Abstract
Objectives
Most authorities recommend daily supplementation of 400 IU vitamin D for all term healthy neonates throughout infancy, however this dose was shown to be inadequate in an earlier study from our institution. We planned to evaluate if supplementation of 800 IU/day in term Indian infants would reduce the prevalence of vitamin D insufficiency (VDI) at 6 months of age.
Methods
In a prospective study, we supplemented 800 IU/day of vitamin D in 70 term infants from birth till 6 months of age. Serum 25-hydroxy cholecalciferol [25(OH)D] was measured at birth and 6 months for all infants; and at 6, 10 and 14 weeks of age in subsets of 23 infants each. The primary outcome was prevalence of VDI (defined as serum 25(OH)D level < 50 nmol/L) at 6 months of age.
Results
A total of 58 out of 70 (83%) infants were followed up until 6 months of age. The median (nmol/L; IQR) serum 25(OH)D at birth and 6 months of age was 25 (12.5–35) and 92.5 (72.5–137.5), respectively. The prevalence of VDI at birth was 91.3% (63/69), which reduced to 6.9% (4/58) at 6 months of age. However, four infants (6.9%, 95% CI 1.9–16.7) developed vitamin D excess (serum 25(OH)D 250–375 nmol/L) requiring reduction of the dose of supplementation. No infant developed vitamin D toxicity (serum 25(OH)D > 375 nmol/L).
Conclusions
Daily supplementation of 800 IU of vitamin D resulted in vitamin D sufficiency in most term healthy infants at 6 months of age but with potential risk of toxicity.
Similar content being viewed by others
Introduction
Vitamin D is a fat-soluble vitamin important for skeletal development and associated with many non-skeletal health outcomes [1]. Despite this recognized importance, there is a lot of controversy over the definition of optimal vitamin D status. The US Endocrine Society defines a 25-hydroxy vitamin D [25(OH)D] level more than 75 nmol/L as the optimal level, while the Institute of Medicine sets this cut-off at 50 nmol/L [2, 3]. Irrespective of the definition, there is a widespread prevalence of vitamin D deficiency (VDD) across all ages and all parts of the world, including pregnant mothers and their infants [4,5,6]. This is even truer and more prevalent in tropical countries like India [7, 8].
Breast milk from mothers not receiving any vitamin D supplementation or adequate sunlight exposure contains only 20–60 IU of antirachitic activity, insufficient to meet the requirements of the infant [9]. Subsequently there have been various recommendations on vitamin D supplementation in infants and children. While most of the authorities recommend a dose of 400 IU/day for breastfed infants, the US Endocrine committee has suggested the intake of 400–1000 IU/day under 1 year of age based on its definition of a higher optimal vitamin D level [2, 6, 10, 11]. Studies comparing different doses of vitamin D in infants have reinforced the need for supplementation but these studies also show the inadequacy of 400 IU/day in some populations and the need for higher doses to achieve vitamin D sufficiency [12,13,14]. Given the higher prevalence of VDD, darker skin and higher breastfeeding rates in India, the optimum dose for vitamin D supplementation is likely to be higher, as was shown in an earlier study from our institution by Deepti et al. (unpublished data). In this study (personal communication), VDI was found in 52% of 111 infants at 14 weeks of age despite daily vitamin D supplementation with 400 IU. However the national guidelines in India recommend a dose of 400 IU/day for infants, like most other guidelines, which is likely to be inadequate [15, 16]. Therefore, we planned this prospective study to evaluate the efficacy of supplementation of 800 IU/day vitamin D in term healthy breastfed infants. The primary objective was to determine the prevalence of vitamin D insufficiency (VDI; defined as 25(OH)D < 50 nmol/L) at 6 months of age in term healthy breastfed infants supplemented with 800 IU of oral vitamin D per day starting from birth.
