Abstract
Purpose of Review
Pregnant women and their offspring are vulnerable to the adverse effects of environmental and psychosocial stressors, individually and in combination. Here, we review the literature on how air pollution and metal exposures may interact with structural and individual-level stressors (including poverty and stressful life events) to impact perinatal and child outcomes.
Recent Findings
The adverse associations between air pollution and metal exposures and adverse infant and child health outcomes are often exacerbated by co-exposure to psychosocial stressors. Although studies vary by geography, study population, pollutants, stressors, and outcomes considered, the effects of environmental exposures and psychosocial stressors on early health outcomes are sometimes stronger when considered in combination than individually.
Summary
Environmental and psychosocial stressors are often examined separately, even though their co-occurrence is widespread. The evidence that combined associations are often stronger raises critical issues around environmental justice and protection of vulnerable populations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cumulative stressors in our environment can have detrimental effects on our health, as evidenced by the emerging literature on the “exposome” [1]. The exposome considers the totality of an individual’s exposures across a lifetime beginning in utero and includes psychosocial factors, chemical exposures, and biological factors. Whereas traditional approaches may consider these exposures and their health impacts individually, the exposome concept highlights the need to consider how multiple factors may interact to contribute to life course health development.
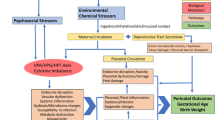
In the current review, we consider the joint impacts of in utero exposures to environmental contaminants and psychosocial stressors in relation to perinatal and child health outcomes. We focus on air pollution and metals as our environmental contaminants of interest because of the strong evidence demonstrating their impacts on children’s health as well as the rapidly expanding literature on co-exposure to psychosocial stressors. Guided by a conceptual framework (Fig. 1) adapted from Morello-Frosch et al. [2•], we consider structural (e.g., poverty) as well as individual-level (e.g., stressful life events) stressors. We added an emerging topic of disasters (e.g., wildfires) which can be natural experiments with combined environmental and psychosocial stressors. Importantly, structural stressors may contribute to individual-level stressors, and both may enhance individual susceptibility to the toxic effects of environmental contaminants [2•]. The “double jeopardy” of exposure to psychosocial stressors and environmental contaminants faced by pregnant women can be further transmitted to their fetuses, with potentially irreversible adverse impacts on health and development [2•]. In epidemiological studies, the combined impacts of stress and environmental exposures have been conceptualized and assessed in a number of ways (e.g., effect modification, statistical interaction, mediation, and clustering). For example, conceptually, associations between environmental exposures and adverse pregnancy outcomes may differ in women experiencing higher or lower psychosocial stress, suggesting effect modification. Alternatively, it is possible that psychosocial stress mediates the relationship between environmental exposures and adverse pregnancy outcomes, requiring alternative analytic approaches.
Conceptual model for how prenatal environmental and psychosocial exposures may impact children’s health (adapted from Morello-Frosch et al. [2•])
Our review of the combined health effects of environmental and psychosocial stressors on children’s health highlights a key environmental justice issue and presents an opportunity to improve health equity. Socioeconomically disadvantaged populations, who often face heightened exposures to structural and individual-level stressors, bear a disproportionate burden of environmental exposures including air pollution and metal contamination. A better understanding of how environmental and psychosocial stressors interact to affect children’s health is needed to inform policy change to alleviate environmental injustice and health inequities [3]. In this review, we focus on air pollution and metals, but additional studies on in utero synthetic chemical exposures and non-chemical stressors have been reviewed elsewhere [4].
Methods
We performed a structured literature review starting with a PubMed search of keywords (medical subject heading and title/abstract terms) to capture (1) any of the following psychosocial stressor terms (depressive disorder, depression, stress, stressor, anxiety, life events, mental health, psychosocial, psychological, prenatal stress, social class, socioeconomic status, poverty, material deprivation, neighborhood deprivation, neighborhood deprivation, residence characteristics, crime, social position, distress, discrimination), and (2) any of the following air pollution and metal exposure terms (metalloids, air pollution, traffic-related pollution, water pollution, particulate matter, nitrogen oxides, nitrogen dioxide, carbon monoxide, ozone, diesel, arsenic, mercury, cadmium, chromium, lead, heavy metals, traffic-related air pollution, polycyclic aromatic hydrocarbons, vehicle emissions, heavy metal) and (3) any of the following pregnancy/childhood health outcome terms (prenatal, perinatal, antenatal, pregnancy, gravidity, gravidity, pregnant, parturition, birth, gestation, foetus, fetus, fetal, infant, newborn, neonate, child). Sets 1, 2, and 3 were combined with the Boolean “AND,” and the terms within each set were combined with the Boolean “OR.”
