Abstract
Objective
Peripherally inserted central catheter (PICC) use continues to increase, leading to the development of a blind bedside technique (BST) for placement. The aim of our study was to compare the BST with the fluoroscopically guided technique (FGT), with specific regard to catheter tip position (CTP).
Materials and methods
One hundred eighty patients were randomized to either the BST or the FGT. All procedures were done by the same interventional team and included postprocedural chest X-ray to assess CTP. Depending on the international guidelines for optimal CTP, patients were classified in three types: optimal, suboptimal not needing repositioning, and nonoptimal requiring additional repositioning procedures. Fisher’s test was used for comparisons.
Results
One hundred seventy-one PICCs were successful inserted. In the BST groups, 23.3% of placements were suboptimal and 30% nonoptimal, requiring repositioning. In the FGT group, 5.6% were suboptimal and 1.1% nonoptimal. Thus, suboptimal and nonoptimal CTP were significantly lower in the FGT group (p < 0.001).
Conclusion
Tip malposition rates are high when using blind BST, exposing the patient to an increased risk of deep venous thrombosis and catheter malfunction. Using the FGT or emerging technologies that could help tip positioning are recommended, especially for long-term indications.
Key points
• Bedside and fluoroscopy guided techniques are commonly used for PICC placement.
• Catheter malposition is the major technical issue with the bedside technique.
• Catheter malposition occurred in 53% of patients with the bedside technique.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Peripherally inserted central catheters (PICC) are increasingly used to provide access to central veins. Indications for using PICCs include administration of antibiotics, total parenteral nutrition, chemotherapy, fluid replacement, and drug administration. First described in 1975 1], PICCs have become a primary vascular access device for both inpatient and outpatient settings. Advantages of PICCs over centrally inserted venous catheters (CIVCs) are the markedly reduced risk of procedure-related trauma (pneumothorax; hemothorax; accidental arterial puncture) [1]. PICCs may be used in an outpatient setting with a lower rate of sepsis than CIVCs [2]. Increasing demand for PICC placement has led to the development of blind placement, or the bedside technique (BST) by specially trained operators, thus reducing the demand for interventional radiology facilities for placement. The BST is based catheter insertion of a set length into the vein according to anthropometric measurements obtained externally on anatomical landmarks. Despite the use of bedside ultrasonography (US) for venous puncture and distal guidance, tip malposition remains an issue, as the superior vena cava (SVC) is not easily accessible for US guidance. Therefore, tip malposition is receiving increased attention because even minor malposition within the SVC can increase the risk of complications, such as catheter malfunction (higher rates of loss of function or occlusion) [2], thrombotic complications [1, 3], arrhythmia, and tamponade [4–6]. The reported rate of tip malposition with the BST varies among studies from 10% to >70% and emerge mainly from retrospective studies [7]. Furthermore, to our knowledge, no consistent data comparing the BST to fluoroscopically guided technique (FGT) exits in the literature. The purpose of this prospective randomized controlled study was to compare the BST with the FGT for PICC placement, with specific regard to catheter tip position (CTP).
Materials and methods
Patient population
The institutional Ethical Committee approved the study protocol, and written informed consent was obtained from each patient. Participation was proposed to all consecutive patients >18 years referred to the interventional radiology department for PICC insertion. Patients were excluded if they were unable or refused to consent to participate.
Patients were randomly assigned in a 1:1 manner to the BST or the FGT using sequentially numbered, opaque, sealed envelopes. From May 2013 to January 2014, 180 patients were enrolled: 90 were randomly assigned to the BST group and 90 to the FGT group.
PICC placements techniques
All PICCs were done in the Interventional Radiology Unit using a low-dose X-ray system (Allura Clarity, Philips Healthcare, Best, The Netherlands) and US guidance (Sparq, Philips) with a 5 to 10-MHz linear-array transducer. All procedures were performed by an interventional radiology team experienced in PICC placement (10 years’ experience, with >800 procedures per year). Operators (SB, FG) were instructed to use a standardized procedure. All operators received specific BST training prior to the study. The PowerPICC2® Solo (4-F single lumen or 5-F dual lumen) device was used for all participants.
