The hearing loss was classified into the following categories: sensorineural (unilateral or bilateral), conductive (unilateral or bilateral) and ANSD (unilateral or bilateral).
Etiological workup after referral from universal neonatal hearing screening screening
A paediatric ENT surgeon in collaboration with a medical geneticist and paediatrician perform the etiological workup.
A detailed history is taken to identify risk factors for congenital hearing loss [1]. Hyperbilirubinemia reaching exchange transfusion levels, use of ototoxic medication and perinatal hypoxia/asphyxia are listed as risk factors for congenital hearing loss and more specifically for ANSD. Clinical examination included tympanoscopy, an examination of the head and face along with a general paediatric examination. Mutations in the otoferlin gene (OTOF) were searched for in those with bilateral ANSD since mutations in the OTOF gene are a major cause of inherited ANSD with an autosomal recessive mode of transmission [21]. Magnetic resonance imaging (MRI) was performed in all infants, and they were all examined at the Department of Ophthalmology. Additional examinations were asked upon clinical indication.
Follow-up and management of infants with ANSD
Those infants with an established diagnosis of unilateral ANSD were offered regular follow-up every 6 months for hearing tests, evaluation of developmental milestones and speech-language development. Children between 6 and 24 months of age may be tested by visual re-inforcement audiometry (VRA), which is based on an orientation reflex towards a new sound source. An experienced audiologist is required to obtain reliable results. At age of 2–4 years, children are tested by play audiometry. The child is conditioned to respond to an auditory stimulus through play activities. Those with bilateral ANSD were treated with hearing aids, auditory verbal therapy, or speech therapy according to the individual needs. Cochlear implantation was considered for infants with a poor response to conventional amplification.
Research question
We performed a retrospective chart review on all neonates assessed in our department after a referral from universal neonatal hearing screening (UNHS) since the start of the programme in 1998 up to December 31, 2014. Parents or caregivers from children that were no longer in follow-up at our department were contacted by phone to obtain information about hearing status and treatment. The screening and audiological data from babies screened at the neonatal intensive care unit (NICU) are analysed separately and not reported here.
Results
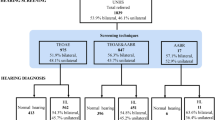
Between the start of the UNHS in Flanders in 1998 and December 31, 2014, 993,796 babies were screened by Kind and Gezin at a well-baby clinic. The referral rate was 0.55 % (5453 babies). We registered data for 791 referrals from the UNHS screening, which represents 14.5 % of the referred infants. At the latest follow-up, 497(62.2 %) babies were found to have normal hearing.
A subgroup of these had a transient hearing loss caused by middle ear effusion that finally resolved either spontaneously or following treatment (tympanocentesis or grommets), resulting in normal hearing. A final conclusion could not be established in 97 babies (12.3 %), and hearing loss was confirmed in 201 (25.4 %) newborns.
The percentage of infants for each hearing loss category in those with confirmed congenital hearing loss (n = 201) is presented in Fig. 1.
During the study period, Kind and Gezin screened 993,796 infants and 14.5 % of babies with a failed UNHS were sent to our hospital. These cases represent 144,100 screened babies. From these data, we calculated that 13/144,100 newborns or 0.09/1000 live births had ANSD.
Amongst 201 infants with confirmed hearing loss upon referral from UNHS at the well-baby clinic, the prevalence of ANSD is 6.5 %.
Individual patient data including risk factors for hearing loss, conclusion of the audiological assessment, associated comorbidity and treatment are presented in Table 1.
A risk factor for hearing loss as defined by the American Academy of Pediatrics [1] could be identified in three patients with ANSD. Two infants had a family history of hearing loss (in one of these, there was a history of otosclerosis in a second-degree relative), and one patient was diagnosed with congenital cytomegalovirus (CMV) infection.
Beyond the identification of risk factors, some children had an associated comorbidity; patient 2 had cerebral palsy and a central visual disturbance. Cochlear nerve deficiency (CND) was identified in one patient with bilateral ANSD and in four infants with unilateral ANSD (Fig. 2). One infant with unilateral ANSD had an arachnoidal cyst at the cerebellopontine angle with compression of the eighth nerve.