Methods
Subjects and setting
We conducted this prospective interventional study in a tertiary care hospital in North India between November 2014 and September 2015. All term infants born with a birth weight of 2500 g or more were eligible for inclusion in the study. Infants with major malformations, severe birth asphyxia (defined as Apgar score of less than 4 at 5 min or need for chest compressions) or need for NICU stay for more than 48 h were excluded. Informed written consent was obtained from the parents before enrolment. The study was approved by the Institute Ethics Committee of AIIMS, New Delhi.
Intervention
Cord blood sample was drawn at birth from the infants for estimation of serum 25(OH)D, parathyroid hormone (PTH), calcium, phosphorus, and alkaline phosphatase (ALP) before starting intervention. All enrolled infants were started on vitamin D supplementation with 800 IU (1 mL) of vitamin D drops (CalShine P drops from Eris Lifesciences Pvt Ltd, Ahmedabad, Gujarat, India) within 48 h of birth. The method of administration of drops and measurement using the dropper provided with the supplement was demonstrated to the mother and care givers by the principal investigator. At discharge, mothers were counselled for exclusive breastfeeding and asked to maintain a compliance sheet with a calendar which were given for ensuring daily administration of the vitamin D drops. The infants were called for four follow-up visits at 6, 10, 14 weeks, and 6 months of age, first three of which were combined with their routine immunisation visits, to avoid extra trips to the hospital. Parents were re-enforced about the importance of vitamin D supplementation and timely follow-up at each visit. Telephonic reminders were employed to ensure compliance and follow-up at each visit. Compliance sheet was checked for any missed doses and vitamin D drops were provided for the duration till further follow-up.
Outcome variables
The primary outcome variable was prevalence of VDI (defined as 25(OH)D < 50 nmol/L) at 6 months of age. Secondary outcomes included the following: serum levels of calcium, phosphorus, alkaline phosphatase, and parathormone at 6 months of age; the proportion of infants with vitamin D excess at each sampling time point; and the postnatal age at which the steady-state concentration of 25(OH)D was attained in supplemented infants. VDI was defined as serum 25(OH)D < 50 nmol/L, VDD as levels < 30 nmol/L, and vitamin D excess as levels of 250–375 nmol/L [3, 17].
Outcome measurement
Blood samples were transported with ice packs immediately to the lab and cold centrifuged at 4 °C, at 3000 rpm for 15 min using the Heraeus multifuge X1R (Thermo scientific, United states). The serum was aliquoted, analysed for 25(OH)D and PTH the same day and the remaining sample was stored at −20 °C for the analysis of calcium, phosphorus and ALP. Serum 25(OH)D was assayed by an autoanalyzer (DiaSorin Liaison, Stillwater, MN) using a chemiluminescent tracer, with a measuring range of 10–375 nmol/L. Serum PTH levels were estimated by using electrochemiluminometric assay using Cobas e411 (Roche Diagnostics, Basel, Switzerland) autoanalyzer, with a measuring range of 1.2–5000 pg/mL. Serum calcium, phosphate and alkaline phosphatase were measured by colorimetric method on Roche Modular P800 auto analyser (Roche diagnostics, Germany).
Statistical analysis
Data was entered in a predesigned proforma in MS Access 2013 (Microsoft corp, Redmond, CA). Statistical analysis was performed by using Stata 11.2 version (StataCorp, College Station, TX). Continuous data were expressed as means and SDs or medians with ranges, whereas categorical variables were expressed as proportions. Paired ‘t’ test was used for the analysis of continuous variables that were normally distributed, whereas Wilcoxon signed rank test was used for skewed data. Categorical variables were analysed by using McNemar test.
Sample size
In an unpublished study from our centre (personal communication) on vitamin D supplementation with 400 IU, VDI was found in around 50% of the infants at 3 months of age. We assumed a prevalence of VDI to be around 20% in Indian infants after being supplemented with 800 IU of vitamin D at 6 months. With the estimated prevalence of 20%, absolute precision of 10%, alpha error of 5%, the required sample size is calculated to be 63. We planned to enrol 70 infants to account for loss to follow-up. For determining steady state age, we planned to estimate serum 25(OH) D levels at additional 3 time points, i.e., at 6, 10 and 14 weeks. However, to minimize the number of blood samplings, we randomly divided sample population into 3 subsets (n = 23, 23 and 24) and measured serum 25(OH) D levels at 6 weeks (in subset 1), 10 weeks (in subset 2) and 14 weeks (in subset 3) along with 25(OH) D levels at birth and 6 months for all infants.
