Abstract
Objective
The aim of the study was to evaluate the incidence of peripheral inserted central catheter (PICC) tip malposition when the catheter is inserted under real-time ultrasound (RTUS) guidance when compared with conventional landmark (CL) technique in neonates. Additional objectives were to evaluate the PICC longevity and central line associated blood stream infections (CLABSI).
Study design
In this randomised controlled trial, neonates were randomised to ‘RTUS’ (n = 40) or ‘CL’ (n = 40) groups. PICC tip was placed under ultrasound guidance in lower third of superior vena cava in the RTUS group. In ‘CL’ group, PICC was inserted as calculated by anatomical landmarks.
Results
The birth weight (1286 (926, 1662) vs. 1061 (889, 1636) g) and gestation (31.12 (3.1) vs. 31.4 (3.6) wks) were comparable among the groups. RTUS guidance during PICC insertion reduced incidence of tip malposition by 52% (67.5 vs. 32.5%; RR: 0.48; 95% CI: 0.29–0.79). The longevity of PICC and episodes of CLABSI were however similar in the two groups.
Conclusions
Real-time ultrasound guidance during PICC placement reduces the incidence of tip malposition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Peripherally inserted central catheters (PICC) have gained popularity in the neonatal intensive care unit (NICU), as a dependable long-term venous access. Particularly, in sick very low birth weight and surgical infants. However, the safe length of their insertion into the central vein varies and cannot be reliably predicted from external landmarks only [1]. Standard radiography when used alone can miss tip dislodgment with movement of extremities, thus causing catheter migration and other complications [2]. In addition, once placed in a non-optimal position, the clinician requires more radiographs after PICC manipulation to confirm position. This not only involves the disruption of nursing care but also unnecessary radiation exposure.
Ultrasound has been shown to decrease the incidence of malposition when used at the time of initial placement of the catheter in adults. Its usage in inserting and locating the umbilical venous and arterial catheters is also well documented [3,4,5,6]. Previous studies have suggested a potential role of ultrasound when compared with plain radiographs for localizing PICC position [3, 4]. In addition, there is a utility of real-time ultrasound in evaluating the tip position after the change in infants’ posture and arm position; a potential advantage over the standard static radiograph [4]. However, these studies had a small sample size [3,4,5] and lacked a good study design, thus making it difficult for the clinician to identify the best tool for confirmation of optimal PICC position.
A recent randomised controlled trial comparing real-time ultrasound (RTUS) with standard radiograph for confirming accurate tip position during line placement suggested that RTUS decreased the duration of time taken for catheter insertion and was associated with fewer manipulations and radiographs when compared with conventional placement. However, this study did not discuss optimal location of the catheter tip. Further, their upper and the lower limb PICCs were unequally distributed between the groups [6].
With this background, a well-designed randomized controlled study was planned with an objective of comparing the incidence of PICC tip malposition of a catheter inserted in upper limb veins under real-time ultrasound guidance with catheter insertion by conventional landmark technique in neonates requiring the catheter for their ongoing clinical care. Additional objectives were to evaluate the longevity of PICC and incidence of central line associated blood stream infections (CLABSI) in both groups.
Methods
Subjects and settings
This superiority open labelled randomized control trial was conducted in the tertiary care neonatal unit, Division of Neonatology, Department of Pediatrics, All India Institute of Medical Sciences, New Delhi from February 2012 to January 2013 (single center study). All infants admitted in NICU during the study period and requiring PICC insertion in upper limb either using basilic or cephalic veins for their clinical management were considered eligible for the study. Infants with neck and chest malformations, bleeding at the time of insertion and unhealthy insertion site were excluded from the study.
The indication for inserting PICC was determined by the clinical team according to unit protocol and the researchers had no role in that decision. The study was approved by the ethics committee of the institute. Informed written parental consent was obtained.
Relevant demographic variables were collected, including the indication for PICC insertion, age at insertion and removal of PICC, calculated PICC length to be inserted (only for ‘CL’ group), the actual length inserted, problems during the procedure (bleeding, difficulty in identifying vein, attempts used), laterality of the arm and the vein chosen for insertion. In addition, the baseline severity scores of all neonates were noted. We used Score for Neonatal Acute Physiology – Perinatal Extension II for assessing the illness severity in these neonates. The variables included in the score were mean arterial blood pressure, lowest temperature, ratio of partial pressure of arterial oxygenation and fractional concentration of oxygen (PaO2/FiO2), urine output, lowest serum pH, occurrence of multiple seizures (yes/ no), Apgar score, birth weight and being small for gestational age (<3rd centile); each parameter being objectively scored according to the respective value (higher score indicating higher severity) [7].