The search was limited to English-language journal articles of human studies published in the last 5 years (January 1, 2015, to December 31, 2019). We examined the titles and abstracts of 1571 articles and identified a subset for full-text review. Only those articles that assessed either air pollution or metals in combination with non-chemical stressors during pregnancy and perinatal or child health outcomes were retained. We excluded studies on tobacco smoke and discussed any articles that were unclear as to their inclusion/exclusion (e.g., stress as an outcome rather than an exposure) to come to a consensus. In addition, we reviewed the references cited in the selected papers to identify additional articles of interest, including any highly relevant older papers. Our search resulted in 30 included studies (Table 1).
Results
Air Pollution
Air pollution originates from natural and anthropogenic sources and relative contributions from these sources vary geographically. Health studies have shown chronic and acute effects of air pollution on mortality, heart disease, and lung disease in children and adults [5]. Prenatal exposure to air pollution is associated with adverse birth outcomes, such as low birth weight, preterm birth, small for gestational age, and birth defects [6]. Emerging evidence also suggests ambient air pollution may affect neurological development in children [7].
The systematic monitoring of air pollution across the USA and many countries worldwide provides rich spatiotemporal data on ambient air pollutants (including nitrogen oxide [NO], nitrogen dioxide [NO2], carbon monoxide [CO], ozone [O3], particulate matter < 10 μm [PM10], and particulate matter < 2.5 μm [PM2.5]) that can then be used in epidemiological research. Based on geocoded maternal residence during pregnancy, estimates of exposure to air pollution during particular windows of pregnancy (e.g., trimester) can be generated, as can pregnancy-long average exposures. Alternative, less commonly used approaches focus on traffic sources such as traffic density, proximity to roadways, and diesel PM [8, 9•], as well as cooking fuel as a source of indoor air pollution [10].
Building on the large literature on exposure to air pollution during pregnancy and adverse birth and childhood outcomes, more recently, a number of studies have considered psychosocial stressors as potential modifiers of these relationships. Most commonly, such studies capitalize on geocoded maternal residence data to estimate measures of neighborhood socioeconomic status (including income, employment, education, and housing at census tract or block group levels [8, 10,11,12,13,14,15,16,17,18,19,20]) as a proxy for psychosocial stress. A smaller number of studies have prospectively assessed individual-level psychosocial stress as reported by women using tools such as stressful life event surveys [21, 22, 23•, 24].
In our review of this literature, we identified 24 papers that investigated the infant and child outcomes following combined maternal exposures to air pollution and psychosocial stress (Table 1). Although most of these studies were conducted in the USA, additional work has examined these relationships in Canada [14, 17, 18], Italy [20], France [13, 15], and Ghana [10]. Low birth weight [10,11,12, 14, 17, 19], preterm birth [8, 13, 16,17,18], and small for gestational age [17, 18] were the most commonly studied outcomes and additional outcomes included infant mortality [15], neural tube defects [9•], early childhood asthma [21, 24], and neurodevelopment [23•]. Most studies considered interactive effects (i.e., effect modification), though some studies examined spatial patterning of cluster effects [12], used ecological approaches [15], performed mediation analyses [10], or identified critical periods using Bayesian-distributed lag interaction models [21, 24].
Area-Level Stressors
Overall, the most common combination of factors in the studies reviewed reported on exposure to air pollution and area-level psychosocial stressors in relation to adverse birth outcomes. Nine of the 13 studies examining air pollution and area-level socioeconomic status (SES) found an increased risk of adverse birth outcomes with combined effects [8, 10, 12,13,14,15,16, 18, 19]. For example, a study of births in British Columbia, Canada (2001–2006), observed a significant interaction between neighborhood PM2.5 and socioeconomic status, with more pronounced negative impacts of PM2.5 on birth weight in lower socioeconomic status neighborhoods [14]. A similar work by Steib et al. (2016) in 2.5 million Canadian births (1999–2008) noted trends towards an association between PM2.5 and preterm birth, small for gestational age, and low birth weight in the two lower tertiles of income [17]. In that study, higher NO2 was also associated with modestly increased odds of small for gestational age and reductions in term birth weight in the lowest tertile of income [18]. In a recent study in Rome [20], risk of preterm birth was examined in relation to air pollution and heat (ambient temperature) with effect modification by socioeconomic status. They did not observe effect modification between air pollution and preterm birth by socioeconomic status, though an increased risk of preterm birth was observed with the combination of heat and low socioeconomic status [20].
Studies in California have revealed interactions between several criteria air pollutants and neighborhood socioeconomic status with regard to risk of preterm birth [8] and spina bifida [9•]. A study of births from 2000 to 2006 in the San Joaquin Valley of California, an area with high air pollution exposures and high income disparity, showed stronger associations (ORs ranged from 2.1 to 4.3) between several pollutants (CO, NO, NO2, PM10, PM2.5) and preterm birth in disadvantaged neighborhoods (based on unemployment rates, use of public assistance, and household income). Similarly, associations between PM10 and spina bifida were stronger in neighborhoods with low median household income and educational attainment (ORs ranged from 2.6 to 5.1). While the preponderance of studies on air pollution and neighborhood-level socioeconomic status has suggested that lower socioeconomic status populations are more vulnerable to the adverse impacts of air pollution, it is worth noting that a smaller number of studies have reported inconsistent results across various pollutants and study populations [9•, 11, 17, 20] or, unexpectedly, increased air pollution–related risks of adverse outcomes in higher socioeconomic status neighborhoods [19].