Results
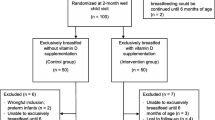
Out of 643 live births during the study period, 490 were born at term gestation. However, we could not obtain cord blood samples in 178 infants (solely done by principal investigator while being posted in delivery area) while consent was denied by parents in 182 cases (who expressed difficulty in follow-up either due to distance, transport facilities or social reasons). After excluding 60 infants based on pre-specified exclusion criteria, we enrolled 70 infants in the study (Fig. 1). A total of 68, 67, 65 and 58 babies completed the 6 week, 10 week, 14 week and 6 month follow-up, respectively, out of which 25(OH)D levels were estimated in 21 (subset 1 at 6 weeks), 20 (subset 2 at 10 weeks), 23 (subset 3 at 14 weeks) and 58 infants at 6 months. The follow-up rates among subsets 1, 2 and 3 at 6 months of age were 83% (19/23), 87% (20/23) and 79% (19/24) respectively.
Baseline characteristics
The mean gestational age of the enrolled infants was 38 weeks and their mean birth weight was 2976 g (±315 g) (Table 1) . One mother received 60,000 IU of vitamin D weekly for 4 weeks during pregnancy. About three-fourths of mothers continued calcium and vitamin D supplements during lactation up to 6 weeks postpartum which fell down to 56% at 14 weeks and 41% at 6 months follow-up. More than 90% and 80% of the infants were exclusively breast fed at discharge and at 6 months, respectively. There was a good compliance rate with vitamin D supplementation in most of the infants who were followed up till 6 months of age with only 5% of doses missed during follow-up period.
Primary outcome
The proportion of infants with VDI was significantly lower at 6 months of age in comparison at birth (6.9% vs. 91.3%; relative risk 0.08, 95% CI: 0.03–0.19; P < 0.001). Serum 25(OH)D levels at 6 months were significantly higher compared with the levels at birth (median [IQR]: 92.5 [72.5–137.5] vs. 25 [12.5–35] nmol/L; P < 0.001) (Table 2).
Secondary outcomes
Markers of vitamin D status
The median serum PTH at birth was 4.4 (3.2–6.2) pg/mL with no infant having hyperparathyroidism, while at 6 months, the median level was 29.9 (23.1–39.2) pg/mL with four infants having hyperparathyroidism (PTH > 65 pg/mL). There were no incidence of hypocalcemia at birth while one infant had hypocalcemia (7.7 mg/dL) at 6 months, without any symptoms or deranged parameters (25(OH)D 120 nmol/L, PTH 27 pg/mL), possibly depicting normal variation in serum calcium levels. The mean phosphorus level was 8.1 mg/dL at birth and 6.5 mg/dL at 6 months of age, reflecting the normal higher values of phosphorus in cord blood (Table 3).
Vitamin D excess
There were four different infants with vitamin D excess during 6 month follow-up, one each at 4 time points, i.e., at 6 (1/21), 10 (1/20) and 14 weeks (1/23) and at 6 months (1/58). The maximum vitamin D level was 270 nmol/L; none of the infants developed vitamin D toxicity. The vitamin D doses were modified when vitamin D level was found to be in the excess range, decreasing it to 400 IU/day for rest of the follow-up period. Post hoc analysis was performed to look for any factors leading to vitamin D excess in four infants and their biochemical parameters of vitamin D status including hypercalcemia. None of the four infants developed hypercalemia and there was no significant difference in maternal vitamin D supplementation during pregnancy or lactation or any postnatal sunlight exposure. The infant whose mother received weekly 60,000 IU vitamin D for 4 weeks did not have any vitamin D excess at birth (22.5 nmol/L), 14 weeks (35 nmol/L) or at 6 months of age (30 nmol/L).