Randomization and Intervention
Mothers of all neonates were given the information sheet when a decision of eligibility was made and then a written consent was obtained from them. All eligible infants were randomized by computer-generated randomization sequence (block size from 4 to 8 with 1:1 allocation ratio) to real-time ultrasound (RTUS) group or the CL group. The random numbers were placed in serially numbered, sealed, opaque envelopes and were opened after obtaining informed consent by the registrar on duty to ensure allocation concealment. Blinding of the primary care givers (nursing professionals) posted in the neonatal unit was not possible due to nature of intervention. However, the consultants interpreting x-rays were blinded to group allocation. All neonates enrolled in the study were monitored in neonatal intensive care unit to ensure compliance to the intervention.
Procedure of PICC line insertion
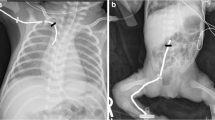
We used 28G (1Fr) size catheter (Polyurethane; Vygon-premiecath®; markings up to 20 cm). The infants enrolled in RTUS group had their catheter inserted from the desired point till it was seen at the defined position on ultrasound (sector probe frequency ranged from 4 Hz to 12 Hz; Aloka model prosound SSD 4000SV, Philips model CX 50 after June 2012) during real-time. The ultrasound was done by getting the images in apical, high parasternal view in parasagittal plane and subcostal long axis views and the catheter tip was positioned in the lower one third of SVC. If investigator was not able to localize the PICC tip on ultrasound in spite of best efforts, then the procedure was considered as ‘failed’ and the PICC was inserted according to conventional landmark technique later. All the ultrasound images were acquired by a single investigator who was trained and his study findings validated by a radiologist before study commencement.
As convention, we preferred inserting PICC in right arm and basilic vein to avoid malposition and decrease the procedure failure rates [8, 9]. If the attempt was unsuccessful on the right side, an attempt was made on the left arm and this was noted separately.
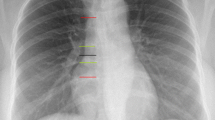
In the “conventional” group, the length of PICC to be inserted was measured as the distance between the point of insertion at the antecubital fossa to the ipsilateral acromion process and then to the ipsilateral manubrium sterni. The catheter was threaded as per the measured length before starting of the procedure. The position was confirmed with a standard radiograph.
Infants were positioned by keeping the upper limb in around 45° shoulder abduction. Radio contrast dye (Iohexol, 0.2 mL) was injected and digital x- ray was taken immediately after injection. Initial assessment of tip position was done by clinical team in NICU and the X-ray was kept for formal reporting for the purpose of the study. If the PICC tip was not clearly visible due to bad quality of X-ray, then the concerned clinical team took a decision for second X-ray. Intravenous fluids were started only after confirmation of the PICC line position.
All other steps including aseptic precautions were done as per unit protocol in both the groups.
Outcome variables and their measurement
PICC tip was considered to be properly positioned if it met all of the following three parameters. First, it crossed the first costochondral junction of the same side, second after crossing first costochondral junction, it should have progressed medially and downwards and should have stayed above the heart base. The point where right atrial hump straightens towards SVC on contrast radiography was considered as heart base. In all other positions the tip was considered to be malpositioned.
The longevity of PICC (expressed in hours) was defined as the time interval from the time of its insertion to its removal. CLABSI was defined by any primary bloodstream infection (BSI) in a neonate with a central catheter in situ (within the 48 h before BSI development) that was not related to an infection at any other site [10].
The radiograph was independently assessed by two experts (one from Neonatology and another from Radiology) who were not aware of the allocated intervention group. A standard operating procedure was developed for interpreting radiograph after consultation with these two experts. The final position of PICC was based on radiograph exposure, rotation and angling, visibility of the tip and the position of the heart base. The estimate of final tip position in all cases, irrespective of group allocation, was made on the basis of x-rays. The final comment on PICC tip position was given in relation to the superior vena cava (SVC) in both groups. In the cases, where heart base identification was difficult, two consultants took a consensus decision regarding the tip position. In addition, wherever there was disagreement in the final PICC tip location a consensus on final outcome was achieved by mutual discussion.