Individual-Level Stressors
Complementing the numerous large-scale studies of co-exposure to air pollution and neighborhood-level stressors is a smaller body of work from prospective pregnancy cohort studies examining individual-level stressors. Although there are many ways to assess individual-level stressors during pregnancy (including but not limited to maternal anxiety, depression, pregnancy-specific stress, and discrimination as well as biomarkers like cortisol), to date, this literature has focused on air pollution in relation to stressful life event scales, including the Crisis in Family Systems (CRISYS) survey which assesses life events occurring during pregnancy across 11 different domains (such as financial, legal, career, relationship, and safety) [25]. In the Boston-based Asthma Coalition on Community, Environment, and Social Stress (ACCESS) cohort, the interaction between air pollutants and stressful life events has been studied in relation to a range of child outcomes. For example, higher exposure to ambient nitrates (NO3) across pregnancy was associated with 2.6-fold increased odds of clinician-diagnosed asthma by age 6 per IQR increase in Ln(NO3), among boys whose mothers also reported higher negative life events during pregnancy [21]. Similar results were reported for the interaction between mid-gestational PM2.5 exposure and prenatal life event stress, with the odds of asthma particularly increased in boys [24]. Further corroboration comes from the Mexico City PROGRESS cohort, whereby associations between first-trimester PM2.5 exposure and child wheeze at age 4 were only present among children of mothers also reporting high prenatal stress [26]. There has been limited, but suggestive, work on other outcomes in this context. For example, although there was no main effect of black carbon exposure in pregnancy on memory and learning scores in children at age 6, among boys with higher exposure to prenatal life event stressors, higher black carbon exposure was associated with lower attention scores [23•].
Several studies by Perera et al. in New York City [27••, 28] and Krakow, Poland [29], have examined co-exposure to stressors (material hardship [28, 29] and psychological distress [27••]) and urinary polycyclic aromatic hydrocarbons (PAHs) in relation to child health outcomes. PAHs are by-products of incomplete combustion of organic substances and air pollution is a major source. In these studies, children with high prenatal PAH exposure and material hardship during pregnancy through childhood had a higher risk of attention-deficit/hyperactivity disorder [28] and lower IQ [29]. In the Polish study, the impacts of maternal report of demoralization on syndromes of anxious/depressed, withdrawn/depressed, rule-breaking, aggressive behavior, and the composite internalizing and externalizing scores were observed only in children with high prenatal PAH exposure [27••].
Disasters
In this era of rapid climate change and increasingly intense weather events, natural disasters such as wildfires, earthquakes, and hurricanes are becoming increasingly common and present a new way to consider joint exposures to air pollution and psychosocial stressors. These dramatic events are often characterized by significant declines in environmental quality (e.g., air, water) as well as intense, acute psychosocial stress for individuals living in and near the afflicted areas, resulting in an increased risk of adverse health outcomes. For example, several studies have looked at birth outcomes following exposure to wildfires in the Western United States. In a study of the 2003 Southern California wildfires, birth weight was 3.3–9.7 g lower among babies who gestated during the fires compared with that among babies who gestated before or after the fires, depending on the trimester of exposure [30]. More recent research in Colorado observed slightly increased odds of preterm birth following exposure to wildfire smoke during gestation [31]. “Unnatural” disasters such as 9/11 can present similarly acute environmental and psychosocial stress exposures, potentially impacting health outcomes. For example, among women in the World Trade Center health registry, performing rescue/recovery work during the bombings and having 9/11-related posttraumatic stress disorder were each associated with an increased likelihood of having a low birth weight or preterm baby, particularly in the 5 years following the disaster [32]. One major limitation of “disaster” research is a difficulty differentiating between the environmental pollutant exposure and the psychosocial stressors to tease apart their individual contributions to adverse outcomes. Such studies can also be limited by a lack of biomarkers of stress or pollutants or direct assessments of stress given the time-sensitive nature of the events.
Metals
Metals are natural components of the environment; nonetheless, industrial, agricultural, and technological changes can impact their presence in the environment relative to naturally occurring levels [33]. Most often, these anthropogenic changes increase the occurrence and variation of metals in food, drinking water, and air; thus, exposure to metals varies by population. By contrast to air pollution which is typically assessed via ambient monitoring, measurement of metal exposure is typically done through collection of biospecimens such as blood and urine. Exposure to metals during the perinatal period has been a particular concern and the subject of extensive research documenting associations with a variety of adverse health outcomes including stillbirth, low birth weight, reduced fetal growth, and altered neurodevelopment [34,35,36,37].
