Abstract
Exposures to environmental chemicals and psychosocial stressors during pregnancy have been individually associated with adverse perinatal outcomes related to birthweight and gestational age, but are not often considered in combination. We review types of psychosocial stressors and instruments used to assess them and classes of environmental chemical exposures that are known to adversely impact perinatal outcomes, and identify studies relevant studies. We discuss the National Institutes of Health’s Environmental influences on Child Health Outcomes (ECHO) program that has combined existing longitudinal cohorts that include more than 50,000 children across the U.S. We describe future opportunities for investigators to use this important new resource for addressing relevant and critical research questions to maternal health. Of the 84 cohorts in ECHO, 38 collected data on environmental chemicals and psychosocial stressors and perinatal outcomes. The diverse ECHO pregnancy cohorts provide capacity to compare regions with distinct place-based environmental and social stressors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Exposures to environmental chemicals and psychosocial stressors are ubiquitous during pregnancy and have been individually associated with adverse pregnancy outcomes, including low birthweight and preterm birth. Due, in part, to structural racism and socioeconomic disadvantages, many of these chemical and stress exposures tend to cluster together, yet few studies have attempted to consider joint effects (i.e., synergistic or additive) [1]. Indeed, the combination, referred to as a “double jeopardy,” may have a greater impact than each individual factor, and result in amplified risk of adverse perinatal outcomes [2].
The objective of this expert-guided review is to discuss measures of environmental chemical and psychosocial exposures during pregnancy and examine the literature on their combined effects on perinatal outcomes and present this opportunity for future investigators to use this important new resource for addressing relevant and critical research questions to maternal health. We describe the National Institutes of Health’s (NIH’s) Environmental influences on Child Health Outcomes (ECHO) program, comprised of 84 pregnancy and childhood cohorts, that can be used to evaluate risk of perinatal outcomes attributable to multiple environmental and psychosocial stressors.
Almost all ECHO cohorts will have information on key perinatal outcomes related to fetal growth (birthweight and weight for gestational age) and prematurity (gestational age at birth). Low birthweight (<2500 g) occurs in 8% of births and the prevalence of preterm birth (<37 weeks gestation) is 10% in the U.S. [3]. These outcomes are associated with increased risk of adverse health outcomes throughout childhood and into adulthood [4]. A large proportion of these outcomes cannot be explained by known risk factors and we have not fully characterized the potential contribution of chemical and stress exposures to adverse perinatal outcomes. Thus, ECHO is well suited to address these risk factors.
Environmental chemical exposures
Pregnant women are exposed to a myriad of environmental chemicals, many of which are known to have adverse health effects, including effects on perinatal outcomes such as low birthweight and preterm birth [5, 6]. Given many environmental chemical exposures, existing exposure assessment where biological samples from pregnant women are available tends to rely mainly on targeted analytic chemistry methods, which focus on compounds selected a priori for analysis [7]. For example, only a few hundred chemicals are routinely measured in humans through targeted methods [5, 8], which are resource- and time intensive to develop, whereas the potential number of chemical exposures that pregnant woman can face is likely much higher, given that there are about 8000 industrial chemicals that are manufactured and used in high quantities in the U.S. and chemical production is about 9 trillion pounds per year [5]. We focus on a subset of classes of environmental chemical exposures measured in the ECHO program, recognizing that others of interest for perinatal outcomes (such as environmental tobacco smoke (ETS) and water disinfection byproducts) as well as new emerging contaminants that merit future research.
Persistent chemicals
Persistent environmental chemicals resist environmental degradation, bioaccumulate in food chains, and have long half-lives in humans. For some of these chemicals (e.g., organochlorine pesticides [OCPs] and perfluoroalkyl substances [PFASs]), exposure is principally via diet (through exposures in drinking water, fish, and meat) [9, 10], while for other chemical classes (e.g., polybrominated diphenyl ethers [PBDEs]) human exposure is through consumer products like textiles, furniture, and electronics [11]. Alarmingly, many persistent chemicals (e.g., polychlorinated biphenyls [PCBs], dichlorodiphenyltrichloroethane and its metabolites, and perfluorooctanesulfonic acid) remain present in measurable levels in virtually all pregnant women despite being phased-out or banned in many parts of the world for decades [5, 6]. Prenatal exposure to these compounds, many of which are endocrine-disrupting chemicals, have been shown to affect fetal growth and other developmental outcomes [12, 13].
Nonpersistent chemicals
In contrast to persistent environmental chemicals, nonpersistent chemicals are rapidly metabolized and excreted by the body, often in a matter of days or even hours. Nevertheless, pregnant women show nearly ubiquitous exposure to several classes of nonpersistent chemicals including phthalates, phenols, and parabens, thus making them pseudo-persistent [6, 14]. These chemicals (e.g., Bisphenol A [BPA] and diethylhexyl phthalate) are found in a wide array of consumer products, including plastics and canned and other food products, where they are introduced during processing and packaging [15, 16]. Certain phthalates, especially diethyl phthalate, and parabens, are also commonly found in personal care products [17, 18]. Phthalates, phenols, and parabens are all known endocrine-disrupting chemicals, interfering with hormone production and/or activity of hormones, particularly during vulnerable periods in development, such as gestation [19].
Heavy metals
Heavy metals enter the human body through primarily ingestion and inhalation [20]. Lead is the most studied metal and is teratogenic prenatally as well as toxic postnatally. Maternal exposure most often occurs through ingestion of dust from lead-based paints or water contaminated by lead pipes [20]. Lead is readily transported across the placenta and can lead to epigenetic changes in the fetus [21]. Maternal and cord blood lead levels have been associated with lower birthweight, shorter birth length, and smaller head circumference [22,23,24,25]. Mercury, which enters the environment through sources such as coal-fired power plants, can enter the body through ingestion of food grown in contaminated soil, or fish that bioaccumulate mercury. Mercury and other heavy metals, such as cadmium and arsenic, have been related to reduced birthweight in a number of populations [26,27,28,29,30,31].
Air pollution
Both indoor and outdoor air pollution, which include air toxics and regularly monitored pollutants such as particulate matter <10 and 2.5 μm in aerodynamic diameter, nitrogen dioxide, carbon monoxide, sulfur dioxide, and ozone, can pose a risk to fetal and infant health. Air pollutants impact the fetus through maternal inhalation; the greatest harm may occur during developmental periods with high oxygen requirement [32]. In addition, increased maternal alveolar ventilation rate during pregnancy can increase exposure to air pollutants. The pathologic effects of these pollutants are believed to be mediated by their effects on local and systemic inflammation among other mechanisms. Higher exposure to air pollution during pregnancy has been associated with respiratory-related and all-cause infant mortality, low birthweight, and preterm birth [33,34,35,36,37].
Biomonitoring of environmental chemicals
Because directly measuring environmental chemical exposures reaching the fetus is typically not feasible, maternal exposures are used as a proxy. Several methods commonly used to assess maternal exposures are discussed below, and detailed information on exposure assessment during pregnancy is available elsewhere [5, 38].
Biomonitoring of maternal matrices for environmental chemicals can provide important measures of internal doses integrated across exposure pathways [39], and commonly used matrices include maternal blood, urine, and hair. Standardized collection, storage, processing, and analytical protocols are critical for meaningful results [5, 40], and appropriate collection protocols vary widely by compound. For example, persistent chemicals may require only one measurement, while multiple measurements are recommended for nonpersistent chemicals due to high within-person variability over time [38].