Post natal age at which steady state is reached
The median serum 25(OH)D was determined at different time points in three subsets of infants. The maximum concentration was attained at 6 weeks of age, i.e., 130 nmol/L with somewhat steady but declining values at subsequent follow-ups (Fig. 2).
Vitamin D levels at various time points during follow-up. *25(OH)D values (median) are depicted as dots and inter-quartile ranges as errorbars at various timepoints during follow up estimated at birth (in 69 infants), 6 weeks (in 21 infants), 10 weeks (in 20 infants), 14 weeks (in 23 infants) and 6 months (in 58 infants). The figures in the graph stand for median 25(OH)D values in nmol/L while figures in parentheses stand for the number of infants who underwent 25(OH)D estimation at various timepoints
Discussion
In our study, we found that infants at 6 months of age with daily supplementation of 800 IU of vitamin D, had vitamin D insufficiency up to 6.9%. There was a significant improvement in the median vitamin D level from 25 (12.5–35) nmol/L at birth to 92.5 (72.5–137.5) nmol/L at 6 months (p < 0.001), which was above the level of 50 nmol/L which is considered sufficient. While the prevalence of vitamin D deficiency (<30 nmol/L) decreased from 68.1% at birth to 3.4% at 6 months and most of them (91%) were in the sufficiency range, four infants developed vitamin D excess (>250 nmol/L) during 6 months follow-up. These results clearly show that a daily dose of 800 IU achieved target serum vitamin D level ≥ 50 nmol/L in more than 90% of infants but is associated with a potential risk of vitamin D toxicity.
Though there are many studies showing a high prevalence of VDD at 3 months in Indian infants without supplementation, there are a few Indian studies in normal birth weight infants with vitamin D supplementation [7, 8, 16]. Ours would be the first Indian study to have looked into the effect of 800 IU vitamin D supplementation in term infants. This high prevalence of VDD in infants can be attributed to the high baseline vitamin D-deficient status of the pregnant lady [18]. Concordance between the maternal and infant vitamin D levels have been well established in multiple studies, though we did not record the maternal vitamin D levels [8, 19].
There are many studies on vitamin D supplementation in infants over the world trying to establish the adequate dose of supplementation with widely varying, even contradictory results. While some conclude that 800 IU/day is sufficient [12,13,14], others suggest that 400 IU or even lesser may be adequate [20,21,22]. These discrepant results can possibly be explained by the wide variation between studies in terms of the population studied, race and skin colour of the enrolled infants and varying breastfeeding rates. We found vitamin D deficiency (<30 nmol/L) in two infants at 6 months, but there were no clinical features suggestive of rickets. Only one of them had elevated PTH levels while none of them had hypocalcemia. Comparing the results of an earlier study from our institute (unpublished data), in term infants with similar vitamin D status at birth, 800 IU definitely proved to be more efficacious than 400 IU as a daily dose for supplementation, achieving sufficiency in 91% of the infants compared to only 52%, respectively. However 800 IU was associated with incidences of vitamin D excess which was not detected in any of the infants receiving 400 IU daily.
In our study, four infants developed vitamin D excess (>250 nmol/L) during 6 months, each occurring at different ages but none of the infants had hypercalcemia at the time of vitamin D excess. These findings are similar to those in other studies of vitamin D supplementation in infants which have shown doses upto 1200 IU, though causing vitamin D excess, do not cause hypercalcemia [12, 13]. Vitamin D dose was reduced to 400 IU, for each of infants with vitamin D excess, in order to bring down their vitamin D levels to normal range.
Ours was the first study trying to evaluate the postnatal age at which the steady-state concentration of 25(OH)D was achieved during supplementation in infants which concluded this age to be within first 6 weeks since there was no increase in vitamin D levels after 6 weeks. However studies in healthy adults have determined this period to be ̴13 weeks which probably reflects different pharmacokinetics in infants with different hepatic maturity [23, 24]. Concentrations of 25(OH)D declined during follow-up in all subsets. This decline may be due to decreased adherence but it appears more likely due to increasing need of vitamin D dose as body weight increases. This pattern is consistent with data from other infant supplementation trials suggesting the need for a vitamin D dose per kilogram in this age group [12, 13].