The most well-studied metal is lead which has multiple sources including lead-based paint in older homes, contaminated soil, household dust, drinking water, and lead-glazed pottery [38]. By contrast to other metals, lead is a criteria air pollutant and is now regulated in water, soil, and food, resulting in a sharp decline in children’s average blood lead levels over the past 50 years [39,40,41]. Nevertheless, chronic low-level lead exposure can have long-lasting adverse impacts, particularly on fetal and child neurodevelopment [42]. In pregnancy, lead is absorbed by the mother and readily transferred to the developing fetus [43, 44]. Prenatal exposure to lead has been associated with reduced birth weight and preterm birth, and with neurodevelopmental deficits [45].
Despite a large environmental justice literature on metal (particularly lead) contamination in disadvantaged neighborhoods, the extent to which prenatal exposures to metals and psychosocial stress interact to impact child health is less well understood. Our review identified three published studies on lead-stress interactions in the context of early childhood cognition and behavior. In the PROGRESS cohort, Tamayo y Ortiz et al. [46••] observed that joint maternal exposure to lead and prenatal negative life events had adverse additive impacts on cognition at age 24 months, as assessed by the Bayley Scales of Infant Development. In a similar analysis of toddler cognition based on a Chinese cohort, the negative impacts of maternal lead on adaptive behavior, language, and social behavior at ages 2–3 were only evident in mothers who also reported high levels of emotional stress in the third trimester [47]. Finally, additional work in the PROGRESS cohort, concurrent exposure to maternal depression and lead in pregnancy predicted a more difficult temperament phenotype at age 2, which translated to a poorer performance on developmental assessments [48]. Importantly, concerns about confounding by socioeconomic status (which is often used as a proxy for psychosocial stress) have been raised in the literature on lead and child outcomes for decades [49, 50] and more explicit consideration as to how the two sets of exposures interact is a necessary next step in this field.
In addition to lead, other common metals, including cadmium and mercury as well as the metalloid arsenic, may adversely affect birth and child outcomes [37]. The PROGRESS study examined synergistic influences of prenatal stress and mercury, a highly potent neurotoxin, on maternal pregnancy physiology. Among women reporting high stress during pregnancy, those who also had above median mercury levels had higher waking cortisol than those with lower mercury levels [51]. These results suggest that not only may mercury directly impact the developing fetal brain (as demonstrated by extensive previous work reviewed in [52]) but it may also do so by altering maternal stress physiology. Most recently, the Fetal Growth Study examined the relationship between metal exposures and psychosocial and demographic factors in relation to fetal growth using two approaches [53]. First, they created a composite index which included lead, mercury, and cadmium exposure; maternal stress and depression; and demographics domains. Second, they constructed weighted quantile sum regression models using those three domains. The study reported consistent associations between the composite index and small for gestational age, while only the sociodemographic domain was significantly associated with small for gestational age in the weighted quantile sum regression models. Novel methods like these for modeling cumulative exposures are important for improving our understanding of how multiple stressors impact pregnancy and childhood health outcomes.
Additional work in the PROGRESS cohort examined the transition metal manganese, which unlike lead and mercury has essential body functions. In that study, manganese and maternal depression in pregnancy were each independently associated with poorer neurodevelopment at age 24 months, and associations between manganese and altered development (particularly language scores) were strongest among mothers also reporting depressive symptoms [54]. Finally, one study examined metal-stress interactions in a unique cohort of women who were pregnant during the 2014 War on Gaza in Palestine and as a result were exposed to high levels of metals from artillery as well as tremendous psychosocial stress [55]. Concentrations of chromium and uranium in maternal hair at delivery predicted negative early emotional development in 6–7-month-old children; however, these associations were not modified by the mother’s own posttraumatic stress.
Discussion
Despite a strong conceptual framework and considerable interest in the joint impacts of prenatal exposures to environmental exposures and psychosocial stressors, the literature on this emerging topic remains small. The most well-developed sub-field by far focuses on the joint impacts of air pollution and socioeconomic status on birth outcomes, capitalizing on the fact that both exposures can be assessed through geocoded maternal residence data. Overall, our review indicates that the impacts of air pollution on birth outcomes may be stronger among women living in lower socioeconomic status neighborhoods, used as a proxy for psychosocial stress. Although some studies found stronger associations with combined effects, many studies also found no association or in the unexpected direction. Many of these studies were not designed (or powered) to detect such associations and were performed post hoc, so publication bias may be present. Though studies overall were inconsistent, findings among some of the studies warrant further investigation. Smaller longitudinal pregnancy cohort studies offer further support for these findings by also considering individual-level stressors. Such studies have typically observed stronger associations between air pollution and child outcomes (most notably asthma and wheeze) in children born to mothers who experienced greater individual-level stress during pregnancy compared with mothers reporting fewer stressors, with sex differences often reported. A much smaller literature on joint exposures to metals and stress similarly suggests that the two exposures may act synergistically to adversely impact health, particularly neurodevelopmental outcomes. While lead has predominated in this small literature, limited evidence suggests that additional metals including mercury and manganese should be considered further.
