Abstract
Objective:
The aim of the study was to better describe incidence, risk factors, and the natural evolution of neonatal portal vein thrombosis (PVT).
Study design:
One hundred and twenty-three premature newborns or with birth weight <1.5 kg were prospectively included in a single center during a one-year period. Three systematic abdominal ultrasound examinations at day 3, day 10, and day 45 (and 1 year in case of persistent PVT) were performed. Clinical and biological data were recorded.
Results:
Seventy neonates (57%) had three normal US examinations. Fifty-three neonates (43%) had a clinical and biological asymptomatic left PVT. No right or extrahepatic portal venous thrombosis was observed. Umbilical vascular catheter (UVC) was removed in case of PVT. No anticoagulation therapy was required. No risk factor was significantly associated with PVT. At 1 year of follow-up, five infants had persistent isolated left PVT (4%).
Conclusion:
A spontaneous favorable evolution of left PVT occurred in more than of 95%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Portal vein thrombosis (PVT) remains unrecognized in the neonatal period. Clinically asymptomatic PVT is sometimes picked up by screening investigations or during routine imaging for other indications. Umbilical catheter placement is often thought to be the cause of PVT [1]. This belief is based on the retrospective observation that many children with extrahepatic portal hypertension have undergone catheterization of the umbilical vein during the newborn period. History of neonatal umbilical vascular catheter (UVC) have been reported in 35% of children with extrahepatic portal vein obstruction, predictive of failure of Meso-Rex bypass [2]. UVC is used in up to 15% of all babies admitted to NICU and 50% of all preterm neonates with very low birth weight [3]. The umbilical vein is available as a site for central venous access for the first week of life for the monitoring and treatment of critically ill neonates. It allows rapid venous access in the delivery room, exchange transfusions, measurement of central venous pressure, and vascular access of last resort [4]. According to the results of prospective studies [5,6,7], properly inserted umbilical venous catheters (UVCs) did not cause portal venous thrombosis. Ultrasonography associated with Doppler is a non-invasive useful tool to detect the position of the UVC and its potential thrombotic complication [1, 8,9,10]. UVC-associated thrombosis was commonly reported in neonates (mainly in the left intrahepatic portal vein) and spontaneous resolution occurs in most cases [11, 12]. Kim et al. [12] showed a 43% rate of neonatal PVT following UVCs when ultrasound (US) examination was completed every 5–7 days while infants were in hospital. The reported incidence of catheter-related thrombosis in infants and children was variable, depending on the arterial or venous position with or without heparin bonding [6, 13]. Poor outcome in neonates with PVT, defined as portal hypertension or lobar atrophy, was diagnosed in 27% of the infants and was significantly more common in those with an initial diagnosis of severe PVT (occlusive PVT involving two branches or single branch PVT associated with parenchymal changes) [1]. In a follow-up case series of Morag et al. [14], among patients who had PVT as neonates, 25% had atrophy of the left lobe of the liver, 7% had splenomegaly (without portal hypertension), and 3% had portal hypertension requiring porta-caval shunt. However, there was no evidence that anticoagulation therapy improves time to resolution or decreases the likelihood of portal hypertension[15].
This prospective study was designed to determine the incidence, risk factors, and the natural evolution of portal venous thrombosis related or not to an UVC in neonates admitted to NICU by systematic serial US.
Material and Methods
Patient population
The study was authorized by the institutional review board of our hospital (2014-A01366-41; 2014-S18) ruled by written information given to parents. Parents’ written consent was obtained. All premature newborns with gestational age under 32 weeks and 6 days and all newborns with birth weight <1.5 kg admitted to the neonatal intensive care unit at our pediatric university hospital from November 2014 to November 2015 were prospectively included.
Umbilical venous catheterization
In our practice, a 4 French diameter UVC was systematically used for venous infusion for infant with birth weight under 1500 g or small for gestational age under 10° percentile or need of emergency venous pathway. Placement was controlled with a standard anteroposterior radiograph at the end of the procedure. The venous position was defined as central tip at or above the junction between the inferior vena cava and the right atrium, or peripheral (under the diaphragm). In case of central position, we recommended to remove the catheter before day 5. Otherwise, in case of abnormal position, UVC should be removed as soon as possible and switch if necessary into a peripherally inserted central catheter. Fluid and nutritional intake are according to the ESPGHAN recommendation. We used a maximum infusion concentration under 1000 mOsm/L and avoided blood transfusion using the UVC. In case of PVT diagnosed on US, UVC was removed and another venous access was required.