Fluoroscopically guided technique
A standardized approach was used for preinsertion assessment of peripheral veins of both upper arms and preparation for puncture site, the side and site of which were at operator discretion. However, in our routine practice, selection is initially based on vein diameter and arm dominance. The preferred site is 10 cm above the antecubital fossa through the basilic, brachial, or cephalic vein in the nondominant arm positioned at 75–90° to the body. After tourniquet placement, the puncture (21 gauge) was performed with B-mode duplex US to identify the target vein and the best access. A 0.018 nitinol with a straight-tip guidewire was then inserted. When using the FGT, guidewire course and position was controlled by fluoroscopy, and the standard peel-away introducer was inserted into the vein. To estimate catheter length for optimal tip position, the guidewire was placed either more than or less than 1 cm into the cavoatrial junction (CAJ) with the arm at 45° and the patient in deep inspiration to better identify the CAJ. An external clamp was used to mark the length of the guidewire at the skin site. Using the guidewire to indicate the desired length, the catheter was prepared to match the measured length using a sterile scalpel. We carefully considered the distance between clamp and skin entrance. Catheter and stylet were inserted as into the sheath as one unit, and the catheter was then advanced. When advancement was achieved, the sheath was removed by splitting and peeling it away. The final CTP was documented with an immediate chest fluoroscopy (posteroanterior projection) with the patient’s arm in adduction and the patient in deep inspiration. The puncture site was dressed using StatLock catheter stabilization device (Bard C.R.).
Bedside technique
Once the insertion site was identified, we estimated catheter length using two cutaneous anatomic landmarks: the right clavicular head and the third intercostal space. The distance was measured between planned insertion sites to the right clavicular head, then down to the third intercostal space, with the shoulder abducted to 90°, as previously described [8] and as recommended by the manufacturer. The same protocol was followed for the FGT.
The PICC was prepared so catheter length matched the measured distance. Catheter introduction was established using the Seldinger technique. During insertion, we attempt to avoid tip malposition by turning the patient’s head toward the insertion site and tilting the chin to the chest [9–12]. Following successful insertion, a US of the jugular vein to confirm CTP. Immediately following successful placement, chest fluoroscopy is obtained in the same manner as for the FGT.
Tip position classification
Based on the European Guidelines [13], we classified CTP into three groups, considering the CAJ as the intersection between the right lateral wall of the SVC, as defined by the right lateral border of the mediastinum; and the right lateral border of the cardiac silhouette, defined by the right atrium [14, 15]:
-
Type 1: optimal tip position located either more than or less than 1 cm from the CAJ
-
Type 2: suboptimal tip location not requiring repositioning, with tip located >1 cm under the CAJ or >1 cm above the CAJ but remaining in the SVC
-
Type 3: nonoptimal tip location requiring repositioning, with tip located >3 cm under the CAJ or not inside the SVC
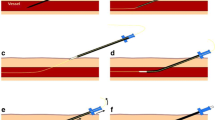
The recommendation of the Ethical Committee was that patients with type 3 undergo repositioning during the same session. Posteroanterior chest X-ray demonstrating CTP is shown in Fig. 1.
Thoracic radiography demarks optimal, suboptimal, and nonoptimal catheter tip position (CTP). In the optimal position (type 1), the tip is positioned <1 cm above or below the cavoatrial junction (CAJ) (black line). In the suboptimal position (type 2), the tip is located >1 cm below or above the CAJ but in the superior vena cava (SVC) (green lines). In type 3 the tip should be repositioned because it is located >3 cm under the CAJ or beyond the SVC (red lines)
Data collection and statistical analysis
Procedural details, including catheter type, patient characteristics, site selection, repositioning, complications, and indications for catheter placement were all recorded. Chest X-rays were interpreted by senior interventional radiologists (SC, SDQ) blinded to the technique used for catheter insertion.
The primary endpoint was defined as the rate of PICCs in a suboptimal position; the secondary endpoint was the rate of PICCs in a nonoptimal location requiring repositioning.
On the basis of the literature, we expected to see a difference between groups of 15% in tip malposition. We therefore calculated that a sample size of 132 patients would be necessary for our study. Considering the possible dropouts or information loss, the final size was estimated to be 180 patients (90 in each group). Clinical data was compared between groups using chi-square or Fisher’s exact test, as appropriate.