All infants were followed during their stay for catheter-related blood stream infections (CLABSI). The indication for removal and the longevity of PICC line were noted by the clinical team whenever the catheter was removed.
Sample size and statistical analysis
The data were entered in Excel datasheet. The data weres coded and analysed statistically using software version 11.1 (Stata Corp, College station, Texas, US). Analysis was performed by intention to treat, that is, all babies were analysed in their allocated groups, regardless of the treatment received. The continuous data with normal distribution were analysed by Student t test and non-normally distributed data by Wilcoxon rank sum test (Mann–Whitney). Categorical data were analysed using Χ2 test or Fisher’s exact test. A p value of < 0.05 was taken as significant.
We also calculated the relative risk (RR), absolute risk reduction (ARR) and number needed to treat (NNT) with 95% confidence intervals and mean difference (95% CI) wherever applicable.
In a previous study done by Fricke et al [8], it was found that the rate of catheter tip malposition was 85.8% after initial placement. We made a conservative estimate of incidence of malposition as 50% in our CL arm. To show a relative reduction of 50% (absolute reduction of 25%) in ‘RTUS’ group when compared with the conventional technique, we needed 66 infants in each arm (with 80% power and 5% alpha error)
Results
A total of 2582 newborns were born during the study period of which 80 were eligible for inclusion (Fig. 1). These 80 neonates were allocated to RTUS (n = 40) and CL (n = 40) through computer-generated randomization.
Cannulation was not possible in one neonate (RTUS group) due to non-availability of good vein for insertion in upper limbs. Procedure could not be completed fully in a total of eight infants, 2 infants in ‘ultrasound’ group and 6 infants in ‘CL’ group, because of the obstruction encountered in the path of PICC. These procedures were considered as “failures”.
The birth weight (g, median (IQR), 1286 (926, 1662) vs. 1061 (889, 1636)) and gestation (wk, mean (SD), 31.12 (3.1) vs. 31.4 (3.6)) were comparable in the in the RTUS and CL groups, respectively. The SNAP-PE II score was, however, higher in the CL group (22.5 (13, 34) vs. 12 (0, 26.5)) respectively as shown in Table 1.
Outcomes
The incidence of malposition of PICC tip was less in RTUS group compared to CL group (32.5% vs. 67.5%; p = 0.002) (Table 2). Real-time ultrasound guidance during PICC insertion reduced incidence of malposition by 52% (RR: 0.48; 95% CI: 0.29 to 0.79, ARR s 0.35 (95% CI: 0.14–0.55) and NNT 3 (95% CI: 2–7). The results were similar with per protocol analysis. The results remained significant even after adjusting for SNAP-PE II scores on logistic regression analysis.
The overall incidence of malposition of PICC tip was 50% (n = 40). Of these 18 (11 in RTUS group and 7 in CL group) were inside right atrium, 13 (9 in RTUS group and 4 in CL group) did not enter the SVC, and there were nine procedure failures which were also considered as malposition’s (three in RTUS group and six in CL group, respectively).
The longevity of PICC was however similar in the two groups (hrs, mean (SD), 154 (14.9) vs. 133 (9.4), p = 0.23) (Table 2). Catheters were removed prematurely (non-electively) in 10 neonates due to infiltration in the arm or due to blockage (four in RTUS and six in CL group). The longevity of PICC was similar in both elective [166 (137, 212) vs. 153 (126, 191) hours; p = 0.41] and non- elective removals [72 (63, 95) vs. 69 (66, 90) hours; p = 0.89] among the groups.
The episodes of CLABSI were also similar in the two groups [4 (10.2%) vs.1 (2.5%); p = 0.24]. Overall, the culture report suggested predominance of gram positive infections (Coagulase negative Staphylococcus aureus (CONS) (three in RTUS group and one in CL group). One infant in ‘CL’ group had gram negative sepsis (Escherichia Coli).