US examination
Three systematic US examinations were provided during hospitalization at Day 3, Day 10, and Day 45 or before being discharged. A grid was completed after each US exam. US included color Doppler US scanning of the portal vein and was performed by pediatric radiologists with a 5–9 multi-MHz linear-array transducer (Voluson S8 General Electric). A pulse Doppler was used to confirm the venous stream. Dimension of the right and left liver was noted. Splenomegaly was reported. The diagnosis of portal venous thrombosis was made by documenting the echogenic intraluminal thrombus at gray-scale US and the absence of flow on color Doppler US images [9, 10, 16]. In the cases with portal venous thrombosis, the location, extent, and size of the thrombus were recorded. Occlusive was defined by hyperechogenic thrombus replacing the entire lumen and no-flow in color Doppler. Partial was defined by non-occlusive hyperechogenic intraluminal thrombus and persistent stream in color Doppler.
Results were classified as normal, occlusive portal venous thrombosis, or partial portal venous thrombosis. A normal longitudinal right to left liver ratio was between 0.92 and 0.95 [17].
Initial and secondary follow-up
If a thrombus was detected within the first three US examination, additional US was provided 6 months later and then at least 1 year later associated with a pediatric hepatologic physician’s consultation.
Study parameters
Potential associated risk factors of PVT were collected such as gestational age, birth weight, antenatal steroid injection, family history of thrombophilia, maternal diabetes, dehydration (weight loss >10% of birth weight during the first week), maximum blood hematocrit in the first week of life, early onset sepsis (positive blood culture or elevation of blood marker inflammation C reactive protein with context of maternal infection or contamination). Liver enzyme was recorded for each patient.
Statistical analysis
Pearson’s Chi-squared tests with Yates’ continuity correction were performed with the R software [18]. A p-value less than 0.05 was regarded as indicating a significant difference. A multiple regression analysis was performed on the data set.
Results
Patient population
One hundred twenty-three neonates (74 girls and 49 boys) were enrolled during the study period. Two neonates died between Day 3 and Day 10 and two children were lost to follow-up during the study (Fig. 1). Gestational ages of the neonates were 25–36 weeks (mean gestational age, 30 weeks; median 31). They weighed 550–2600 g (mean weight, 1290 g).
Umbilical venous catheterization
Nineteen (15.4 %) neonates had no UVC. They were older (median gestational weeks of 31.8 versus 30.6, p < 0.0001) and heavier (median birth weight of 1702 versus 1207 g, p < 0.0001) than those who had UVC. Among the 70 neonates with normal US examinations, 53 had a UVC. Among the 53 neonates with left PVT, 51 had a UVC. The frequency of left PVT was 49% among children with UVC (51/104) and 10.5% in children without UVC. UVC was significantly associated with PVT (p = 0.001).
The location of the UVC tip was in central position in 55 cases, in peripheral position in 49. Details and duration of the utilization of the UVC are reported in Table 1. A peripheral position of the UVC was significantly associated with higher rate of PVT (61% versus 38%, p = 0.031). In case of peripheral position, UVC was removed as soon as possible, within the next 24th hour. The UVC were placed for 1 day to 7 days (mean, 79 h).
US examination
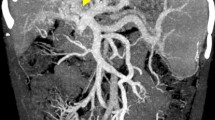
Seventy neonates (57%) had three normal US examinations. Fifty-three neonates (43%) had a left portal venous thrombosis observed in at least one US examination (Fig. 2). No right or extrahepatic portal venous thrombosis was observed. In all cases, the liver and the spleen were not enlarged, and neither ascites nor extrahepatic collateral vessels were noted.
a Normal hepatic ultrasound image with visualization of the end of the umbilical venous catheter in the left portal vein (thin white arrow). b Normal hepatic portal bifurcation color Doppler image with homogeneous colored right and left porta venous. c Partial left venous thrombosis, represented by partial hyperechogenic left portal venous obstruction (thick white arrow). d Total left venous thrombosis with total obstruction of the left portal vein by hyperechogenic thrombus (black star)
Left portal venous thrombosis was detected in 33 (26.8%) of the 123 neonates during the first (Day 3) or second (Day 10) US examination, with a spontaneous resolution on the third (Day 45 or before discharge) US examination. The results of the three systematic US examinations are provided in Table 2.
Among 17 neonates with partial (n = 8) or occlusive (n = 9) left PVT on the third US examination, 11 had normal US examination at 6 months.