We acknowledge certain limitations in our study. Though adequate efforts were made to ensure a good rate of follow up, by repeated reminders and clubbing study visits with immunization visits, follow up rate at 6 months was 83%. Maternal vitamin D levels were not measured at birth or follow up and thus a correlation between maternal and infant levels were not possible. As high level of vitamin D deficiency in pregnant and lactating mothers has been well established in multiple studies from India prior to ours, we presumed it was unnecessary to repeat the same [18, 19]. Some of the risk factors for deficiency such as sun exposure of mothers during pregnancy and lactation, details of dietary calcium, phytate and vitamin D intake of mothers were not recorded. We were unable to do any meaningful analysis of the effect of sunlight exposure on vitamin D level, however this is unlikely to play a significant role in achieving optimal serum levels alone [16]. Testing for features of vitamin D excess in the form of urinary calcium creatinine ratio or ultrasonographic evaluation for nephrocalcinosis was not carried out as there were no incidences of vitamin D toxicity (>375 nmol/L) or hypercalcemia [2]. Finally we did not study the effects of vitamin D deficiency or supplementation on bone mineralization.
Conclusion
A daily supplementation of 800 IU/day of oral vitamin D resulted in vitamin D sufficiency in most term healthy infants at 6 months of age. However, a small but significant proportion of infants developed vitamin D excess requiring dose modification. Given the potential risk of toxicity in a few infants and a possible risk of insufficiency in a few others, supplementation of 800 IU/day oral vitamin D cannot be universally recommended. Alternative approaches including correction of vitamin D deficiency at birth by appropriate bolus dose followed by routine supplementation with a lower dose (400–600 IU) should be explored as a possible strategy to achieve vitamin D sufficiency in infants.
References
Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–73. https://doi.org/10.4065/81.3.353.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. https://doi.org/10.1210/jc.2011-0385.
National Institute of Health. Dietary reference intakes for calcium and vitamin D. In: Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Institute of Medicine (US) Committee to review dietary reference intakes for vitamin D and calcium. Washington, DC: National Academies Press; 2011. http://www.ncbi.nlm.nih.gov/books/NBK56070/. Accessed 30 March 2017.
Merewood A, Mehta SD, Grossman X, Chen TC, Mathieu JS, Holick MF, et al. Widespread vitamin D deficiency in urban Massachusetts newborns and their mothers. Pediatrics. 2010;125:640–7. https://doi.org/10.1542/peds.2009-2158.
Merewood A, Mehta SD, Grossman X, Chen TC, Mathieu J, Holick MF, et al. Vitamin D status among 4-month-old infants in New England: a prospective cohort study. J Hum Lact J Int Lact Consult Assoc. 2012;28:159–66. https://doi.org/10.1177/0890334411434802.
Braegger C, Campoy C, Colomb V, Decsi T, Domellof M, Fewtrell M, et al. Vitamin D in the healthy European paediatric population. J Pediatr Gastroenterol Nutr. 2013;56:692–701. https://doi.org/10.1097/MPG.0b013e31828f3c05.
Agarwal R, Virmani D, Jaipal ML, Gupta S, Gupta N, Sankar MJ, et al. Vitamin D status of low birth weight infants in Delhi: a comparative study. J Trop Pediatr. 2012;58:446–50. https://doi.org/10.1093/tropej/fms013.
Marwaha RK, Tandon N, Chopra S, Agarwal N, Garg MK, Sharma B, et al. Vitamin D status in pregnant Indian women across trimesters and different seasons and its correlation with neonatal serum 25-hydroxyvitamin D levels. Br J Nutr. 2011;106:1383–9. https://doi.org/10.1017/S000711451100170X.
Hollis BW, Roos BA, Draper HH, Lambert PW. Vitamin D and its metabolites in human and bovine milk. J Nutr. 1981;111:1240–8.
Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–52. https://doi.org/10.1542/peds.2008-1862.
Canadian Paediatric Society. Vitamin D supplementation: recommendations for Canadian mothers and infants. Position statements and practice points. Ottawa, ON: Canadian Paediatric Society. http://www.cps.ca/documents/position/vitamin-d. Accessed 30 March 2017.
Gallo S, Comeau K, Vanstone C, Agellon S, Sharma A, Jones G, et al. Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. JAMA. 2013;309:1785–92. https://doi.org/10.1001/jama.2013.3404.
Grant CC, Stewart AW, Scragg R, Milne T, Rowden J, Ekeroma A, et al. Vitamin D during pregnancy and infancy and infant serum 25-hydroxyvitamin D concentration. Pediatrics. 2014;133:e143–53. https://doi.org/10.1542/peds.2013-2602.
Holmlund-Suila E, Viljakainen H, Hytinantti T, Lamberg-Allardt C, Andersson S, Mäkitie O. High-dose vitamin d intervention in infants--effects on vitamin d status, calcium homeostasis, and bone strength. J Clin Endocrinol Metab. 2012;97:4139–47. https://doi.org/10.1210/jc.2012-1575.
Balasubramanian S, Dhanalakshmi K, Amperayani S. Vitamin D deficiency in childhood—a review of current guidelines on diagnosis and management. http://www.indianpediatrics.net/july2013/july-669-675.htm. Accessed 26 February 2017.
Chandy DD, Kare J, Singh SN, Agarwal A, Das V, Singh U, et al. Effect of vitamin D supplementation, directly or via breast milk for term infants, on serum 25 hydroxyvitamin D and related biochemistry, and propensity to infection: a randomised placebo-controlled trial. Br J Nutr. 2016;116:52–58. https://doi.org/10.1017/S0007114516001756.
Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016;101:394–415. https://doi.org/10.1210/jc.2015-2175.
Sahu M, Bhatia V, Aggarwal A, Rawat V, Saxena P, Pandey A, et al. Vitamin D deficiency in rural girls and pregnant women despite abundant sunshine in northern India. Clin Endocrinol. 2009;70:680–4. https://doi.org/10.1111/j.1365-2265.2008.03360.x.
Jain V, Gupta N, Kalaivani M, Jain A, Sinha A, Agarwal R. Vitamin D deficiency in healthy breastfed term infants at 3 months & their mothers in India: seasonal variation & determinants. Indian J Med Res. 2011;133:267–73.
Pittard WB, Geddes KM, Hulsey TC, Hollis BW. How much vitamin D for neonates? Am J Dis Child. 1991;145:1147–9.
Shakiba M, Sadr S, Nefei Z, Mozaffari-Khosravi H, Lotfi MH, Bemanian MH. Combination of bolus dose vitamin D with routine vaccination in infants: a randomised trial. Singap Med J. 2010;51:440–5.
Siafarikas A, Piazena H, Feister U, Bulsara MK, Meffert H, Hesse V. Randomised controlled trial analysing supplementation with 250 versus 500 units of vitamin D3, sun exposure and surrounding factors in breastfed infants. Arch Dis Child. 2011;96:91–95. https://doi.org/10.1136/adc.2009.178301.
Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–10.
Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr. 2001;73:288–94.
Acknowledgements
We acknowledge the contributions by Mrs Shiji Binu, Mr Leslie James and Mr Bijoy Jose for assistance in analysis of blood samples. We thank all the babies enrolled in the study and their families for being part of the study and the nursing staff at AIIMS, New Delhi.
Funding
Indian Council of Medical Research (ICMR) funded the drug; laboratory tests were available as free in-house facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Priyadarshi, M., Sankar, M.J., Gupta, N. et al. Efficacy of daily supplementation of 800 IU vitamin D on vitamin D status at 6 months of age in term healthy Indian infants. J Perinatol 38, 1566–1572 (2018). https://doi.org/10.1038/s41372-018-0216-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-018-0216-6
- Springer Nature America, Inc.