Results
Patient characteristics, procedural details, and chest X-rays were available for all patients and are reported in Table 1. There was no significant difference between groups regarding baseline demographics, indications for catheter placement, ability to accomplish venous puncture, and successful PICC introduction. Successful introduction was accomplished in 95.5% in the FGT group and 95.6% in the BST group. There was no significant difference in complication rate between groups. All complications comprised hematomas near the insertion area that were treated conservatively. There was no significant difference between groups regarding access site, and a higher percentage of PICCs were inserted in the left arm in both groups (66.7% in the FGT and 77.8% in the BST) (Table 1).
Regarding tip position, 79 of 89 (88.8%) attempted PICCs in the FGT group resulted in an optimal CTP (type 1) compared with 38 of 90 (42.2%) attempted PICCs in the BST group, with a statistically significant difference between groups (p < 0.001) (Fig. 2). Five of the 89 procedures (5.6%) met criteria for type 2 following the FGT (suboptimal tip position not requiring repositioning). In the BST group, 21 of 90 procedures (23.3%) were type 2 (Fig. 3). The difference between groups was again statistically significant (p < 0.001). There was one nonoptimal (type 3) CTP in following the FGT and 27 (30%) following BST (Fig. 4). Regarding comparison between the left arm access and the right arm access, a significant difference was also observed in the percentage of nonoptimal positioning that necessitates repositioning (type 3) (p < 0.001). Precise PICC tip locations for types 2 and 3 are reported in Table 2.
Discussion
PICC malposition is a well-known problem, with reported rates for the BST ranging from 10 to >70% [16–18] in retrospective studies. To our knowledge, our study is the first prospective randomized controlled study comparing the BST with the FGT in terms of malposition rate. To limit confounding factors that could influence results, we conducted the study with the same product, the same team of operators, and the same facilities in consecutive patients from the same institution. Our study demonstrated a high rate of tip malposition with the BST (53%) versus the FGT (6.7%). Furthermore, the need for catheter repositioning was high in the BST (30%). Our findings relative to the BST are consistent with some reported in the literature [7, 19, 20] but are higher than others [17]. Johnston et al. [19] and Venkastan et al. [21] reported a malposition rate, respectively, of 76 and 63%, whereas Ng et al. [22] and Trerotola et al. [17] found a malposition rate 37 and 10%, respectively. As described by Johnston et al. [19], the differences between these rates are probably multifactorial but can be explained by differences in insertion technique, patient population, operator experience, method used to determine catheter length, and the definition used to describe malposition. Concerning patient population, there is an increased risk of malposition rate in intensive care unit (ICU) compared with non-ICU patients, probably explained by the difficulty in positioning the patient, the presence of other central venous catheters, and differences in venous flow characteristics secondary to mechanical ventilation [23].
One of the major issues in PICC placement is the method used to determine catheter length. We were surprised by the limited data in the literature, particularly of randomized controlled trials on this subject, despite widespread use of anthropometric measurements, to define optimal placement technique. Different anthropometric measurement techniques have been used: Johnston et al. estimated insertion length by measuring the distance between insertion point and midclavicle and added the distance between the suprasternal notch and the acromioclavicular joint [19]. Venkatesan et al. estimated catheter length by measuring the distance from insertion site on the arm to the angle of Louis [21]. Schweickert et al. trimmed the catheter length on measurements from the site of venipuncture to 1–2 cm below the suprasternal notch [12].
In our, we followed the instructions for use from the manufacturer, as recommended by the Infusion Nurse Society’s 2006 standard of practice [24] and other authors [8, 25], being convinced that this technique was the most widely used worldwide. The second issue was certainly the definition used to describe malposition, which is still controversial and is absence a consistent definition of optimal tip position. In the North American guidelines, optimal tip location is the low SVC or CAJ [19, 24, 26]; European guidelines indicate the ideal tip position is in the mid or low SVC, at the CAJ, or in the high right atrium [13]. Other authors accept any position in the SVC [21, 22]. These differences in definition might help explain differences between reported malposition rates.
How position is assessed is also of concern. Defining tip location on plain X-rays is difficult and subject to significant interobserver variations. Indeed, identification of the CAJ using landmarks derived from fluoroscopy or chest X-ray is imprecise and open to interpretation [14].