None of our patients had a mutation in the otoferlin gene.
Hearing aids and speech/language therapy were proposed to those with bilateral hearing loss. In one patient (case 4), hearing thresholds normalised over time—he had been treated with ventilation tubes for middle ear effusion. The infants with unilateral disease were observed with periodic testing to confirm the presence of a normal hearing in the unaffected ear and evaluate their speech and language development.
One patient with bilateral ANSD was found to have bilateral CND on MRI complemented with high-resolution computed tomography. Since the cochlear nerve appeared completely absent on the right side and rather hypoplastic on the left, he received a cochlear implant at the left ear at an age of 7 months and he responds to sounds at 10 months post-implantation (aided thresholds at 25 dB HL for 500–1000 Hz and at 30 dB HL for 2000–4000 Hz.).
Discussion
The estimated incidence of ANSD in the population of newborn infants at the well-baby clinic was 0.09/1000 live births. A risk factor for hearing loss could be identified in three patients. Seven babies had bilateral ANSD and six had a unilateral condition.
The prevalence of ANSD in newborns with confirmed hearing loss detected through UNHS at the well-baby clinic was 6.5 %. In about half of the patients, an underlying anatomical abnormality could be identified through magnetic resonance imaging. The novelty of our data is that we excluded NICU infants and only included referrals from a well-baby clinic. Our data may therefore provide an estimate on the prevalence of ANSD in infants with congenital hearing loss without a history of NICU admission.
In the past, several studies reported upon the prevalence of ANSD identified through a newborn hearing screening programme but most studies included NICU infants.
Sininger reviewed the incidence of ANSD in NICU infants and found a range from 5.3 to 14.8 % with a mean of 10.8 % [23].
ANSD after universal neonatal hearing screening was found in 0.027 to 0.06 % of screened infants [11, 17]. These figures are in line with the present report where we found an estimated incidence of ANSD of 0.09/1000 live births.
Dowley et al. described the results from a newborn hearing screening programme based upon TOAE screening in UK [11]. Over a 5-year time period (2002–2007), 45,050 infants were screened; 30 were diagnosed with severe to profound hearing loss, and 12 of these 30 had ANSD. The incidence of ANSD in this study was 0.27/1000 live births. This figure is threefold higher than our present data but might be explained by the fact that all children with ANSD in the study reported by Dowley et al. were admitted to a NICU. Moreover, Dowley et al. reported on the incidence of ANSD amongst children with severe to profound hearing loss. We considered all children with confirmed congenital hearing loss ranging from moderate to severe/profound hearing loss and including both unilateral and bilateral cases which may be another explanation for the lower prevalence rates.
Ngo et al. looked at the results of a universal newborn hearing screening programme using AABR and found 52 cases of hearing loss amongst 14,807 screened infants. Nine cases (17.3 %) of the newborns with hearing loss had an ANSD profile [17]. Four were born premature and six cases had neonatal jaundice. Only two infants, both with unilateral ANSD, had no risk factor or associated condition. In that study, the incidence of ANSD was 0.6/1000 live births.
Kirkim et al. investigated the prevalence of ANSD amongst infants referred for a second OAE and AABR screening after initial referral from a newborn hearing screening programme in Turkey [13]. Amongst 107 refers after the second screening, 28 were lost to follow-up, 14 had normal hearing, 55 had hearing loss and another 10 had ANSD. In that study, the percentage of ANSD in babies with confirmed hearing loss was 15.4 %. Hyperbilirubinemia was the main risk factor in 70 % of the cases and only three babies had no risk factor. The incidence after UNHS was found to be 0.44/1000 live births.
Our estimated ANSD incidence of 0.09/1000 live births is somewhat lower than these authors reported, although in our population non-genetic causes of ANSD such as hyperbilirubinemia and ototoxic drugs were much less frequently encountered than in the previous studies due to the fact NICU infants were excluded in our study.
Risk factors for hearing loss as identified by the American Academy of Pediatrics [1] were identified in only three patients of our study group, a family history of hearing loss (n = 2) and congenital cytomegalovirus infection (n = 1).
Specific risk factors for ANSD have been described in the past such as prematurity, hyperbilirubinemia, sepsis, birth asphyxia, cerebral palsy and ototoxic medication (gentamycin, vancomycin and furosemide) [3, 5, 11].