Study parameters
We recorded four (3.2%) neonates with familial histories of thrombophilia and 11 (9.1%) with maternal diabetes. Sepsis was found in 41 cases. Eighty-six newborns received antenatal steroid injections (69.9%). Dehydration occurred in 41 cases (33%) and mean maximum hematocrit was 49.1% (34.5–63.2 %). Eight (6.5%) newborns suffered from early onset sepsis. Sepsis was found in 41 cases (33.8%), five had necrotizing enterocolitis (Bell’s score ≥2). No elevated liver transaminases were observed. All these criteria were not significantly associated with a higher risk of PVT. No anticoagulation therapy was initiated. Our population was homogeneous and no significant differences exist between premature newborn with or without UVC. Details are provided in Table 3.
Follow-up
The five patients with persistent PVT at one year had a pediatric hepatologic physician’s consultation after the US exam. All clinical and biological examinations were normal. All infants with persistent occlusive left PVT had UVC for 3 days (n = 1) to 5 days (n = 3). No significant risk factor was found among the five patients. The left hepatic lobe in the four cases with occlusive left PVT was hypotrophic (decrease of the longitudinal right/left liver ratio). Details of the liver ultrasonographic measurements are shown in Table 4.
Discussion
Left portal venous thrombosis was a frequent event, reported in 26.8% of the 123 neonates within the tenth first days. We have observed spontaneous resolution of partial or occlusive left PVT, sometimes very quickly between two US examinations. Left PVT remained clinically and biologically asymptomatic.
Except UVC, no other significant risk factor of PVT was found. The global incidence of 43% of left PVT was consistent to those previously reported in children with UVC: 32% in Yadav et al. [5], 34% in Sakha et al. [11], and 43% in Kim et al. studies [12]. The differences could partially be explained by the timing and frequency of US monitoring and by size, location, and duration of the UVC. If the ductus venosus is not always perfectly aligned to the umbilical vein, the left portal vein may be the recipient of the UVC tip during placement [19]. Although left venous portal thrombosis has occurred without any UVC, a peripheral position of the UVC was significantly associated with higher rate of PVT. The improper placement of the catheter should be avoided and corrected as soon as possible.
We could recommend a control of the catheter position, by ultrasonographic examination, or radiograph if unavailable, to be sure that the UVC is not terminating in the liver. A short duration of use of UVC seems to limit the frequency of PVT. In case of PVT during the use of a UVC, removing the UVC was the first treatment and no anticoagulation therapy was required. To our knowledge, there are no evidence-based guidelines on anticoagulation treatment of neonatal PVT (prophylactic use of heparin, thrombolytic therapy, surgical thrombectomy) and its utility is still debated [15]. Complementary to previous studies, we also noticed a non-negligible proportion of PVT in children without any UVC (10%).
At 1 year of follow-up, four infants had persistent occlusive left PVT (2.8%) associated with left liver hypotrophy, still without clinical or biological significance or risk factor. It seems possible that the lower size of the left hepatic lobe could be explained by a lack of sufficient arterial supplementation. We have noticed that during the follow-up of our study, the left PVT did not extended to the main portal vein but have planned a follow-up of these infants to be sure that no delayed complications would emerge.
A limitation is the modality of PVT diagnosis. US examination was performed by one pediatric radiologist. US examination is operator-dependent. Technical factors (skill level of the radiologist, neonate cooperation, small size of the structures, the presence of abdominal gas and anatomic variations) may sometimes induce variability, but US remains a useful non-invasive bed-side examination.
Conclusion
Left portal venous thrombosis was often observed in premature newborn children. UVC was a major risk factor. We could suggest a short duration of a UVC in the central position with an US position control to limit the apparition of PVT. No other risk factor of PVT or persistent PVT was observed. In case of left PVT, a spontaneous favorable evolution occurred in more than 90% of cases, without any anticoagulation therapy, after removing the UVC. No clinical or biological effect was observed, even in case of persistent left PVT at 1 year of age.
References
Morag I, Epelman M, Daneman A, Moineddin R, Parvez B, Shechter T, et al. Portal vein thrombosis in the neonate: risk factors, course, and outcome. J Pediatr. 2006;148:735–9.
Guérin F, Bidault V, Gonzales E, Franchi‐Abella S, De Lambert G, Branchereau S. Meso‐Rex bypass for extrahepatic portal vein obstruction in children. Br J Surg. 2013;100:1606–13.
Veldman A, Nold MF, Michel-Behnke I. Thrombosis in the critically ill neonate: incidence, diagnosis, and management. Vasc Health Risk Manag. 2008;4:1337–48.
Seguin J, Fletcher MA, Landers S, Brown D, Macpherson T. Umbilical venous catheterizations: audit by the Study Group for Complications of Perinatal Care. Am J Perinatol. 1994;11:67–70.