Our study was designed to address these limitations and limit their impact in interpretation. First, the radiographic landmark we choose to localize the CAJ (inflection of the right atrial border) was demonstrated as one of the closest radiographic landmarks to the CAJ by Ridge et al. [15]. Using echocardiography (ECG)-gated computed tomography angiography (CTA), the authors reported that the inflection of the right atrial border was located a mean of 1 cm above the CAJ [standard deviation (SD) of more than or less than 0.8 cm. Second, two experienced radiologists read in consensus all chest X-rays; patients were classified regarding CTP on chest X-ray using the three-level classification described earlier. Twenty-three percent of patients in the BST arm were classified type 2 and 30% as type 3, which remains unacceptably high in comparison with the FGT (5.6% type 2 and 1.1% type 3). These data clearly demonstrate that blind bedside PICC insertion is not accurate for optimal tip positioning. However, the FGT also has limitations: It exposes the patient and operator to X-ray radiation; it is not practical in critically ill patients; and in patients classified for type 2 and 3 FGT positioning, , there are underlying difficulties recognizing the CAJ.
Emerging X-ray-free technologies, such as ECG placement techniques such as intracavitary (IC) ECG, IC-ECG associated with magnetic tracking (Sherlock 3CG tip confirmation system), and IC-ECG associated with Doppler findings, seem to be very promising for reducing malposition rates. Indeed, Baldinelli et al. demonstrated a rate of suboptimal CTP of 7.14% using IC-ECG technique [8]; Johnston et al. reported a rate of 21% using the Sherlock 3CG [7]. These techniques require p-wave recognition and are easy to use in many clinical situations (atrial fibrillation; cardiac pacemaker; dilated cardiomyopathy); however, they are unable to help establish optimal PICC length.
The clinical impact of tip position is still debated, even though a direct relationship with tip location and PICC-related complications exists. With available technologies, we believe that tip position type is to be considered with specific regard to the target population. Type 2 tip positions are of greatest concern in patients at high risk of thrombosis (systemic comorbidities such as cancer; infusate type); those requiring a long-term PICC should be optimally selected for insertion method. Further studies are needed regarding patient selection for optimal procedure determination, depending on the patient’s clinical history, type of infusate, and access duration. Further developments are needed to obtain optimal PICC insertion, navigation, and positioning.
In conclusion, PICCs are increasingly used in routine practice in various indications. This study clearly demonstrates that techniques used for placement are not equal for attaining optimal CTP. Considering the importance of the CTP, the FGT should be considered at least for patients at high risk of complications. Further evaluations are needed to better select patients for the optimal placement technique, and technological advances will aid in greater CTP accuracy when using the the BST.
Abbreviations
- PICC:
-
Peripherally inserted central catheters
- BST:
-
Blind bedside technique
- FGT:
-
Fluoroscopically guided technique
- CTP:
-
Catheter tip position
- CICV:
-
Centrally inserted venous catheters
- SVC:
-
Superior vena cava
- CAJ:
-
Cavoatrial junction
- ICU:
-
Intensive care unit
References
Amerasekera SS, Jones CM, Patel R, Cleasby MJ (2009) Imaging of the complications of peripherally inserted central venous catheters. Clin Radiol 64:832–840
Maki DG, Kluger DM, Crnich CJ (2006) The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc 81:1159–1171
Caers J, Fontaine C, Vinh-Hung V et al (2005) Catheter tip position as a risk factor for thrombosis associated with the use of subcutaneous infusion ports. Support Care Cancer 13:325–331
Verdino RJ (2006) The evolution of atrial fibrillation ablation from triggers to substrate. J Electrocardiol 39:S184–S187
Bivins MH, Callahan MJ (2000) Position-dependent ventricular tachycardia related to a peripherally inserted central catheter. Mayo Clin Proc 75:414–416