These risk factors are more commonly encountered amongst NICU graduates and were not present in any of our patients.
Hyperbilirubinemia is recognised as a major risk factor for ANSD, also in late preterm and term infants [5, 16, 19, 22]. Saluja et al. suggested to perform a comprehensive auditory evaluation in all late preterm and term infants with severe hyperbilirubinemia (with total serum concentration at which exchange transfusion may be considered) to identify infants with ANSD [22]. In our study population, none of the patients had hyperbilirubinemia reaching levels requiring exchange transfusion. Only one of the patients with unilateral ANSD had a risk factor for hearing loss (second-degree relative with a history of otosclerosis). This is in line with data published by Beutner et al. These authors performed a risk factor analysis in 37 ANSD children (mean age 2 years) [5]. The majority of patients in that study (56.8 %) had perinatal complications, but in seven children (18.9 %), no risk factor could be identified (six of these seven had unilateral disease).
The aetiology is unknown in 40 % of individuals with ANSD [24].
Bilateral ANSD cases may be related to systemic pathology. Rance et al. presented clinical findings for 20 young children with ANSD [19]. Twelve of them were identified through an early hearing loss identification programme for children at increased risk of hearing loss (based upon neonatal or family history). The remaining eight children were referred following concerns regarding hearing status or speech/language development. Four out of the 20 children had other disabilities relating to sensory motor deficits (cerebral palsy n = 3, unilateral facial palsy n = 1). One of our bilateral ANSD patients had a history of cerebral palsy and a central visual disturbance, and another had a congenital CMV infection that might have contributed to the clinical picture. In the remainder, no systemic pathology could be found.
In unilateral cases, structural anomalies may be more common. Structural abnormalities were encountered in five patients with unilateral ANSD. This illustrates the importance of imaging studies to be performed upon confirmation of an ANSD diagnosis. MRI is the first-choice modality [20]. Roche et al. reviewed the MRI images of 183 ears with ANSD and found evidence for definite or possible CND in 28 % [20]. Buchman et al. found that 9 out of 51 (18 %) children presenting with ANSD had CND and 5 of them were affected in only one ear [8]. In our series, CND is also the most frequent anatomical abnormality occurring 5 of the 13 infants (38 %). Cochlear nerve deficiency was especially common in the unilateral ANSD infants in our series, and only one infant was affected on both sides. One infant with unilateral ANSD had a unique presentation of a cerebellopontine angle arachnoidal cyst with compression on the cochlear nerve [6]. Audiological assessment revealed an ANSD on the affected side. A complete recovery of the hearing was observed following microsurgical resection of the cyst wall and marsupialisation.
Parents of babies diagnosed with ANSD are informed that the management of their baby’s hearing impairment requires a team approach and that this is usually more challenging than in children with typical sensorineural (cochlear) hearing loss. Parents are informed that both ABR and behavioural thresholds are poor predictors of speech discrimination ability and that there may be improvement over time but that there is much uncertainty around prognosis. Careful monitoring and follow-up are required at regular intervals, and for most children, a combination of communication systems incorporating auditory stimulation and visual support is appropriate. A number of children may benefit from hearing aids, and there are increasing number of children who benefit from a cochlear implant. This option should be considered when children are not making progress with hearing aids, but implantation should be delayed until audiological test results are stable and demonstrate unequivocal evidence of permanent ANSD.
Interestingly, in one of our patients (case 4) with an initial diagnosis of ANSD, the auditory function recovered during the first year of life and normal hearing status could be documented by audiometry. In three other children (cases 5, 6 and 7), a statement on normal hearing in the long term was only obtained by parental report and not confirmed by audiometry because these children were lost to follow-up. A delayed maturation of the auditory nerve has been described earlier [2, 18], and in some infants, the absence of auditory brain stem responses and the presence of OAEs at hearing screening may reflect the delayed maturation of both the brain stem and the auditory nerve. Alternatively, it is well known that auditory abilities of children with ANSD may vary ranging from mild to severe [4] and that about 5 % of the patients have a mild condition requiring no intervention for hearing or developing speech and language. Parents of this small subgroup may have the impression that their child has a normal hearing.