While biomonitoring may not offer insight into sources of exposure, maternal questionnaires are widely used to assess chemical exposures during pregnancy, including the presence, duration, frequency, and pattern of consumer product and pesticide use [5, 38]. Questionnaires may be used when biospecimen collection or other personal exposure monitoring methods (such as household air and dust monitoring) are not logistically or financially feasible, thus allowing a larger study sample size than possible with more direct measurement techniques. Limitations to questionnaires include recall bias and the challenge of capturing multiple exposures and sources [38, 41]. In contrast to the extensive set of validated instruments to assess psychosocial stressors during pregnancy (see below), few validated questionnaires assess environmental chemical exposures on a personal level beyond analyzing biospecimens or monitoring personal environments directly.
Place-based assessment of environmental exposures
Geographic information systems (GIS) can be used to integrate environmental monitoring data or other contextual spatial datasets into epidemiological analyses [42, 43]. A typical use of GIS for perinatal research is to estimate exposure to outdoor air pollution using monitoring data collected at or near study subjects’ residences and workplaces [44,45,46]. The precision of GIS and environmental monitoring data as proxies for environmental chemical exposures depends on the availability of data on how individuals move through and interact with their environment [40]. Novel methods combining satellite data and modeling techniques allow daily estimates of air pollution, temperature, and greenspace with fine spatial and temporal resolution across the U.S. [47].
Psychosocial stressors
Pregnant women are exposed to psychosocial stressors via many routes, including poverty and low socioeconomic status (SES), major life events, pregnancy-related stressors, racial discrimination, and the presence of placed-based chronic stressors. These psychosocial stressors have been associated with adverse perinatal outcomes including low birthweight, small for gestational age, and preterm birth [48]. We focus on psychosocial risk factors most relevant to perinatal outcomes and the ECHO program goals of improving child health.
Poverty and low SES
The association between low SES and increased risk for adverse perinatal outcomes is evident at both individual and neighborhood levels [49, 50]. Neighborhood socioeconomic factors may affect demographic groups differently; one study found stronger neighborhood SES effects for African Americans and Asian Americans in urban areas [51]. Mechanisms that may explain these socioeconomic-perinatal health links include reduced access to prenatal care [50], variations in levels of bacterial vaginosis and cigarette smoking [52], as well as increased rates of exposure to stressful life events [53]. Living in high poverty and highly segregated communities can further compound the effect of individual-level poverty on adverse perinatal outcomes through exposures to distressed physical environments (e.g., environmental hazards and dilapidated housing), fragmented social networks (lack of social support and political power), and limited health-related resources (health care, access to healthy food, recreational spaces, and transportation) [2].
Perceived and pregnancy-related stress
Self-report measures have been used to capture individual experience or perception of stress and worry, including both general stress and stress related to specific experiences. General levels of stress are captured by self-reported measures such as the perceived stress scale (PSS) or the state-trait anxiety inventory (STAI). These measures are widely used across many studies with both healthy and clinical samples. Measures of pregnancy-specific perceived stress, such as the Prenatal Distress Questionnaire (PDQ) and Pregnancy-specific Anxiety Scale (PSAS), focus on concerns about birth and the baby, over body weight or image, and emotional or relationship changes [54]. These questionnaires take into consideration how the individual feels about normative changes that occur during pregnancy, such as physical discomfort or shifts in social relationships, whereas a general self-report scale such as the PSS or STAI do not specify worries related to this specific life experience. The most significant limitation of measures such as the PDQ and PSAS is that they do not measure perceived stress about experiences unrelated to pregnancy.
Measures of pregnancy-specific stress, focusing on concerns about the baby’s health and birth outcomes, are distinct and clearly independent of the effects of more general stress [54]. Perinatal outcomes, such as preterm birth, are more reliably predicted by pregnancy-related stress than more general stress measures [55, 56].
Stressful life events
Major life events, such as death of a family member, job loss, or divorce, may contribute to stress, including during pregnancy. Perhaps the most commonly employed self-report measure of stressful life events in pregnancy is the Pregnancy Risk Assessment Monitoring System (PRAMS), which includes questions about financial, partner-related, emotional, and traumatic life stressors. Centers for Disease Control and Prevention surveillance studies that use PRAMS suggest that more than half of all U.S. women report experiencing one or more stressful life events in the year before the birth of their child [57].
Prospective pregnancy cohort studies have employed a variety of life event checklists, or they have collected data on exposure to stressful life events reported in a clinical interview for assessment of post-traumatic stress disorder [58]. Other event-based assessment tools include the Life Events Checklist or the Traumatic Life Events Questionnaire.
An alternative method of studying and assessing psychosocial stress during pregnancy is the utilization of convenience samples of women who have collectively experienced a stressful life event during pregnancy. These events include natural disasters or other adverse events such as the 1998 Quebec Ice Storm [59], the Dutch Famine [60], or the 2001 World Trade Center attacks [61]. A recent meta-analysis examining stress during pregnancy noted small but significant associations between adverse perinatal outcomes and both maternal reports of stressful life events and natural disasters during pregnancy. Of note, each of these effect sizes were smaller than that noted between pregnancy-specific stress and perinatal outcomes [62]. Recent discoveries related to transgenerational epigenetic processes have also focused attention on the potential impact of maternal childhood trauma on offspring development [63]. In addition to checklists assessing potentially stressful life events in proximity to the pregnancy, reports about early life stress, including childhood maltreatment and adversity, are often used as well. The Childhood Trauma Questionnaire [64] and Adverse Childhood Experience questionnaire [65] are brief and easy to use measures that have been widely validated for assessment of these early experiences in pregnant populations [66].
Discrimination
Measures such as the Krieger Experiences of Discrimination Scale (EOD) [67] have been employed to assess discrimination-related stressors. The EOD includes both a self-report of event-based EOD and questions about worry related to discrimination. The EOD has been used in studies of racial discrimination during pregnancy [68], but has not been formally validated for use in pregnant populations.
A recent study that directly compared stressors experienced by non-Hispanic White and Black women in pregnancy found that non-Hispanic Black women experienced higher levels of discrimination, and also had higher levels of biological indicators of stress (C-reactive protein and adrenocorticotropic hormones), relative to White women in the sample at the same SES level [69]. These findings suggest that chronic stressors, specific to African American women, may impact their biological functioning during pregnancy, which may in turn be associated with their risk for adverse birth outcomes, a phenomenon known as “weathering” [70].
Biomarkers and potential mediators of exposures to psychosocial stressors
Recent studies exploring the mechanisms relating prenatal exposure to psychosocial stressors and perinatal outcomes have focused on biologic measures of the stress response, including corticotrophin-releasing hormone (CRH) and telomere biology. CRH plays a critical role in the physiologic response to stress by regulating release of adrenocorticotropic hormone from the anterior pituitary lobe, which in turn stimulates cortisol secretion from the adrenal cortex [71]. Evidence suggests positive associations between psychosocial stress and increased levels of CRH in maternal plasma during pregnancy [72, 73]. CRH produced in the placenta has been implicated as one of the primary endocrine mediators in the physiology of pregnancy and of spontaneous labor and studies suggest that it may be associated with increased risk of fetal growth restriction [74,75,76].