Yadav S, Dutta AK, Sarin SK. Do umbilical vein catheterization and sepsis lead to portal vein thrombosis? A prospective, clinical, and sonographic evaluation. J Pediatr Gastroenterol Nutr. 1993;17:392–6.
Schwartz DS, Gettner PA, Konstantino MM, Bartley CL, Keller MS, Ehrenkranz RA, et al. Umbilical venous catheterization and the risk of portal vein thrombosis. J Pediatr. 1997;131:760–2.
Guimarães H, Castelo L, Guimarães J, Cardoso A, d’Orey C, Mateus M, et al. Does umbilical vein catheterization to exchange transfusion lead to portal vein thrombosis? Eur J Pediatr. 1998;157:461–3.
Oppenheimer DA, Carroll BA, Garth KE. Ultrasonic detection of complications following umbilical arterial catheterization in the neonate. Radiology. 1982;145:667–72.
Van Gansbeke D, Avni EF, Delcour C, Engelholm L, Struyven J. Sonographic features of portal vein thrombosis. AJR Am J Roentgenol. 1985;144:749–52.
Tessler FN, Gehring BJ, Gomes AS, Perrella RR, Ragavendra N, Busuttil RW, et al. Diagnosis of portal vein thrombosis: value of color Doppler imaging. AJR Am J Roentgenol. 1991;157:293–6.
Sakha SH, Rafeey M, Tarzamani MK. Portal venous thrombosis after umbilical vein catheterization. Indian J Gastroenterol. 2007;26:283–4.
Kim JH, Lee YS, Kim SH, Lee SK, Lim MK, Kim HS. Does umbilical vein catheterization lead to portal venous thrombosis? Prospective US evaluation in 100 neonates. Radiology. 2001;219:645–50.
Krafte-Jacobs B, Sivit CJ, Mejia R, Pollack MM. Catheter-related thrombosis in critically ill children: comparison of catheters with and without heparin bonding. J Pediatr. 1995;126:50–4.
Morag I, Shah PS, Epelman M, Daneman A, Strauss T, Moore AM. Childhood outcomes of neonates diagnosed with portal vein thrombosis. J Paediatr Child Health. 2011;47:356–60.
Williams S, Chan AKC. Neonatal portal vein thrombosis: diagnosis and management. Semin Fetal Neonatal Med. 2011;16:329–39.
Seibert JJ, Taylor BJ, Williamson SL, Williams BJ, Szabo JS, Corbitt SL. Sonographic detection of neonatal umbilical-artery thrombosis: clinical correlation. AJR Am J Roentgenol. 1987;148:965–8.
Konuş OL, Ozdemir A, Akkaya A, Erbaş G, Celik H, Işik S. Normal liver, spleen, and kidney dimensions in neonates, infants, and children: evaluation with sonography. AJR Am J Roentgenol. 1998;171:1693–8.
Team RC. R: A language and environment for statistical computing. Vienna, Austria; 2014. http://www.R-Proj.Org/2015
Schlesinger AE, Braverman RM, DiPietro MA. Pictorial essay. Neonates and umbilical venous catheters: normal appearance, anomalous positions, complications, and potential aid to diagnosis. AJR Am J Roentgenol. 2003;180:1147–53.
Acknowledgements
The authors thank John Sheath for English language assistance.
Author Contributions
BM, MC, AB, and DS conceptualized and designed the study, recruited patients, drafted the initial manuscript, and approved the final manuscript as submitted. MC and AF designed the data collection instruments, coordinated and supervised data collection, recruited patients for the study, and drafted the initial manuscript. CS-T, LM, AF, GF, PB, and ES recruited patients for the study, reviewed and revised the manuscript, and approved the final manuscript as submitted. BM and DS designed the data collection instruments, recruited patients for the study, coordinated and supervised data collection, critically reviewed the manuscript, and approved the final manuscript as submitted. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cabannes, M., Bouissou, A., Favrais, G. et al. Systematic ultrasound examinations in neonates admitted to NICU: evolution of portal vein thrombosis. J Perinatol 38, 1359–1364 (2018). https://doi.org/10.1038/s41372-018-0132-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-018-0132-9
- Springer Nature America, Inc.
This article is cited by
-
Epidemiology and risk factors for thrombosis in children and newborns: systematic evaluation and meta-analysis
BMC Pediatrics (2023)
-
Portal vein thrombosis and food protein-induced allergic proctocolitis in a premature newborn with hypereosinophilia: a case report
BMC Pediatrics (2021)
-
Adverse events associated with umbilical catheters: a systematic review and meta-analysis
Journal of Perinatology (2021)
-
Epidemiology of thrombosis in Canadian neonatal intensive care units
Journal of Perinatology (2020)