Because follow-up data are lacking on these three patients, we cannot conclude whether these are cases of spontaneous resolution and maturation or rather mild forms of ANSD. In cases of maturation, recovery would normally be complete by 12–18 months.
ANSD may be underdiagnosed because of lack of familiarity on the part of professionals or the methodology used for neonatal hearing screening [11].
Although the number of children with ANSD in a well-baby population is small, a timely and correct identification of these children is important to start early intervention. In countries where neonatal screening is based upon OAE detection, these children will pass the screening and one may expect that a delayed diagnosis of hearing loss will be made because they present with difficulties in speech-language development at later age. For both screening methods (OAE and AABR), the detection threshold is 35 dB HL and mild hearing loss may be missed. Korver et al. estimated that in the Netherlands, where well babies are screened with OAE in the first screening stage, between 11 and 54 children (0.06–0.3 per 1000) will be missed with hearing impairment due to ANSD [14]. AABR screening is more expensive compared to OAE screening, but the total cost of the screening is reduced due to a reduction in false positives that are referred for full audiometric assessment [15]. Based upon data from literature and our present findings, we recommend AABR as the first screening method in all newborns (NICU and well babies).
Although the UNHS programme in Flanders has a high sensitivity (94.02 %) and specificity (99.96 %), false positive test results may cause unnecessary anxiety in parents and a request for additional examinations. At the last follow-up, 493 out of 791 (62.3 %) of the referred babies were found to have normal hearing. However, a subgroup (232 infants) had a transient hearing loss caused by middle ear effusion, leaving 251 infants (31.7 %) for whom a normal hearing could be immediately confirmed.
During the study period, 993,796 well babies had a UNHS in Flanders. We estimated that 14.5 % of them were referred to our hospital (144,100) babies. From these data, we can calculate that 251 out of 144,100 screened babies (0.17 %) had a false positive test result and should not have been referred.
Also, the problem of transient hearing loss related to middle ear effusion has been reported earlier. Amongst 152 UNHS referrals, 55.3 % of them were found to have a transient hearing loss related to middle ear effusion [7].
Our study has several limitations because of its retrospective nature. Long-term follow-up data were not available for three infants with ANSD. They were contacted by phone but the parents refused a reassessment and told that their child did not have any apparent hearing problem. Although we cannot exclude a referral bias, we believe that the population described in this paper is representative for ANSD in babies from a well-baby clinic.
Conclusions
The estimated incidence of ANSD identified through UNHS in well babies is 0.09/1000 live births. Although this number is substantially lower than in NICU graduates, AABR is the first-choice screening method as it would allow for early identification of these infants with ANSD. Magnetic resonance imaging has a high diagnostic yield and revealed an underlying anatomical abnormality explaining the condition in about half the patients.
Abbreviations
- AABR:
-
Automatic ABR
- ABR:
-
Auditory brain stem responses
- ASSR:
-
Auditory steady state responses
- CM:
-
Cochlear microphonic
- CMV:
-
Cytomegalovirus
- CND:
-
Cochlear nerve deficiency
- ENT:
-
Ear-nose-throat
- HL:
-
Hearing loss
- MRI:
-
Magnetic resonance imaging
- nHL:
-
Normal hearing level
- NICU:
-
Neonatal intensive care unit
- TOAEs:
-
Transient evoked otoacoustic emissions
- UNHS:
-
Universal neonatal hearing screening
References
American Academy of Pediatrics, Joint Committee on Infant Hearing (2007) Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics 120:898–921. doi:10.1542/peds.2007-2333