Telomeres are DNA-protein complexes that cap the end of chromosomes and protect the cell’s genomic stability [76]. Telomere length is a biomarker of cellular aging since telomeres shorten with cell replication. Telomere length is regulated by both genetic and environmental factors including chronic stress exposure. For example, shorter telomere length has been associated with conditions of chronic adversity, such as longer working hours [77], chronic caregiving [78], and lower SES [79]. The mechanisms linking stress to telomere shortening are uncertain, but immune and inflammatory pathways along with oxidative stress production are suspected [76]. Shorter maternal telomeres have been associated with intrauterine adversity or growth restriction in animal [80, 81] and human [82, 83] studies. Researchers also seek to better understand the extent to which biomarkers of stress response such as telomere length mediate observed relationships between maternal perceptual and place-based stress exposures and developmental outcomes in offspring [84].
Place-based sources of stress
Neighborhood environments can be important contributors to maternal chronic psychosocial stress that can function independently of individual-level stressors to impact health [85, 86]. A review of 28 studies found consistent relationships between poor neighborhood conditions and adverse perinatal outcomes after accounting for individual characteristics [87], including neighborhood-level poverty [88], and crime [89]. Evidence also suggests living in neighborhoods with higher rates of domestic violence increase the risk of giving birth to a small for gestational age infant compared with living in areas with low rates of domestic violence [90]. A systematic review and meta-analysis found racial residential segregation to be associated with higher risk of preterm birth and low birthweight, particularly for African American women [91]. Built environment characteristics, such as urban greenspace, may promote maternal health and reduce stress and has been associated with increased birthweight [92,93,94], while other residential environments, such as housing damage, have been associated with small for gestational age and lower birthweight [95].
Human studies on combined effects of psychosocial and environmental stressors during the prenatal period
A systematic review of human studies investigated combined effects of prenatal environmental chemical exposures and maternal psychosocial stressors on perinatal outcomes and found a paucity of research that addressed this question [1]. The review identified only 17 papers published between 1971 and 2013. Twelve studies examined smoking and ETS (two of which also included alcohol consumption); four studies evaluated air pollution, traffic density, and/or highway proximity; and one occupational study assessed benzene as the chemical exposure of interest. Most studies measured maternal prenatal psychosocial stress in terms of SES ascertained from maternal characteristics, such as educational attainment, social class, household income, as well as neighborhood poverty level, and/or food affordability. Maternal race/ethnicity was also evaluated to a lesser extent. One study examined low prenatal mood (question from PRAMS) as a measure of psychosocial stress.
In general, prenatal chemical exposures exerted stronger effects on perinatal outcomes than did maternal stress measures; however, the effects of environmental chemicals on birthweight were more pronounced for women who were also experiencing higher psychosocial stress exposures (e.g., low SES) during pregnancy. For this current review, we focus on environmental chemicals and psychosocial stressors excluding smoking and ETS. The majority of combined studies have focused on air pollution and socioeconomic stressors.
Since the systematic review was conducted in 2013, we have identified eight additional human studies that assessed the combined effect of maternal stress and prenatal environmental chemicals excluding smoking and ETS exposure [35, 96,97,98,99,100,101,102,103]. These newer studies are primarily focused on air pollution as an environmental exposure and SES as the psychosocial stressor. Few studies also included other stressors including educational attainment [98] and race/ethnicity [100, 101] and one study examined exposure to biomass fuel and unsafe water [98]. Most recent studies indicate that environmental chemical exposures interact with maternal psychosocial stressors during pregnancy to adversely affect perinatal outcomes (e.g., low birthweight, preterm birth, and stillbirth) and have combined effects in either mediation analyses or statistical interactions, yet few studies have quantitatively examined these associations beyond studies of air pollution. Few studies found unexpected results with higher air pollution exposures, or stronger associations between air pollution and adverse birth outcomes in higher income areas [99, 102].
In two studies on air pollution, modest inverse associations between air pollution and birthweight were reported, with stronger effects observed in black versus white women [33, 104]. Studies examining birth outcomes in relation to traffic-related pollution and SES were less consistent. Two studies on traffic-related air pollution (TRAP) effects and perinatal outcomes found an increased risk of preterm birth [35] and low birthweight [105], with stronger effects of TRAP among pregnant women with lower compared with higher SES. A third study reported that pregnant women with higher SES were more likely to experience preterm birth, small for gestational age and especially low birthweight associated with TRAP [106]. Discrepancies between studies may be attributable to differences in SES variable definitions (e.g., individual versus neighborhood measures of household income, poverty, unemployment, income from public assistance, and maternal educational attainment), population differences in geography, as well as sample size given the modeling of interaction terms.
Few studies have assessed the combined effects of work-related stress and occupational exposures to chemicals among pregnant women. One study found that work stress combined with occupational exposure to benzene was associated with reductions in birthweight while each stressor alone did not show an association in a cohort of pregnant women working at a large petrochemical company [107].
A limited number of studies have examined a full range of prenatal psychosocial stressors as we have outlined above—factors such as discrimination, adverse events, depression, and past trauma in combination with environmental chemical exposures. Further, the range of environmental chemicals analyzed has been limited and has not included compounds such as flame retardants, PFASs, BPA, and phthalates, for which the main sources of exposure are diet and consumer products.
Biological mechanisms for a combined effect
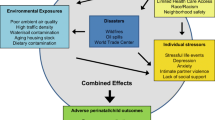
Figure 1 presents a schematic of proposed biological mechanisms shared and possibly potentiated by the combined exposures to psychosocial and environmental stressors affecting perinatal outcomes. Environmental chemicals can enter the maternal circulation and/or reproductive tract secretions through direct skin/mucosal contact, ingestion, or inhalation. Through maternal circulation chemicals may come into direct contact with the placenta and cause either direct tissue damage or subtler but equally harmful inflammatory perturbations, interference with the microbial colonization of the placenta, as well as downstream epigenetic changes that may be either directly caused by the chemicals or be microbiome-mediated [108]. Placental inflammation and disturbances in maternal microbiomes have been implicated in low birthweight and preterm birth [109,110,111], and placental measures have been documented as moderators of most known maternal factors/processes that affect birthweight (maternal age, parity, SES, and race/ethnicity) [112]. In addition to directly harming the placenta, prenatal environmental chemical exposures can negatively influence maternal health, including possible effects on the kidney, liver, cardiovascular, metabolic, and immune functions that can lead to or exacerbate gestational disease or hypertension, which in turn may lead to adverse perinatal outcomes. Other chemicals may lead to disturbances in the maternal microbiome [113], which in turn is implicated in poor perinatal outcomes including adverse fetal growth and preterm birth [109, 114].
Endocrine-disrupting chemicals may affect the hypothalamic–pituitary–adrenal (HPA), hypothalamic–pituitary–gonada, hypothalamic–pituitary–thyroid, and gluco-psychosocial axes [5, 115], which could translate into downstream immuno-endocrine disturbances and inflammatory activation. The molecular and systemic mechanisms of chemical damage to mother and placenta may be shared with and potentiated by the psychosocial stressors. Evidence shows the link between psychosocial stress and inflammation through activation of the HPA axis and imbalance of pro-inflammatory cytokines and growth factors, which are under neuroendocrine control at both systemic and reproductive tract levels [116]. Increased levels of cytokines have been detected in peripheral blood in association with psychosocial stress and depression [115, 117,118,119,120]. Cytokines produced in the central nervous system in response to psychosocial stress can change responsiveness within the HPA axis, thus making it more vulnerable to other stressors and potentially provoking cytokine cascades in the periphery that are known to modulate different aspects of the reproductive function [121,122,123]. Among the cytokines induced by psychosocial stress is interleukin-6, which has been implicated in spontaneous preterm delivery [124].