Discussion
PICCs have been used in the care of the critically ill neonates for over 30 years for parenteral nutrition, and administration of fluids and medications. Real-time ultrasound is a noninvasive technique providing real-time images to localize intravascular catheters while minimally disturbing the neonate. Ultrasound use in neonates for this purpose is not novel, however the evaluation as a tool, to date, has been limited.
This study evaluated two different techniques of PICC insertion and suggested that real-time ultrasound during PICC insertion decreases the incidence of tip malposition. The longevity of PICC and the episodes of CLABSI were, however, similar in between the two groups.
The usage of real-time ultrasound when compared with conventional landmark technique (using contrast radiography) decreased the incidence of tip malposition. Our study results are comparable with previous studies done in neonates [3, 4, 6, 11]. However, the studies had small sample size [3,4,5] and lacked a good study design [3,4,5]; thus limiting their generalizability. The two initial studies [3, 4] were done as feasibility studies, while the latest study [5] documented a poor concordance between real-time ultrasound and plain radiographs and demonstrated the superiority of real-time ultrasound in localizing the catheter tip in contrast to a single static image of a radiograph. A recent randomized controlled trial evaluated these two techniques and inferred that the numbers of manipulations were lesser in the ultrasound group and its utility enabled early identification of catheter tip [6]. However, optimal catheter location was defined in relation to superior vena cava or inferior vena cava only, the position of which is likely to change during various phases of respiration. The authors in this study used plain radiographs and not contrast radiography (a more specific method) for PICC tip confirmation [12,13,14] and in addition PICCs were inserted in both upper and the lower limbs; thus increasing the error in tip position judgment. We used contrast radiography for confirming the catheter tip position and used a more objective definition for localizing tip position.
The duration of PICC stay was similar in the two groups but overall was less than previously reported in literature [15, 16]. The lower duration of PICC line use can be explained by our unit’s aggressive enteral nutrition policy. The incidence of CLABSI was also similar in the two groups and overall incidence is comparable to that described in literature [16, 17]. Though we did not individually analyze the predisposing factors incidence of infections in the two groups; the higher incidence of infection with CONS infection is comparable with other studies [18, 19]. This relates to the adhesive characteristic properties of this bacterium.
In our experience, the time taken for PICC insertion was more in the RTUS group. Whether this increase in procedure time will predisposes them for infection risk remains to be evaluated.
The strength of our study is its robust design and a prospective follow up of all enrolled infants. The method of primary outcome measurement, incidence of malposition, was clearly laid down before the initiation of the study to avoid potential bias. Two consultants independently reviewed X-rays. Allocation concealment was ensured though blinding was not possible due to the nature of the intervention.
There are, however, a few limitations too. We did not specifically note the duration of the two procedures in this study. Since all the RTUS were done by a single investigator, it is not possible to evaluate the operator dependence of real-time ultrasound from the present study. Additionally, the trial was stopped early because of time constraints. The formal introduction of this technology requires evaluation of cost-benefit analysis of the technique, the requirement of trained staff to perform the procedure, and ongoing quality assurance to maintain these skills.
Conclusions
Ultrasound guidance during the initial placement of PICC reduced the incidence of malposition by 52%. Real-time ultrasound guidance may hence be recommended routinely for PICC catheter insertion.
References
McGee WT, Ackerman BL, Rouben LR, Prasad VM, Bandi V, Mallory DL. Accurate placement of central venous catheters: a prospective, randomized, multicenter trial. Crit Care Med. 1993;21:1118–23.
Racadio JM, Doellman DA, Johnson ND, Bean JA, Jacobs BR. Pediatric peripherally inserted central catheters: complication rates related to catheter tip location. Pediatrics. 2001;107:E28.
Ohki Y, Nako Y, Morikawa A, Maruyama K, Koizumi T. Percutaneous central venous catheterization via the great saphenous vein in neonates. Acta Paediatr Jpn. 1997;39:312–6.
Madar RJ, Deshpande SA. Reappraisal of ultrasound imaging of neonatal intravascular catheters. Arch Dis Child Fetal Neonatal Ed. 1996;75:F62–4.
Jain A, McNamara PJ, Ng E, El-Khuffash A. The use of targeted neonatal echocardiography to confirm placement of peripherally inserted central catheters in neonates. Am J Perinatol. 2012;29:101–6.
Katheria AC, Fleming SE, Kim JH. A randomized controlled trial of ultrasound-guided peripherally inserted central catheters compared with standard radiograph in neonates. J Perinatol. 2013;33:791–4.