Attias J, Raveh E (2007) Transient deafness in young candidates for cochlear implants. Audiol Neurotol 12:325–333. doi:10.1159/000103271
Berg AL, Spitzer JB, Towers HM, Bartosiewicz C, Diamond BE (2005) Newborn hearing screening in the NICU: profile of failed auditory brainstem response/passed otoacoustic emission. Pediatrics 116:933–938. doi:10.1542/peds.2004-2806
Berlin CI, Hood LJ, Morlet T, Wilensky D, Li L, Mattingly KR, Taylor-Jeanfreau J, Keats BJ, John PS, Montgomery E, Shallop JK, Russell BA, Frisch SA (2010) Multi-site diagnosis and management of 260 patients with auditory neuropathy/dys-synchrony (auditory neuropathy spectrum disorder). Int J Audiol 49:30–43. doi:10.3109/14992020903160892
Beutner D, Foerst A, Lang-Roth R, von Wedel H, Walger M (2007) Risk factors for auditory neuropathy/auditory synaptopathy. ORL J Otorhinolaryngol Relat Spec 69:239–244. doi:10.1159/000101545
Boudewyns AN, Declau F, De Ridder D, Parizel PM, van den Ende J, Van de Heyning PH (2008) Case report: “auditory neuropathy” in a newborn caused by a cerebellopontine angle arachnoid cyst. Int J Pediatr Otorhinolaryngol 72:905–909. doi:10.1016/j.ijporl.2008.02.003
Boudewyns A, Declau F, Van den Ende J, Van Kerschaver E, Dirckx S, Hofkens-Van Den Brandt A, Van de Heyning P (2011) Otitis media with effusion: an underestimated cause of hearing loss in infants. Otol Neurotol 32:799–804. doi:10.1097/MAO.0b013e31821b0d07
Buchman CA, Roush PA, Teagle HF, Brown CJ, Zdanski CJ, Grose JH (2006) Auditory neuropathy characteristics in children with cochlear nerve deficiency. Ear Hear 27:399–408. doi:10.1097/01.aud.0000224100.30525.ab
Declau F, Boudewyns A, Van den Ende J, Peeters A, van den Heyning P (2008) Etiologic and audiologic evaluations after universal neonatal hearing screening: analysis of 170 referred neonates. Pediatrics 121:1119–1126. doi:10.1542/peds.2007-1479
Declau F, Boudewyns A, Van den Ende J, van de Heyning P (2013) Auditory neuropathy: a challenge for diagnosis and treatment. B-Ent Suppl 21:65–79
Dowley AC, Whitehouse WP, Mason SM, Cope Y, Grant J, Gibbin KP (2009) Auditory neuropathy: unexpectedly common in a screened newborn population. Dev Med Child Neurol 51:642–646. doi:10.1111/j.1469-8749.2009.03298.x
King AM, Purdy SC, Dillon H, Sharma M, Pearce W (2005) Australian hearing protocols for the audiological management of infants who have auditory neuropathy. Aust N Z J Audiol 27:69–77
Kirkim G, Serbetcioglu B, Erdag TK, Ceryan K (2008) The frequency of auditory neuropathy detected by universal newborn hearing screening program. Int J Pediatr Otorhinolaryngol 72:1461–1469. doi:10.1016/j.ijporl.2008.06.010
Korver AM, van Zanten GA, Meuwese-Jongejeugd A, van Straaten HL, Oudesluys-Murphy AM (2012) Auditory neuropathy in a low-risk population: a review of the literature. Int J Pediatr Otorhinolaryngol 76:1708–1711. doi:10.1016/j.ijporl.2012.08.009
Lin HC, Shu MT, Lee KS, Lin HY, Lin G (2007) Reducing false positives in newborn hearing screening program: how and why. Otol Neurotol 28:788–792
Madden C, Rutter M, Hilbert L, Greinwald JH Jr, Choo DI (2002) Clinical and audiological features in auditory neuropathy. Arch Otolaryngol Head Neck Surg 128:1026–1030
Ngo RY, Tan HK, Balakrishnan A, Lim SB, Lazaroo DT (2006) Auditory neuropathy/auditory dys-synchrony detected by universal newborn hearing screening. Int J Pediatr Otorhinolaryngol 70:1299–1306. doi:10.1016/j.ijporl.2005.12.004
Psarommatis I, Riga M, Douros K, Koltsidopoulos P, Douniadakis D, Kapetanakis I, Apostolopoulos N (2006) Transient infantile auditory neuropathy and its clinical implications. Int J Pediatr Otorhinolaryngol 70:1629–1637. doi:10.1016/j.ijporl.2006.05.005
Rance G, Beer DE, Cone-Wesson B, Shepherd RK, Dowell RC, King AM, Rickards FW, Clark GM (1999) Clinical findings for a group of infants and young children with auditory neuropathy. Ear Hear 20:238–252
Roche JP, Huang BY, Castillo M, Bassim MK, Adunka OF, Buchman CA (2010) Imaging characteristics of children with auditory neuropathy spectrum disorder. Otol Neurotol 31:780–788