The ECHO program
ECHO is a research program launched by the NIH in 2016 to understand the effects of a broad range of early environmental factors on child health and development. ECHO includes 84 existing longitudinal cohorts with more than 50,000 children from diverse racial, geographic, and socioeconomic backgrounds across the U.S. Together, many of these cohorts follow participants from before they are born through childhood and into adolescence.
The greatest strength of ECHO is its ability to pool data from multiple cohorts to increase sample sizes to the thousands rather than the more typical hundreds. This increase in statistical power allows for examination of multiple exposures and multiple outcomes. An additional strength is the diverse geographic representation with varied demographics that exists across the ECHO cohorts. This variability improves generalizability as well as the ability to compare regions with distinct psychosocial and environmental stressors (Fig. 2).
The ECHO Data Analysis Center (DAC) developed several surveys to ascertain the collection of common data elements, including psychosocial stressors and environmental chemical exposures, from the ECHO cohorts. The cohorts specified whether data elements were ever collected for mothers, fathers, and children and the timing of the data collection (e.g., prenatal life stage). The current paper uses survey data on recruitment site locations (Module 2 release date: 01/31/2018); perinatal outcomes and psychosocial stressors in the mother’s prenatal life stage (Module 3 release date: 03/23/2018); and assays for stress biomarkers and chemical exposures (Module 4 release date: 05/01/2018). All assays have already been run or the cohorts noted that they will be run in the future on banked specimens. The response rate for these surveys was 100%. SAS (version 9.3) was used for data analyses and ESRI ArcGIS (version 10.5.1) was used to map recruitment sites to the zip code centroid.
ECHO will have the ability to examine various psychosocial and/or environmental stressors in relation to key perinatal outcomes. Here we describe exposure data from 80 ECHO cohorts that have data on both gestational age at birth and birthweight (Table 1, Fig. 3). Sixty cohorts had at least one psychosocial stress measure. Specifically, 39 cohorts measured perceived stress (e.g., PSS, racial discrimination, economic stress, psychological stress, social networks, interpersonal violence, neighborhood violence, and stressful life events); 17 have data on stress biomarkers (e.g., any stress assay, CRH, cortisol, adrenocorticotropic hormone, and/or telomere length) in mothers (prenatal, infant life stage) and/or infants; and 50 cohorts have place-based measures of stress (e.g., address data that may be combined with other datasets and/or built environment measures [food-related, activity-related, and proximity to alcohol and tobacco outlets]). In addition, 38 cohorts have data on environmental chemical exposure during the prenatal life stage. Specifically, 17 cohorts have measured levels of at least one persistent chemical (e.g., perfluorinated compounds, PBDEs, PCBs, OCPs, and/or organophosphate insecticides); 22 cohorts have measured nonpersistent chemicals (e.g., phenols and phthalates); 15 cohorts have measured metals or metalloids; and 49 cohorts have data on air pollution (i.e., polycyclic aromatic hydrocarbons, measured/estimated or planned indoor/personal air pollution, and/or measured/estimated or planned outdoor air pollution). Of note, 38 cohorts have existing data on prenatal exposure to psychosocial stressors and environmental chemicals as well as perinatal outcome data.
Analyses of these combinations of factors will depend on the research question of interest. Potential options include investigations of effect modification (i.e., statistical interaction) where the effects of environmental chemicals on perinatal outcomes may differ among women with different levels of psychosocial stress. Mediation analyses may also be of interest to quantify whether environmental chemical exposures mediate the relationship between psychosocial stressors and perinatal outcomes (e.g., low SES leading to higher environmental chemical exposures and subsequent perinatal outcomes) or vice versa (e.g., environmental chemical exposures affecting psychological stress and subsequent perinatal outcomes).
Challenges exist to harmonize extant data with differences in methods for chemical exposure or psychosocial stress assessment including laboratory protocols, timing during pregnancy, and questionnaire/instrument. For example, these methods may present differing levels of measurement error across different studies. For data collected prospectively in ECHO, additional challenges remain to quantify estimates for joint or multiple exposures, which may include mixtures (e.g., chemicals) and/or correlated variables. Statistical methods to examine cumulative risk of environmental chemical and psychosocial stressors have been reviewed elsewhere [125].
ECHO will also include coordinated measurement of chemical exposures from banked biospecimens (such as breast milk, placenta, microbiome samples, maternal and child hair, children’s teeth, as well as urine and blood) collected by participating cohorts, and program-wide collection of additional questionnaire data on psychosocial stressors is planned. Biospecimens may be used to assess biomarkers of stress response in addition to environmental chemical exposures. These data will be made public to a broader community of children’s health researchers and provide unprecedented opportunities for multidisciplinary teams to conduct novel explorations and robust analyses that advance scientific understand of both social and environmental determinants of perinatal outcomes and children’s future health.
Conclusion
The combined effects of environmental chemicals and psychosocial stressors are emerging as potentially important yet understudied factors that affect perinatal and children’s future health. Specifically, environmental chemicals beyond air pollution and psychosocial stressors, such as discrimination are understudied. We focused on perinatal outcomes as critical predictors of infant morbidity and mortality and chronic diseases in adulthood, though examination of combined effects of environmental chemicals and psychosocial stressors could be applied to other health outcomes.
We recommend future examinations of The ECHO program has a unique opportunity to integrate and evaluate nearly 40 cohorts with prenatal data on multiple chemical and psychosocial stress exposure. Leveraging these publicly available data from the ECHO cohorts, researchers will be able to develop a more comprehensive understanding of the complex effects of important prenatal exposures on development, thereby informing interventions and prevention to reduce adverse perinatal outcomes.
References
Vesterinen HM, Morello-Frosch R, Sen S, Zeise L, Woodruff TJ. Cumulative effects of prenatal-exposure to exogenous chemicals and psychosocial stress on fetal growth: Systematic-review of the human and animal evidence. PLOS ONE. 2017;12:e0176331.
Morello-Frosch R, Shenassa ED. The environmental “riskscape” and social inequality: implications for explaining maternal and child health disparities. Environ Health Perspect. 2006;114:1150–3.
Martin JAHB, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2016. Hyattsville, MD: National Center for Health Statistics; 2018.
Barker DJ. The developmental origins of adult disease. Eur J Epidemiol. 2003;18:733–6.
Wang A, Padula A, Sirota M, Woodruff TJ. Environmental influences on reproductive health: the importance of chemical exposures. Fertil Steril. 2016;106:905–29.
Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003-4. Environ Health Perspect. 2011;119:878–85.
Dennis KK, Marder E, Balshaw DM, Cui Y, Lynes MA, Patti GJ, et al. Biomonitoring in the Era of the Exposome. Environ Health Perspect. 2017;125:502–10.
Centers for Disease Control and Prevention. The fourth national report on human exposure to environmental chemicals. Atlanta, GA: U.S. Centers for Disease Control and Prevention; 2017.
D’Hollander W, de Voogt P, De Coen W, Bervoets L. Perfluorinated substances in human food and other sources of human exposure. Rev Environ Contam Toxicol. 2010;208:179–215.