The take-away message of this emergent literature—that the adverse impacts of environmental exposures are often heightened in the face of psychosocial stress—is further evidence of the “double jeopardy” faced by mothers and children in vulnerable communities. Unfortunately, there are numerous examples of heightened exposures to chemical and non-chemical stressors in disadvantaged neighborhoods. For example, a number of poor urban communities around the USA (including most publicly Flint, MI; Newark, NJ; and Pittsburgh, PA) have gone through recent crises of lead contamination in public drinking water supplies. These communities are at an increased risk of lead contamination given aging infrastructure and housing stock as well as limited municipal resources to conduct ongoing testing and improve infrastructure as needed. It is clear that these communities bear the burden of tremendous structural stressors (related to poverty as well as discrimination, safety, food insecurity, and health care access) and determining the extent to which these translate into individual-level stressors (including concerns about exposures to environmental hazards) is an important next step.
Air pollution provides a second clear example of this phenomenon. A 2018 study reported that compared with the general US population, those living in poverty and African Americans had 35% and 54% higher burden of PM2.5, respectively [56]. Multiple factors may explain these disparities in exposure. For example, industrial sites, where chemicals are produced, used, emitted, and discarded, are more likely to be situated in low-income communities [57]. This may be due to the relatively low cost of land development leading to heightened industrial construction in low-income neighborhoods or alternatively may reflect migration of wealthier residents out of neighborhoods when factories or industrial plants are built nearby [58]. Indeed, home values often decrease when environmental hazards are discovered, resulting in further socioeconomic segregation and heightened exposures among poorer residents. One exception to this is that some large metropolitan areas (for instance, New York, NY; or San Francisco, CA) may face poorer air quality due to population density and also extremely high housing prices favoring high-income residents [59].
Research Gaps and Future Directions
In addition to an overall need for more research that considers chemical and non-chemical exposures in tandem, several high priority research areas are noted. First, although numerous mechanistic studies have examined these exposures individually, better understanding the mechanisms by which in utero environmental and psychosocial exposures may jointly impact children’s development is vital. Several important biological pathways that are impacted by both air pollution and psychosocial stress have garnered attention (including oxidative stress, endocrine disruption, and inflammation, as reviewed in [34]), but there are likely more worth considering. For instance, Brunst et al. [22] observed that PM2.5 and maternal lifetime trauma each individually reduced mitochondrial DNA copy numbers in cord blood and placenta, suggesting mitochondrial dysfunction as a potential mechanism of interest [22]. A similar study examined the relationship between PM, mitochondrial DNA copy number, and telomere length, an additional biomarker of stress, in mothers in relation to fetal growth [60]. PM10 exposure during the first pregnancy trimester was associated with increased maternal mitochondrial DNA copy numbers, reduced telomere length, and reduced birth weight, but interactive effects of PM10 and telomere length on birth weight were not assessed [60].
Better knowledge of the relationships between chemical and non-chemical exposures is needed to inform modeling strategies. For example, evidence suggests that in some contexts, air pollution may be on the causal pathway between socioeconomic status and birth outcomes [10]. At the same time, environmental exposures may impact maternal mental health. For instance, a recent study reported that low-level maternal lead exposure was associated with increased anxiety and depression in pregnancy, raising the possibility that maternal psychosocial status may also be a mediator of the impact of lead exposure on children’s development [61]. Similarly, another study found that air pollution exposure during pregnancy was associated with emotional stress [62]. Adding to the complexity, just as approaches to assess mixtures of chemical exposures (such as weighted quantile sum regression and Bayesian kernel machine regression) are increasingly utilized by environmental epidemiologists, so should novel methods for assessing “mixtures” of chemical and non-chemical stressors be developed [53, 63]. Additional statistical tools such as hierarchical cluster analysis, factor analysis, and principal components analysis may prove to be valuable tools in this context given the large number of variables considered [63].
With the exception of air pollution studies that can use address-based data to assess exposure in large populations, to date, studies of co-exposure to environmental contaminants and psychosocial stressors have been limited by relatively small numbers of participants, often recruited from a single geographic area. With the inception of the National Institutes of Health’s Environmental Influences on Child Health Outcomes (ECHO) program which creates a harmonized “cohort of cohorts” of roughly 50,000 mother-child dyads, comes opportunities to study joint exposures in ways that have not been previously possible in the USA [64]. This large and diverse national-level cohort, in which extensive environmental and psychosocial data are collected, is uniquely situated to address issues of co-exposure, interactions, and confounding in relation to priority child health issues including pre-, peri-, and postnatal outcomes, obesity, neurodevelopment, airway health, and positive health [65].