Richardson DK, Corcoran JD, Escobar GJ, Lee SKSNAP-II. and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138:92–100.
Fricke BL, Racadio JM, Duckworth T, Donnelly LF, Tamer RM, Johnson ND. Placement of peripherally inserted central catheters without fluoroscopy in children: initial catheter tip position. Radiology. 2005;234:887–92.
Camara D. Minimizing risks associated with peripherally inserted central catheters in the NICU. MCN Am J Matern Child Nurs. 2001;26:17–21.
Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2011;36:309–33.
Mavilde LGP. Placement of peripherally inserted central venous catheters (PICC) in children guided by ultrasound. http://clinicaltrials.gov/show/NCT01279642. Accessed April 2016.
Odd DE, Page B, Battin MR, Harding JE. Does radio-opaque contrast improve radiographic localisation of percutaneous central venous lines? Arch Dis Child Fetal Neonatal Ed. 2004;89:F41–3.
Reece A, Ubhi T, Craig AR, Newell SJ. Positioning long lines: contrast versus plain radiography. Arch Dis Child Fetal Neonatal Ed. 2001;84:F129–30.
Webster NJ, Page B, Kuschel CA, Battin MR. Digital imaging does not improve localization of percutaneously inserted central lines in neonates. J Paediatr Child Health. 2005;41:256–9.
Birch P, Ogden S, Hewson M. A randomised, controlled trial of heparin in total parenteral nutrition to prevent sepsis associated with neonatal long lines: the Heparin in Long Line Total Parenteral Nutrition (HILLTOP) trial. Arch Dis Child Fetal Neonatal Ed. 2010;95:F252–7.
Shah PS, Kalyn A, Satodia P, Dunn MS, Parvez B, Daneman A, et al. A randomized, controlled trial of heparin versus placebo infusion to prolong the usability of peripherally placed percutaneous central venous catheters (PCVCs) in neonates: the HIP (Heparin Infusion for PCVC) study. Pediatrics. 2007;119:e284–91.
Harms K, Herting E, Kron M, Schiffmann H, Schulz-Ehlbeck H. Randomized, controlled trial of amoxicillin prophylaxis for prevention of catheter-related infections in newborn infants with central venous silicone elastomer catheters. J Pediatr. 1995;127:615–9.
Sengupta A, Lehmann C, Diener-West M, Perl TM, Milstone AM. Catheter duration and risk of CLA-BSI in neonates with PICCs. Pediatrics. 2010;125:648–53.
Tsai M-H, Hsu J-F, Lien R, Huang H-R, Chiang C-C, Chu S-M, et al. Catheter management in neonates with bloodstream infection and a percutaneously inserted central venous catheter in situ: removal or not? Am J Infect Control. 2012;40:59–64.
Author contributions
TO: Writing the protocol, collecting data and having written the first draft of the manuscript. RA: Conceptualized the study, helped in writing protocol, final analysis and contributed to the final manuscript. AKG: Helped in interpretation of outcome measurement and contributed to final manuscript. SV: Helped in sample size calculation and statistical analysis and contributed to the final manuscript. AT: Helped in writing protocol, forming standard of practice for implementation of study and writing final manuscript. JSM: Helped in writing initial protocol and final analysis of the data and contributed to the final manuscript. AKD and VKP gave critical inputs in designing and execution of the study and critically reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Oleti, T., Jeeva Sankar, M., Thukral, A. et al. Does ultrasound guidance for peripherally inserted central catheter (PICC) insertion reduce the incidence of tip malposition? – a randomized trial. J Perinatol 39, 95–101 (2019). https://doi.org/10.1038/s41372-018-0249-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-018-0249-x
- Springer Nature America, Inc.
This article is cited by
-
Point-of-Care Ultrasound in Neonatology in India: The Way Forward
Indian Pediatrics (2023)
-
Agitated saline contrast to delineate central venous catheter position in neonates
Journal of Perinatology (2021)
-
Use of Point of Care Ultrasound for Confirming Central Line Tip Position in Neonates
Indian Pediatrics (2020)
-
Point of care ultrasound (POCUS) in Canadian neonatal intensive care units (NICUs): where are we?
Journal of Ultrasound (2019)