Yu Y, Li C, Zhang X, Zhang X, Pang Y, Zhang S, et al. Route-specific daily uptake of organochlorine pesticides in food, dust, and air by Shanghai residents, China. Environ Int. 2012;50:31–7.
Johnson-Restrepo B, Kannan K. An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere. 2009;76:542–8.
Blum A, Balan SA, Scheringer M, Trier X, Goldenman G, Cousins IT, et al. The Madrid Statement on Poly- and Perfluoroalkyl Substances (PFASs). Environ Health Perspect. 2015;123:A107–111.
Kezios KL, Liu X, Cirillo PM, Cohn BA, Kalantzi OI, Wang Y, et al. Dichlorodiphenyltrichloroethane (DDT), DDT metabolites and pregnancy outcomes. Reprod Toxicol. 2013;35:156–64.
Meeker JD, Cantonwine DE, Rivera-Gonzalez LO, Ferguson KK, Mukherjee B, Calafat AM, et al. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environ Sci Technol. 2013;47:3439–47.
Sakhi AK, Lillegaard IT, Voorspoels S, Carlsen MH, Loken EB, Brantsaeter AL, et al. Concentrations of phthalates and bisphenol A in Norwegian foods and beverages and estimated dietary exposure in adults. Environ Int. 2014;73:259–69.
Schecter A, Lorber M, Guo Y, Wu Q, Yun SH, Kannan K, et al. Phthalate concentrations and dietary exposure from food purchased in New York State. Environ Health Perspect. 2013;121:473–94.
Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, Hauser R. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J Expo Sci Environ Epidemiol. 2014;24:459–66.
Harley KG, Kogut K, Madrigal DS, Cardenas M, Vera IA, Meza-Alfaro G, et al. Reducing Phthalate, paraben, and phenol exposure from personal care products in adolescent girls: findings from the HERMOSA Intervention Study. Environ Health Perspect. 2016;124:1600–7.
Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015;36:E1–E150.
Papanikolaou NC, Hatzidaki EG, Belivanis S, Tzanakakis GN, Tsatsakis AM. Lead toxicity update. A brief review. Med Sci Monit. 2005;11:RA329–336.
Sen A, Heredia N, Senut MC, Land S, Hollocher K, Lu X, et al. Multigenerational epigenetic inheritance in humans: DNA methylation changes associated with maternal exposure to lead can be transmitted to the grandchildren. Sci Rep. 2015;5:14466.
Gundacker C, Frohlich S, Graf-Rohrmeister K, Eibenberger B, Jessenig V, Gicic D, et al. Perinatal lead and mercury exposure in Austria. Sci Total Environ. 2010;408:5744–9.
Osman K, Akesson A, Berglund M, Bremme K, Schutz A, Ask K, et al. Toxic and essential elements in placentas of Swedish women. Clin Biochem. 2000;33:131–8.
Tao Y, Bai X, Zhang H, Liu J. [Effect of lead exposure in prenatal and postnatal duration on infant growth]. Wei Sheng Yan Jiu. 2001;30:102–4.
Zhu M, Fitzgerald EF, Gelberg KH, Lin S, Druschel CM. Maternal low-level lead exposure and fetal growth. Environ Health Perspect. 2010;118:1471–5.
Everson TM, Kappil M, Hao K, Jackson BP, Punshon T, Karagas MR, et al. Maternal exposure to selenium and cadmium, fetal growth, and placental expression of steroidogenic and apoptotic genes. Environ Res. 2017;158:233–44.
Finley C. Mandatory continuing education-a survey of current activity. A special communication. Phys Ther. 1988;68:374–7.
Koslowski L, Schmolke M. [Proceedings: do the history and clinical findings allow any conclusion on the stage of appendicitis? (author’s transl)]. Langenbecks Arch Chir. 1973;334:851–8.
Liao KW, Chang CH, Tsai MS, Chien LC, Chung MY, Mao IF, et al. Associations between urinary total arsenic levels, fetal development, and neonatal birth outcomes: a cohort study in Taiwan. Sci Total Environ. 2018;612:1373–9.
Luo Y, McCullough LE, Tzeng JY, Darrah T, Vengosh A, Maguire RL, et al. Maternal blood cadmium, lead and arsenic levels, nutrient combinations, and offspring birthweight. BMC Public Health. 2017;17:354.
Rahman ML, Valeri L, Kile ML, Mazumdar M, Mostofa G, Qamruzzaman Q, et al. Investigating causal relation between prenatal arsenic exposure and birthweight: are smaller infants more susceptible? Environ Int. 2017;108:32–40.
Hackley B, Feinstein A, Dixon J. Air pollution: impact on maternal and perinatal health. J Midwifery Women’s Health. 2007;52:435–43.
Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115:1118–24.
Dadvand P, Parker J, Bell ML, Bonzini M, Brauer M, Darrow LA, et al. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ Health Perspect. 2013;121:267–373.
Padula AM, Mortimer KM, Tager IB, Hammond SK, Lurmann FW, Yang W, et al. Traffic-related air pollution and risk of preterm birth in the San Joaquin Valley of California. Ann Epidemiol. 2014;24:888–95. e884.
Sram RJ, Binkova B, Dejmek J, Bobak M. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect. 2005;113:375–82.
Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res. 2012;117:100–11.
Malinowski AK, Ananth CV, Catalano P, Hines EP, Kirby RS, Klebanoff MA, et al. Research standardization tools: pregnancy measures in the PhenX Toolkit. Am J Obstet Gynecol. 2017;217:249–62.
Schoeters GE, Den Hond E, Koppen G, Smolders R, Bloemen K, De Boever P, et al. Biomonitoring and biomarkers to unravel the risks from prenatal environmental exposures for later health outcomes. Am J Clin Nutr. 2011;94 6 Suppl:1964S–1969S.
Robinson O, Basagana X, Agier L, de Castro M, Hernandez-Ferrer C, Gonzalez JR, et al. The pregnancy exposome: multiple environmental exposures in the INMA-Sabadell Birth Cohort. Environ Sci Technol. 2015;49:10632–41.
Nieuwenhuijsen MJ. Design of exposure questionnaires for epidemiological studies. Occup Environ Med. 2005;62:272–80. 212–274.
Akkus C, Ozdenerol E. Exploring childhood lead exposure through GIS: a review of the recent literature. Int J Environ Res Public Health. 2014;11:6314–34.
Nuckols JR, Ward MH, Jarup L. Using geographic information systems for exposure assessment in environmental epidemiology studies. Environ Health Perspect. 2004;112:1007–15.
Aguilera I, Pedersen M, Garcia-Esteban R, Ballester F, Basterrechea M, Esplugues A, et al. Early-life exposure to outdoor air pollution and respiratory health, ear infections, and eczema in infants from the INMA study. Environ Health Perspect. 2013;121:387–92.
Chiu YH, Hsu HH, Wilson A, Coull BA, Pendo MP, Baccarelli A, et al. Prenatal particulate air pollution exposure and body composition in urban preschool children: Examining sensitive windows and sex-specific associations. Environ Res. 2017;158:798–805.
Lee A, Leon Hsu HH, Mathilda Chiu YH, Bose S, Rosa MJ, Kloog I, et al. Prenatal fine particulate exposure and early childhood asthma: effect of maternal stress and fetal sex. J Allergy Clin Immunol. 2018;141:1880–6.