Ultimately, improved understanding of interactions between environmental and psychosocial stressors can help to inform policy- and practice-oriented solutions to improve children’s health. One approach suggested by this line of research is heightened monitoring and enforcement of environmental health standards in disadvantaged communities. Given finite resources, efforts to improve environmental quality (e.g., reducing air pollution, stringent water quality testing) may be most impactful in disadvantaged communities who may bear a higher burden of psychosocial stress as well. Recognizing that improving environmental quality in disadvantaged communities is challenging, interventions to reduce psychosocial stress in the most vulnerable populations represent another strategy, for instance through improved mental health screening and treatment options in pregnancy and the postpartum. Ultimately, a multi-pronged approach to improving environmental quality, combating structural stressors, and reducing individual stressors is likely to make the greatest impact on perinatal and child outcomes. Given the increasing disparities, aging infrastructure, and climate-related issues now facing the USA, disadvantaged populations are likely to face even greater (and more disproportionate) environmental challenges—and by extension, adverse child health outcomes—if trends continue.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomark Prev. 2005;14:1847–50.
• Morello-Frosch R, Shenassa ED. The environmental “riskscape” and social inequality: implications for explaining maternal and child health disparities. Environ Health Perspect. 2006;114:1150–3. Introduces a framework for thinking about the “double jeopardy” of co-exposure to environmental hazard and place-based stressors.
Perera F. Pollution from fossil-fuel combustion is the leading environmental threat to global pediatric health and equity: solutions exist. Int J Environ Res Public Health. 2017;15.
Barrett ES, Padula AM. Joint impact of synthetic chemical and non-chemical stressors on children’s health. Curr Environ Health Rep 2019.
Schraufnagel DE, Balmes JR, Cowl CT, et al. Air pollution and noncommunicable diseases: a review by the Forum of International Respiratory Societies’ Environmental Committee, part 1: the damaging effects of air pollution. Chest. 2019;155:409–16.
Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res. 2012;117:100–11.
Vrijheid M, Casas M, Gascon M, Valvi D, Nieuwenhuijsen M. Environmental pollutants and child health-a review of recent concerns. Int J Hyg Environ Health. 2016;219:331–42.
Padula AM, Mortimer KM, Tager IB, et al. Traffic-related air pollution and risk of preterm birth in the San Joaquin Valley of California. Ann Epidemiol. 2014;24:888–895e884.
• Padula AM, Yang W, Carmichael SL, Tager IB, Lurmann F, Hammond SK, et al. Air pollution, neighbourhood socioeconomic factors, and neural tube defects in the San Joaquin Valley of California. Paediatr Perinat Epidemiol. 2015;29:536–45. An example of stronger associations between air pollution and neural tube defects in low SES areas.
Amegah AK, Damptey OK, Sarpong GA, et al. Malaria infection, poor nutrition and indoor air pollution mediate socioeconomic differences in adverse pregnancy outcomes in Cape Coast. Ghana PLoS One. 2013;8:e69181.
Shmool JL, Bobb JF, Ito K, Elston B, Savitz DA, Ross Z, et al. Area-level socioeconomic deprivation, nitrogen dioxide exposure, and term birth weight in New York City. Environ Res. 2015;142:624–32.
Coker E, Liverani S, Ghosh JK, Jerrett M, Beckerman B, Li A, et al. Multi-pollutant exposure profiles associated with term low birth weight in Los Angeles County. Environ Int. 2016;91:1–13.
Deguen S, Ahlers N, Gilles M, et al. Using a clustering approach to investigate socio-environmental inequality in preterm birth-a study conducted at fine spatial scale in Paris (France). Int J Environ Res Public Health. 2018;15:1895.
Erickson AC, Ostry A, Chan HM, Arbour L. Air pollution, neighbourhood and maternal-level factors modify the effect of smoking on birth weight: a multilevel analysis in British Columbia. Canada BMC Public Health. 2016;16:585.
Padilla CM, Kihal-Talantikit W, Vieira VM, Deguen S. City-specific spatiotemporal infant and neonatal mortality clusters: links with socioeconomic and air pollution spatial patterns in France. Int J Environ Res Public Health. 2016;13.
Padula AM, Huang H, Baer RJ, et al. Environmental pollution and social factors as contributors to preterm birth in Fresno County. Environ Health. 2018;17:70.
Stieb DM, Chen L, Beckerman BS, Jerrett M, Crouse DL, Omariba DW, et al. Associations of Pregnancy Outcomes and PM2.5 in a National Canadian Study. Environ Health Perspect. 2016;124:243–9.
Stieb DM, Chen L, Hystad P, et al. A national study of the association between traffic-related air pollution and adverse pregnancy outcomes in Canada, 1999-2008. Environ Res. 2016;148:513–26.
Tu J, Tu W, Tedders SH. Spatial variations in the associations of term birth weight with ambient air pollution in Georgia, USA. Environ Int. 2016;92-93:146–56.