Orme RM, McSwiney MM, Chamberlain-Webber RF (2007) Fatal cardiac tamponade as a result of a peripherally inserted central venous catheter: a case report and review of the literature. Br J Anaesth 99:384–388
Johnston AJ, Holder A, Bishop SM, See TC, Streater CT (2014) Evaluation of the Sherlock 3CG Tip Confirmation System on peripherally inserted central catheter malposition rates. Anaesthesia 69:1322–1330
Baldinelli F, Capozzoli G, Pedrazzoli R, Marzano N (2015) Evaluation of the correct position of peripherally inserted central catheters: anatomical landmark vs. electrocardiographic technique. J Vasc Access 0:0
Ragasa J, Shah N, Watson RC (1989) Where antecubital catheters go: a study under fluoroscopic control. Anesthesiology 71:378–380
LaRue GD (1995) Improving central placement rates of peripherally inserted catheters. J Intraven Nurs 18:24–27
Burgess GE 3rd, Marino RJ, Peuler MJ (1977) Effect of head position on the location of venous catheters inserted via basilic veins. Anesthesiology 46:212–213
Schweickert WD, Herlitz J, Pohlman AS, Gehlbach BK, Hall JB, Kress JP (2009) A randomized, controlled trial evaluating postinsertion neck ultrasound in peripherally inserted central catheter procedures. Crit Care Med 37:1217–1221
Pittiruti M, Hamilton H, Biffi R, MacFie J, Pertkiewicz M (2009) ESPEN Guidelines on Parenteral Nutrition: central venous catheters (access, care, diagnosis and therapy of complications). Clin Nutr 28:365–377
Vesely TM (2003) Central venous catheter tip position: a continuing controversy. J Vasc Interv Radiol 14:527–534
Ridge CA, Litmanovich D, Molinari F, Bankier AA, Eisenberg RL (2013) Radiographic evaluation of central venous catheter position: anatomic correlation using gated coronary computed tomographic angiography. J Thorac Imaging 28:129–133
Fricke BL, Racadio JM, Duckworth T, Donnelly LF, Tamer RM, Johnson ND (2005) Placement of peripherally inserted central catheters without fluoroscopy in children: initial catheter tip position. Radiology 234:887–892
Trerotola SO, Thompson S, Chittams J, Vierregger KS (2007) Analysis of tip malposition and correction in peripherally inserted central catheters placed at bedside by a dedicated nursing team. J Vasc Interv Radiol 18:513–518
Minkovich L, Djaiani G, McCluskey SA, Mitsakakis N, Gilbert RW, Beattie WS (2011) Frequent malpositions of peripherally inserted central venous catheters in patients undergoing head and neck surgery. Can J Anaesth 58:709–713
Johnston AJ, Bishop SM, Martin L, See TC, Streater CT (2013) Defining peripherally inserted central catheter tip position and an evaluation of insertions in one unit. Anaesthesia 68:484–491
Pittiruti M, Bertollo D, Briglia E et al (2012) The intracavitary ECG method for positioning the tip of central venous catheters: results of an Italian multicenter study. J Vasc Access 13:357–365
Venkatesan T, Sen N, Korula PJ et al (2007) Blind placements of peripherally inserted antecubital central catheters: initial catheter tip position in relation to carina. Br J Anaesth 98:83–88
Ng PK, Ault MJ, Maldonado LS (1996) Peripherally inserted central catheters in the intensive care unit. J Intensive Care Med 11:49–54
Pinsky MR (1990) The effects of mechanical ventilation on the cardiovascular system. Crit Care Clin 6:663–678
(2006) Infusion Nursing Standards of Practice. J Infus Nurs 29:S1–92
Tian G, Chen B, Qi L, Zhu Y (2011) Modified insertion of a peripherally inserted central catheter: taking the chest radiograph earlier. Crit Care Nurse 31:64–69
Funaki B (2002) Central venous access: a primer for the diagnostic radiologist. AJR Am J Roentgenol 179:309–318
Acknowledgements
The scientific guarantor of this publication is Salah Dine Qanadli. The authors of this manuscript declare relationships with the following companies: Salah Dine Qanadli was a consultant for C. R Bard Inc. during the last 3 years. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Glauser, F., Breault, S., Rigamonti, F. et al. Tip malposition of peripherally inserted central catheters: a prospective randomized controlled trial to compare bedside insertion to fluoroscopically guided placement. Eur Radiol 27, 2843–2849 (2017). https://doi.org/10.1007/s00330-016-4666-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4666-y