Kloog I, Chudnovsky AA, Just AC, Nordio F, Koutrakis P, Coull BA, et al. A New hybrid spatio-temporal model for estimating daily multi-year PM2.5 concentrations across Northeastern USA using high resolution aerosol optical depth data. Atmos Environ. 2014;95:581–90.
Hobel CJ, Goldstein A, Barrett ES. Psychosocial stress and pregnancy outcome. Clin Obstet Gynecol. 2008;51:333–48.
Blumenshine P, Egerter S, Barclay CJ, Cubbin C, Braveman PA. Socioeconomic disparities in adverse birth outcomes: a systematic review. Am J Prev Med. 2010;39:263–72.
Luo ZC, Wilkins R, Kramer MS. Fetal, Infant Health Study Group of the Canadian Perinatal Surveillance S. Effect of neighbourhood income and maternal education on birth outcomes: a population-based study. CMAJ. 2006;174:1415–20.
Pearl M, Braveman P, Abrams B. The relationship of neighborhood socioeconomic characteristics to birthweight among 5 ethnic groups in California. Am J Public Health. 2001;91:1808–14.
Kramer MS, Seguin L, Lydon J, Goulet L. Socio-economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatr Perinat Epidemiol. 2000;14:194–210.
Adler NE, Stewart J. Health disparities across the lifespan: meaning, methods, and mechanisms. Ann N Y Acad Sci. 2010;1186:5–23.
Yali AM, Lobel M. Coping and distress in pregnancy: an investigation of medically high risk women. J Psychosom Obstet Gynaecol. 1999;20:39–52.
Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol. 2008;27:604–15.
Roesch SC, Schetter CD, Woo G, Hobel CJ. Modeling the types and timing of stress in pregnancy. Anxiety, Stress Coping. 2004;17:87–102.
Burns ER, Farr SL, Howards PP, Centers for Disease C, Prevention. Stressful life events experienced by women in the year before their infants’ births-United States, 2000–2010. MMWR Morb Mortal Wkly Rep. 2015;64:247–51.
Chen MJ, Grobman WA, Gollan JK, Borders AE. The use of psychosocial stress scales in preterm birth research. Am J Obstet Gynecol. 2011;205:402–34.
Dancause KN, Laplante DP, Oremus C, Fraser S, Brunet A, King S. Disaster-related prenatal maternal stress influences birth outcomes: project Ice Storm. Early Hum Dev. 2011;87:813–20.
Schulz LC. The Dutch Hunger Winter and the developmental origins of health and disease. Proc Natl Acad Sci USA. 2010;107:16757–8.
Yehuda R, Engel SM, Brand SR, Seckl J, Marcus SM, Berkowitz GS. Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. J Clin Endocrinol Metab. 2005;90:4115–8.
Bussières EL, Tarabulsy GM, Pearson J, Tessier R, Forest JC, Giguère Y. Maternal prenatal stress and infant birth weight and gestational age: a meta-analysis of prospective studies. Dev Rev. 2015;36:179–99.
Gudsnuk KM, Champagne FA. Epigenetic effects of early developmental experiences. Clin Perinatol. 2011;38:703–17.
Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abus Negl. 2003;27:169–90.
Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–58.
Schreier HM, Enlow MB, Ritz T, Coull BA, Gennings C, Wright RO, et al. Lifetime exposure to traumatic and other stressful life events and hair cortisol in a multi-racial/ethnic sample of pregnant women. Stress. 2016;19:45–52.
Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med. 2005;61:1576–96.
Dominguez TP, Strong EF, Krieger N, Gillman MW, Rich-Edwards JW. Differences in the self-reported racism experiences of US-born and foreign-born Black pregnant women. Soc Sci Med. 2009;69:258–65.
Borders AE, Wolfe K, Qadir S, Kim KY, Holl J, Grobman W. Racial/ethnic differences in self-reported and biologic measures of chronic stress in pregnancy. J Perinatol. 2015;35:580–4.
Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96:826–33.
Petraglia F, Imperatore A, Challis JR. Neuroendocrine mechanisms in pregnancy and parturition. Endocr Rev. 2010;31:783–816.
Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999;180 1 Pt 3:S257–63.
Tse AC, Rich-Edwards JW, Koenen K, Wright RJ. Cumulative stress and maternal prenatal corticotropin-releasing hormone in an urban U.S. cohort. Psychoneuroendocrinology. 2012;37:970–9.
Zoumakis E, Kalantaridou SN, Makrigiannakis A. CRH-like peptides in human reproduction. Curr Med Chem. 2009;16:4230–5.
Wadhwa PD, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, et al. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;191:1063–9.
Epel E, Daubenmier J, Moskowitz JT, Folkman S, Blackburn E. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34–53.
Parks CG, DeRoo LA, Miller DB, McCanlies EC, Cawthon RM, Sandler DP. Employment and work schedule are related to telomere length in women. Occup Environ Med. 2011;68:582–9.
Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101:17312–5.
Steptoe A, Hamer M, Butcher L, Lin J, Brydon L, Kivimaki M, et al. Educational attainment but not measures of current socioeconomic circumstances are associated with leukocyte telomere length in healthy older men and women. Brain Behav Immun. 2011;25:1292–8.
Tarry-Adkins JL, Chen JH, Smith NS, Jones RH, Cherif H, Ozanne SE. Poor maternal nutrition followed by accelerated postnatal growth leads to telomere shortening and increased markers of cell senescence in rat islets. FASEB J. 2009;23:1521–8.
Tarry-Adkins JL, Martin-Gronert MS, Chen JH, Cripps RL, Ozanne SE. Maternal diet influences DNA damage, aortic telomere length, oxidative stress, and antioxidant defense capacity in rats. FASEB J. 2008;22:2037–44.
Biron-Shental T, Sukenik Halevy R, Goldberg-Bittman L, Kidron D, Fejgin MD, Amiel A. Telomeres are shorter in placental trophoblasts of pregnancies complicated with intrauterine growth restriction (IUGR). Early Hum Dev. 2010;86:451–6.
Jones CW, Gambala C, Esteves KC, Wallace M, Schlesinger R, O’Quinn M, et al. Differences in placental telomere length suggest a link between racial disparities in birth outcomes and cellular aging. Am J Obstet Gynecol. 2017;216:294 e291–294 e298.
Provenzi L, Scotto di Minico G, Giorda R, Montirosso R. Telomere length in preterm infants: a promising biomarker of early adversity and care in the neonatal intensive care unit? Front Endocrinol. 2017;8:295.
Diez-Roux AV. Bringing context back into epidemiology: variables and fallacies in multilevel analysis. Am J Public Health. 1998;88:216–22.
Macintyre S, Ellaway A, Cummins S. Place effects on health: how can we conceptualise, operationalise and measure them? Soc Sci Med. 2002;55:125–39.
Metcalfe A, Lail P, Ghali WA, Sauve RS. The association between neighbourhoods and adverse birth outcomes: a systematic review and meta-analysis of multi-level studies. Paediatr Perinat Epidemiol. 2011;25:236–45.
Grady SC. Racial disparities in low birthweight and the contribution of residential segregation: a multilevel analysis. Soc Sci Med. 2006;63:3013–29.
Morenoff JD. Neighborhood mechanisms and the spatial dynamics of birth weight. AJS. 2003;108:976–1017.
Felker-Kantor E, Wallace M, Theall K. Living in violence: neighborhood domestic violence and small for gestational age births. Health Place. 2017;46:130–6.