Asta F, Michelozzi P, Cesaroni G, et al. The modifying role of socioeconomic position and greenness on the short-term effect of heat and air pollution on preterm births in Rome, 2001-2013. Int J Environ Res Public Health. 2019;16:2497.
Bose S, Chiu YM, Hsu HL, di Q, Rosa MJ, Lee A, et al. Prenatal nitrate exposure and childhood asthma. Influence of maternal prenatal stress and fetal sex. Am J Respir Crit Care Med. 2017;196:1396–403.
Brunst KJ, Sanchez-Guerra M, Chiu YM, Wilson A, Coull BA, Kloog I, et al. Prenatal particulate matter exposure and mitochondrial dysfunction at the maternal-fetal interface: effect modification by maternal lifetime trauma and child sex. Environ Int. 2018;112:49–58.
• Cowell WJ, Bellinger DC, Coull BA, et al. Associations between prenatal exposure to black carbon and memory domains in urban children: modification by sex and prenatal stress. PLoS One. 2015;10:e0142492. A review of the animal and human literature on combined exposure to air pollution and non-chemical stressors a focus on neurodevelopmental outcomes.
Lee A, Leon Hsu HH, Mathilda Chiu YH, Bose S, Rosa MJ, Kloog I, et al. Prenatal fine particulate exposure and early childhood asthma: effect of maternal stress and fetal sex. J Allergy Clin Immunol. 2018;141:1880–6.
Shalowitz MU, Berry CA, Rasinski KA, Dannhausen-Brun CA. A new measure of contemporary life stress: development, validation, and reliability of the CRISYS. Health Serv Res. 1998;33:1381–402.
Rosa MJ, Just AC, Kloog I, et al. Prenatal particulate matter exposure and wheeze in Mexican children: effect modification by prenatal psychosocial stress. Ann Allergy Asthma Immunol. 2017;119:232–7 e231.
•• Perera FP, Wang S, Rauh V, Zhou H, Stigter L, Camann D, et al. Prenatal exposure to air pollution, maternal psychological distress, and child behavior. Pediatrics. 2013;132:e1284–94. An early and impactful study combining PAH exposure and materal stress to examine joint effects on child behavior in Poland.
Perera FP, Wheelock K, Wang Y, Tang D, Margolis AE, Badia G, et al. Combined effects of prenatal exposure to polycyclic aromatic hydrocarbons and material hardship on child ADHD behavior problems. Environ Res. 2018;160:506–13.
Vishnevetsky J, Tang D, Chang HW, et al. Combined effects of prenatal polycyclic aromatic hydrocarbons and material hardship on child IQ. Neurotoxicol Teratol. 2015;49:74–80.
Holstius DM, Reid CE, Jesdale BM, Morello-Frosch R. Birth weight following pregnancy during the 2003 Southern California wildfires. Environ Health Perspect. 2012;120:1340–5.
Abdo M, Ward I, O’Dell K, et al. Impact of wildfire smoke on adverse pregnancy outcomes in Colorado, 2007-2015. Int J Environ Res Public Health. 2019;16.
Maslow CB, Caramanica K, Li J, Stellman SD, Brackbill RM. Reproductive outcomes following maternal exposure to the events of September 11, 2001, at the World Trade Center, in New York City. Am J Public Health. 2016;106:1796–803.
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Exp Suppl. 2012;101:133–64.
Ferguson KK, Chin HB. Environmental chemicals and preterm birth: biological mechanisms and the state of the science. Curr Epidemiol Rep. 2017;4:56–71.
Kim Y, Ha EH, Park H, Ha M, Kim Y, Hong YC, et al. Prenatal lead and cadmium co-exposure and infant neurodevelopment at 6 months of age: the Mothers and Children’s Environmental Health (MOCEH) study. Neurotoxicology. 2013;35:15–22.
Llanos MN, Ronco AM. Fetal growth restriction is related to placental levels of cadmium, lead and arsenic but not with antioxidant activities. Reprod Toxicol. 2009;27:88–92.
Rahman A, Kumarathasan P, Gomes J. Infant and mother related outcomes from exposure to metals with endocrine disrupting properties during pregnancy. Sci Total Environ. 2016;569-570:1022–31.
Abadin H, Ashizawa A, Stevens YW et al. In Toxicological profile for lead. Atlanta (GA): 2007.
McClure LF, Niles JK, Kaufman HW. Blood lead levels in young children: US, 2009-2015. J Pediatr. 2016;175:173–81.
Muntner P, Menke A, DeSalvo KB, Rabito FA, Batuman V. Continued decline in blood lead levels among adults in the United States: the National Health and Nutrition Examination Surveys. Arch Intern Med. 2005;165:2155–61.
Pirkle JL, Brody DJ, Gunter EW, Kramer RA, Paschal DC, Flegal KM, et al. The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES). JAMA. 1994;272:284–91.
Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–9.