Rodriguez-Ballesteros M, Reynoso R, Olarte M, Villamar M, Morera C, Santarelli R, Arslan E, Meda C, Curet C, Volter C, Sainz-Quevedo M, Castorina P, Ambrosetti U, Berrettini S, Frei K, Tedin S, Smith J, Cruz Tapia M, Cavalle L, Gelvez N, Primignani P, Gomez-Rosas E, Martin M, Moreno-Pelayo MA, Tamayo M, Moreno-Barral J, Moreno F, del Castillo I (2008) A multicenter study on the prevalence and spectrum of mutations in the otoferlin gene (OTOF) in subjects with nonsyndromic hearing impairment and auditory neuropathy. Hum Mutat 29:823–831. doi:10.1002/humu.20708
Saluja S, Agarwal A, Kler N, Amin S (2010) Auditory neuropathy spectrum disorder in late preterm and term infants with severe jaundice. Int J Pediatr Otorhinolaryngol 74:1292–1297. doi:10.1016/j.ijporl.2010.08.007
Sininger Y (2004) Overview of auditory neuropathy. Audiology Online, July 8
Starr APTKR (2001) Pathophysiology of auditory neuropathy. In: Starr A, Sininger Y (eds) Auditory neuropathy: a new perspective on hearing disorders. Singular Thomson Learning, San Diego, pp 1–15
Acknowledgments
The authors would like to thank Luc Stappaerts from Kind and Gezin who provided the data on the UNHS screening for Flanders.
Author’s contribution
All authors contributed to data collection, interpretation of the data and preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None of the authors received any financial support or funding for the present study.
Conflict of interest
An Boudewyns declares that she has no conflict of interest.
Frank Declau declares that he has no conflict of interest.
Jenneke van den Ende declares that she has no conflict of interest.
Anouk Hofkens declares that she has no conflict of interest.
Sara Dirckx declares that she has no conflict of interest.
Paul van de Heyning declares that he has no conflict of interest.
Ethical approval
The study was performed in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the caregivers of all individual infants included in the study.
Additional information
Communicated by Peter de Winter
Appendix: Cochlear microphonic
Appendix: Cochlear microphonic
The cochlear microphonic is a preneural response from the cochlear outer hair cells. The presence of a cochlear microphonic at or below a sound level that does not evoke a recordable ABR is an indication for ANSD. The cochlear microphonic is a more robust criterion for the diagnosis of ANSD compared to transient evoked otoacoustic emissions because these may disappear with time or may be absent in cases where there is also a conductive component to the hearing loss (such as with middle ear effusion).
The recommended method to detect a CM is the use of separate, replicated runs of condensation and rarefaction polarity at a stimulus level of 80 dB nHL.
A CM is considered present when a waveform appears in the first few milliseconds of the tracing and is the only part of the waveform that reverses polarity from rarefaction to condensation. A control trial is then performed with the sound tube of the insert phone clamped to rule out transducer artefacts.
An example of a cochlear microphonic is provided below Fig. 3. More detailed information may be found at Lightfoot G (ed). 2011. Guidelines for cochlear microphonic testing. NHSP Clinical Group (http://www.thebsa.org.uk/wp-content/uploads/2015/02/CM_Guidance_v2_2109111.pdf).
An example of an ABR tracing for the right ear, illustrating a cochlear microphonic. Using alternating click stimuli at intensities of 80 and 70 dB nHL, no ABR tracing is present (red line). However, using runs of condensation and rarefaction polarity at the same stimulus intensities reveals the presence of a cochlear microphonic (black arrow) appearing after 1 ms
Rights and permissions
About this article
Cite this article
Boudewyns, A., Declau, F., van den Ende, J. et al. Auditory neuropathy spectrum disorder (ANSD) in referrals from neonatal hearing screening at a well-baby clinic. Eur J Pediatr 175, 993–1000 (2016). https://doi.org/10.1007/s00431-016-2735-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-016-2735-5