Mehra R, Boyd LM, Ickovics JR. Racial residential segregation and adverse birth outcomes: a systematic review and meta-analysis. Soc Sci Med. 2017;191:237–50.
Dadvand P, Sunyer J, Basagana X, Ballester F, Lertxundi A, Fernandez-Somoano A, et al. Surrounding greenness and pregnancy outcomes in four Spanish birth cohorts. Environ Health Perspect. 2012;120:1481–7.
Dadvand P, Wright J, Martinez D, Basagana X, McEachan RR, Cirach M, et al. Inequality, green spaces, and pregnant women: roles of ethnicity and individual and neighbourhood socioeconomic status. Environ Int. 2014;71:101–8.
Ward Thompson C, Aspinall P, Roe J, Robertson L, Miller D. Mitigating stress and supporting health in deprived urban communities: the importance of green space and the social environment. Int J Environ Res Public Health. 2016;13:440.
Miranda ML, Messer LC, Kroeger GL. Associations between the quality of the residential built environment and pregnancy outcomes among women in North Carolina. Environ Health Perspect. 2012;120:471–7.
Amegah AK, Damptey OK, Sarpong GA, Duah E, Vervoorn DJ, Jaakkola JJK. Malaria Infection, poor nutrition and indoor air pollution mediate socioeconomic differences in adverse pregnancy outcomes in Cape Coast, Ghana. PLoS ONE. 2013;8:e69181.
Amegah AK, Jaakkola JJ. Work as a street vendor, associated traffic-related air pollution exposures and risk of adverse pregnancy outcomes in Accra, Ghana. Int J Hyg Environ Health. 2014;217:354–62.
Amegah AK, Näyhä S, Jaakkola JJK. Do biomass fuel use and consumption of unsafe water mediate educational inequalities in stillbirth risk? An analysis of the 2007 Ghana Maternal Health Survey. BMJ Open. 2017;7:e012348.
Habermann M, Gouveia N. Socioeconomic position and low birth weight among mothers exposed to traffic-related air pollution. PLoS ONE. 2014;9:e113900.
Pereira G, Belanger K, Ebisu K, Bell ML. Fine particulate matter and risk of preterm birth in connecticut in 2000–2006: a longitudinal study. Am J Epidemiol. 2014;179:67–74.
Rappazzo KM, Daniels JL, Messer LC, Poole C, Lobdell DT. Exposure to elemental carbon, organic carbon, nitrate, and sulfate fractions of fine particulate matter and risk of preterm birth in New Jersey, Ohio, and Pennsylvania (2000–2005). Environ Health Perspect. 2015;123:1059–65.
Stieb DM, Chen L, Hystad P, Beckerman BS, Jerrett M, Tjepkema M, et al. A national study of the association between traffic-related air pollution and adverse pregnancy outcomes in Canada, 1999–2008. Environ Res. 2016;148 Supplement C:513–26.
Tu J, Tu W, Tedders SH. Spatial variations in the associations of term birth weight with ambient air pollution in Georgia, USA. Environ Int. 2016;92 Supplement C:146–56.
Morello-Frosch R, Jesdale BM, Sadd JL, Pastor M. Ambient air pollution exposure and full-term birth weight in California. Environ Health. 2010;9:44.
Zeka A, Melly SJ, Schwartz J. The effects of socioeconomic status and indices of physical environment on reduced birth weight and preterm births in Eastern Massachusetts. Environ Health. 2008;7:60.
Généreux M, Auger N, Goneau M, Daniel M. Neighbourhood socioeconomic status, maternal education and adverse birth outcomes among mothers living near highways. J Epidemiol Community Health. 2008;62:695.
Chen D, Cho S-I, Chen C, Wang X, Damokosh AI, Ryan L, et al. Exposure to benzene, occupational stress, and reduced birth weight. Occup Environ Med. 2000;57:661–7.
Tomlinson MS, Bommarito PA, Martin EM, Smeester L, Fichorova RN, Onderdonk AB, et al. Microorganisms in the human placenta are associated with altered CpG methylation of immune and inflammation-related genes. PLoS One. 2017;12:e0188664.
Prince AL, Ma J, Kannan PS, Alvarez M, Gisslen T, Harris RA, et al. The placental microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. Am J Obstet Gynecol. 2016;214:627.e621–627.e616.
Galinsky R, Polglase GR, Hooper SB, Black MJ, Moss TJM. The consequences of chorioamnionitis: preterm birth and effects on development. J Pregnancy. 2013;2013:412831.
Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213:S29–S52.
Baptiste-Roberts K, Salafia CM, Nicholson WK, Duggan A, Wang NY, Brancati FL. Maternal risk factors for abnormal placental growth: the national collaborative perinatal project. BMC Pregnancy Childbirth. 2008;8:44.
Ribado JV, Ley C, Haggerty TD, Tkachenko E, Bhatt AS, Parsonnet J. Household triclosan and triclocarban effects on the infant and maternal microbiome. EMBO Mol Med. 2017;9:1732–41.
Vinturache AE, Gyamfi-Bannerman C, Hwang J, Mysorekar IU, Jacobsson B. Maternal microbiome – a pathway to preterm birth. Semin Fetal Neonatal Med. 2016;21:94–9.
Kim YK, Jung HG, Myint AM, Kim H, Park SH. Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J Affect Disord. 2007;104:91–5.
Haimovici F, Anderson JL, Batesb GW, Racowsky C, Ginsburg ES, Simovicic D, et al. Stress, anxiety and depression of both partners in infertile couples are associated with cytokine levels and adverse IVF outcome. Am J Reprod Immunol. 2018;79:e12832.
Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev. 2012;36:764–85.
Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depression anxiety. 2013;30:297–306.
McNamara RK, Lotrich FE. Elevated immune-inflammatory signaling in mood disorders: a new therapeutic target? Expert Rev neurotherapeutics. 2012;12:1143–61.
Maes M, Anderson G, Kubera M, Berk M. Targeting classical IL-6 signalling or IL-6 trans-signalling in depression? Expert Opin therapeutic targets. 2014;18:495–512.
Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol therapeutics. 2011;130:226–38.
Ozkan ZS, Deveci D, Kumbak B, Simsek M, Ilhan F, Sekercioglu S, et al. What is the impact of Th1/Th2 ratio, SOCS3, IL17, and IL35 levels in unexplained infertility? J Reprod Immunol. 2014;103:53–8.
Altun T, Jindal S, Greenseid K, Shu J, Pal L. Low follicular fluid IL-6 levels in IVF patients are associated with increased likelihood of clinical pregnancy. J Assist Reprod Genet. 2011;28:245–51.
Taylor BD, Holzman CB, Fichorova RN, Tian Y, Jones NM, Fu W, et al. Inflammation biomarkers in vaginal fluid and preterm delivery. Hum Reprod. 2013;28:942–52.
Huang H, Wang A, Morello-Frosch R, Lam J, Sirota M, Padula A, et al. Cumulative risk and impact modeling on environmental chemical and social stressors. Curr Environ Health Rep. 2018;5:88–99.