Allen KA. Is prenatal lead exposure a concern in infancy? What is the evidence? Adv Neonatal Care. 2015;15:416–20.
Neal AP, Guilarte TR. Mechanisms of lead and manganese neurotoxicity. Toxicol Res (Camb). 2013;2:99–114.
Perng W, Tamayo-Ortiz M, Tang L, et al. Early Life Exposure in Mexico to ENvironmental Toxicants (ELEMENT) Project. BMJ Open. 2019;9:e030427.
•• Tamayo-Ortiz M, Tellez-Rojo MM, Wright RJ, et al. Longitudinal associations of age and prenatal lead exposure on cortisol secretion of 12-24 month-old infants from Mexico City. Environ Health. 2016;15:41. One of several articles on the PROGRESS cohort has examined lead and other metals with prenatal stress and depression and their combined effects on child health outcomes in Mexico City.
Zhou L, Xu J, Zhang J, et al. Prenatal maternal stress in relation to the effects of prenatal lead exposure on toddler cognitive development. Neurotoxicology. 2017;59:71–8.
Stroustrup A, Hsu HH, Svensson K, et al. Toddler temperament and prenatal exposure to lead and maternal depression. Environ Health. 2016;15:71.
Bellinger DC. Lead neurotoxicity and socioeconomic status: conceptual and analytical issues. Neurotoxicology. 2008;29:828–32.
Cassidy-Bushrow AE, Sitarik AR, Havstad S, Park SK, Bielak LF, Austin C, et al. Burden of higher lead exposure in African-Americans starts in utero and persists into childhood. Environ Int. 2017;108:221–7.
Schreier HM, Hsu HH, Amarasiriwardena C, et al. Mercury and psychosocial stress exposure interact to predict maternal diurnal cortisol during pregnancy. Environ Health. 2015;14:28.
Grandjean P, Herz KT. Methylmercury and brain development: imprecision and underestimation of developmental neurotoxicity in humans. Mt Sinai J Med. 2011;78:107–18.
Zilversmit Pao L, Harville EW, Wickliffe JK, et al. The cumulative risk of chemical and nonchemical exposures on birth outcomes in healthy women: the fetal growth study. Int J Environ Res Public Health. 2019;16.
Munoz-Rocha TV, Tamayo YOM, Romero M, et al. Prenatal co-exposure to manganese and depression and 24-months neurodevelopment. Neurotoxicology. 2018;64:134–41.
Vanska M, Diab SY, Perko K, et al. Toxic environment of war: maternal prenatal heavy metal load predicts infant emotional development. Infant Behav Dev. 2019;55:1–9.
Mikati I, Benson AF, Luben TJ, et al. Disparities in distribution of particulate matter emission sources by race and poverty status. Am J Public Health. 2018;108:480–5.
Solomon GM, Morello-Frosch R, Zeise L, Faust JB. Cumulative environmental impacts: science and policy to protect communities. Annu Rev Public Health. 2016;37:83–96.
Macey GP, Her X, Reibling ET, Ericson J. An investigation of environmental racism claims: testing environmental management approaches with a geographic information system. Environ Manag. 2001;27:893–907.
Benfer EA. Health justice: a framework (and call to action) for the elimination of health inequity and social injustice. Am Univ Law Rev. 2015;65:275–351.
Iodice S, Hoxha M, Ferrari L, et al. Particulate air pollution, blood mitochondrial DNA copy number, and telomere length in mothers in the first trimester of pregnancy: effects on fetal growth. Oxidative Med Cell Longev. 2018;2018:5162905.
Li S, Xu J, Liu Z, Yan CH. The non-linear association between low-level lead exposure and maternal stress among pregnant women. Neurotoxicology. 2017;59:191–6.
Lin Y, Zhou L, Xu J, et al. The impacts of air pollution on maternal stress during pregnancy. Sci Rep. 2017;7:40956.
Huang H, Wang A, Morello-Frosch R, et al. Cumulative risk and impact modeling on environmental chemical and social stressors. Curr Environ Health Rep. 2018;5:88–99.
Padula AM, Monk C, Brennan PA, Borders A, Barrett ES, McEvoy C, et al. A review of maternal prenatal exposures to environmental chemicals and psychosocial stressors-implications for research on perinatal outcomes in the ECHO program. J Perinatol. 2020;40:10–24.
Gillman MW, Blaisdell CJ. Environmental influences on Child Health Outcomes, a research program of the National Institutes of Health. Curr Opin Pediatr. 2018;30:260–2.
Funding
NIH P30ES005022, R00ES021470
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Early Life Environmental Health
Rights and permissions
About this article
Cite this article
Padula, A.M., Rivera-Núñez, Z. & Barrett, E.S. Combined Impacts of Prenatal Environmental Exposures and Psychosocial Stress on Offspring Health: Air Pollution and Metals. Curr Envir Health Rpt 7, 89–100 (2020). https://doi.org/10.1007/s40572-020-00273-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40572-020-00273-6