Acknowledgements
Research reported in this publication was supported by the ECHO program, Office of The Director, NIH, under Award numbers U2COD023375 (Coordinating Center), U24OD023382 (DAC); UG3OD023272 (AMP, TJW, RM-F, and JV); R00ES021470 (AMP), UG3OD023328, UG3OD023316 (CM, SF, and PD), UG3OD023318 (PAB), UG3OD023319 (AB); UG3OD023349, UGOD023271, UGOD023305, P30 ES005022 (ESB); UG3OD023288 (CTM); UG3OD023251 (AA); P42ES017198 (RW); UG30D023349, UG3OD23285 (CS); UG3OD023285 (RF); U24OD023382 (AK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors wish to thank our ECHO colleagues; the medical, nursing, and program staff; as well as the children and families participating in the ECHO cohorts.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
See Appendix for full listing of collaborators
Appendix
Appendix
The authors wish to thank our ECHO colleagues, the medical, nursing and program staff, as well as the children and families participating in the ECHO cohorts. We also acknowledge the contributions of the following ECHO program collaborators:
ECHO Components
Coordinating Center: Duke Clinical Research Institute, Durham, North Carolina: Benjamin DK, Smith PB, Newby KL
Data Analysis Center: Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland: Jacobson LP; Research Triangle Institute, Durham, North Carolina: Parker CB
Person-Reported Outcomes Core: Northwestern University, Evanston, Illinois: Gershon R, Cella D
Children’s Health and Exposure Analysis Resource: Icahn School of Medicine at Mount Sinai, New York City, New York: Teitelbaum SL; Wright RO; Wadsworth Center, Albany, New York: Aldous, KM, RTI International, Research Triangle Park, North Carolina: Fennell T; University of Minnesota, Minneapolis, Minnesota: Hecht SS, Peterson L; Westat, Inc., Rockville, Maryland: O’Brien B
Idea States Pediatric Trials Network: University of Arkansas for Medical Sciences, Little Rock: Lee JY, Snowden J
ECHO Awardees
Albert Einstein College of Medicine, Bronx, New York: Aschner JL, Teitelbaum SL
Brigham & Women's Hospital, Boston, Massachusetts: Litonjua AA
Columbia University, New York, New York: Perera FP
Dartmouth College, Hanover, New Hampshire: Karagas MR,
Drexel University, Philadelphia, Pennsylvania: Newschaffer CJ
Emory University, Atlanta, Georgia: Dunlop AL, Brennan PA, Corwin EJ
Feinstein Institute for Medical Research, Manhasset, New York: Gregersen PK, Diamond B
Harvard Pilgrim Health Care, Boston, Massachusetts: Oken E, Kleinman KP
Icahn School of Medicine at Mount Sinai, Boston, Massachusetts: Wright RO
Kaiser Permanente, Oakland, California: Ferrara A, Croen LA
Massachusetts General Hospital, Boston: Camargo CA
Medical University of South Carolina, Charleston: Vena JE, Wapner R
Memorial Hospital of Rhode Island, Pawtucket: Deoni S, Mueller HG
Michigan State University, East Lansing, Michigan: Paneth N, Barone C, Copeland GE, Elliott MR, Ruden DM
New York State Psychiatric Institute at Columbia University, New York: Duarte CS, Canino GJ, Monk CE, Posner JE
New York University, New York, New York: Blair CB
New York University School of Medicine, New York, New York: Trasande L
Oregon Health & Science University, Portland, Oregon: McEvoy CT, Spindel ER
Northeastern University, Boston, Massachusetts: Alshawabkeh AN
Avera Research, Sioux Falls, South Dakota: Elliott AJ
University of California, Berkeley: Eskenazi B
University of California, Davis: Hertz-Picciotto I, Bennett DH, Schweitzer JB
University of Chicago, Illinois: Claud EC
University of Colorado Anschutz Medical Campus, Aurora, Colorado: Dabelea D
University of Illinois, Urbana: Schantz SL
University of Oregon, Eugene: Leve LD
University of New Mexico, Albuquerque: Lewis JL, MacKenzie D, Begay M-G
University of North Carolina, Chapel Hill: O’Shea M, Fry R
University of Pittsburgh, Pennsylvania: Hipwell AE, Keenan KE
University of Rochester, New York: O’Connor TG, Buss C, Miller RK, Wadhwa PD
University of Southern California, Los Angeles: Gilliland FD, Breton CV
University of Utah, Salt Lake City: Stanford JB, Clark EB, Porucznik C
University of Washington, Seattle: Karr C, Bush NR, Lewinn KZ
University of Wisconsin, Madison: Gern J
Women & Infants Hospital of Rhode Island, Providence: Lester B
ECHO Cohorts
Brigham & Women's Hospital, Boston, Massachusetts: Gold D; Weiss ST
Children's Hospital of Philadelphia, Pennsylvania: Schultz RT
Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio: Hershey N
Columbia University, New York, New York: Miller R; Herbstman JB
Emory University, Atlanta, Georgia: Marsit CJ
George Washington University, Washington D.C.: Ganiban JM
Henry Ford Health System, Detroit, Michigan: Johnson C, Ownby D, Zoratti E
Icahn School of Medicine at Mount Sinai, New York, New York: Stroustrup A
Inova Health Care System, Falls Church, Virginia: Huddleston K
Johns Hopkins University, Baltimore, Maryland: Volk HE
Kennedy Krieger Institute, Baltimore, Maryland: Landa RJ
Medical University of South Carolina, Charleston: Hunt K
Penn State, University Park, Pennsylvania: Neiderhiser JM
Seattle Children's Research Institute, Washington: Sathyanarayana S
University of Arizona, Tucson, Arizona: Martinez F, Wright A
University of California, San Francisco: Keller R; Woodruff TJ
University of California, Davis: Schmidt RJ, Ozonoff S
University of Chicago, Illinois: Andrews B
University of Miami, Coral Gables, Florida: Messinger DS
University of Michigan, Ann Arbor, Michigan: Padmanabhan V
University of North Carolina, Chapel Hill: Piven J
University of Pittsburgh. Magee-Women's Hospital, Pennsylvania: Simhan HN
University of Rochester, Rochester, New York: Pryhuber G
University of Tennessee Health Science Center: Tylavsky FA
University of Washington, Seattle: Dager SR; Maycock D; Stone WL
University of Wisconsin, Madison: Jackson D, Seroogy C
Vanderbilt University, Nashville, Tennessee: Hartert T; Moore P
Washington University, St. Louis, Missouri: Bacharier L; Botteron KN
Rights and permissions
About this article
Cite this article
Padula, A.M., Monk, C., Brennan, P.A. et al. A review of maternal prenatal exposures to environmental chemicals and psychosocial stressors—implications for research on perinatal outcomes in the ECHO program. J Perinatol 40, 10–24 (2020). https://doi.org/10.1038/s41372-019-0510-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-019-0510-y
- Springer Nature America, Inc.
This article is cited by
-
The association between increased fetal movements in the third trimester and perinatal outcomes; a systematic review and meta-analysis
BMC Pregnancy and Childbirth (2024)
-
Reproductive and Social Policies, Sociopolitical Stress, and Implications for Maternal and Child Health Equity
Current Environmental Health Reports (2024)
-
Prenatal Cannabis Use and Offspring Autism-Related Behaviors: Examining Maternal Stress as a Moderator in a Black American Cohort
Journal of Autism and Developmental Disorders (2024)
-
Perinatal health effects of herbicides exposures in the United States: the Heartland Study, a Midwestern birth cohort study
BMC Public Health (2023)
-
Addressing systemic problems with exposure assessments to protect the public’s health
Environmental Health (2023)