Abstract
Background
Evidence of the acute effects of high-level air pollution on small airway function and systemic inflammation in adults is scarce.
Objective
To examined the associations of short-term (i.e., daily) exposure to multiple air pollutants with lung function and inflammatory markers.
Methods
We assessed short-term (daily) effects of air pollutants, including particulate matter with aerodynamic diameter less than 2.5 μm (PM2.5) and 10 μm (PM10), nitrogen dioxide (NO2), sulfur dioxide (SO2) and carbon monoxide (CO), on lung function and peripheral immune cell counts over various lag times using generalized linear regression models.
Results
A total of 4764 adults were included from the general community-dwelling population in Shanghai, China. Exposure to air pollutants and lung function were negatively correlated. Decline in FEF between 25% and 75% of vital capacity (FEF25–75%) were found associated with PM2.5, SO2, and CO, and decline in forced expiratory volume in 3 s (FEV3) to forced vital capacity (FVC) ratio were associated with all examined pollutants, indicating obstruction in small airways. Obstructed airflow in large and middle airways as indicated by decline in FEV1/FVC were also associated with all pollutants. In subgroup analysis, significant negative associations between the five pollutants and SAD parameters were found only in males but not in females. The difference in the associations of SO2 with FEF75% between males and females achieved statistical significance. Additionally, all examined pollutants were significantly associated with lower peripheral neutrophil count.
Impact statement
-
Acute exposure to air pollutants were associated with airflow-limitation.
-
Both small airways and proximal airways were affected.
-
Acute exposure to air pollutants were accompanied with a lower neutrophil count.

Similar content being viewed by others
Introduction
Small airways are generally defined as airways with an internal diameter less than 2 mm [1]. Small airway dysfunction (SAD) has been shown as an important contributor to the occurrence and development of asthma [2] and chronic obstructive pulmonary disease (COPD) [3, 4], which are most common chronic airway diseases characterized by obstructive ventilation dysfunction due to reversible or irreversible airway narrowing. Notably, several prospective longitudinal studies have suggested that the development of asthma and COPD might begin with SAD without obvious symptoms in the early stage [5, 6]. A recent study reported that the overall prevalence of spirometry-defined SAD was 43.5% in China, accounting for more than 400 million people [7]. Thus, identifying modifiable risk factors and underlying mechanisms of SAD is critical to reduce the burden of disease and to prevent early lung function impairment.
Epidemiological studies have linked long-term (e.g., annual) or short-term (e.g., daily) exposure to air pollution to changes in spirometric indicators of the large and middle airways among healthy subjects and patients with asthma or COPD [8,9,10,11]. However, only a few studies have examined the chronic or acute effects of air pollution on small airways. A recent study has shown that long-term exposure to ambient fine particulate matter (particles with a diameter less than 2.5 μm, PM2.5) was a risk factor for spirometry-defined SAD in China [7]. Only one large-scale cohort study has demonstrated that acute increases in concentrations of nitrogen dioxide (NO2) and particles with a diameter less than 10 μm (PM10) were associated with SAD [12]. However, this study was conducted in a developed country with relatively low air pollution exposure levels. More evidence from developing countries is still needed to evaluate the impacts of high-levels exposure on SAD. Additionally, air pollution is a complex mixture, and therefore a comprehensive understanding of the effects of various air pollutants on lung function is essential.
The respiratory tract connects the immune system to the external atmospheric environment. Inhaled gaseous and particulate pollutants may stimulate airway epithelial cells and immune cells residing in the airway and trigger systematic immune responses involving different immune cell types, including neutrophils and eosinophils, which may contribute to the pathogenesis of air pollution-related pulmonary diseases [13, 14]. Activation of these immune cells by ambient air pollutants may be partially reflected in their peripheral blood cell counts. In other words, peripheral blood cell counts might be used to explore the associations between air pollutants and the airway inflammatory response.
In this study, we examined the associations of short-term (i.e., daily) exposure to multiple air pollutants, including PM2.5, PM10, sulfur dioxide (SO2), carbon monoxide (CO), and NO2, with lung function and inflammatory markers (i.e., peripheral white blood cell, neutrophil, and eosinophil counts). Further, we sought to identify susceptible subgroups in the study population.
Materials and Methods
Study design and participants
Data for this study was collected from permanent residents aged 20 years or older in Shanghai, China (defined as living in Shanghai for 1 year or longer), as part of a previous national cross-sectional study (the China Pulmonary Health study), details of which have been reported elsewhere [15]. Participants were recruited from the general community-dwelling population in four different communities between June 2012 and February 2014. Two of them were randomly selected from urban districts, and the others were from rural townships. Information on demographic characteristics, residential address, history of pulmonary and other chronic diseases, cigarette smoke exposure, occupational exposure, and biofuel use was recorded by a standardised questionnaire in this study.
Given that the data of PM2.5 concentrations were monitored since January 1, 2013, 449 participants who had spirometry tests prior to this date were excluded due to lack of exposure data. Another 68 subjects were excluded for missing data on smoking history. Finally, there were 4764 subjects included in the final analysis (Fig. S1). The ethics review committee of Beijing Capital Medical University approved the study (No. 11-ke-42), and all participants signed informed consent.
Spirometry test and blood cell counts
Spirometry tests were conducted as previously reported [10]. Quality-control checks for all the results of spirometry tests were performed by an experienced technician and a specialist physician. The National Lung Health Education Program (NLHEP) was followed to assure the repeatability of spirometry tests [16]. The greatest values of forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) were recorded. Other lung function parameters (i.e., forced expiratory flow (FEF) at 75% of vital capacity (FEF75%)) of the same procedure of FVC and FEV1 were recorded. All participants underwent bronchial dilation test to assess reversibility. The results of spirometry tests before bronchodilator inhalation were used for modeling. Lung function outcomes including FEV1 and FEV1/FVC as indicators for large airway obstruction, FEF75%, FEF between 25% and 75% of vital capacity (FEF25–75%), and forced expiratory flow in 3 seconds (FEV3)/FVC as indicators of small airway function [7, 17], and FVC as the indicator for restrictive ventilation dysfunction. COPD was defined as the post-bronchodilator FEV1/FVC < 70% [18]. We calculated the ratios of observed to predicted FEV1 based on US general population references and used them to stage the degree of obstruction (GOLD stage I, ≥ 80% predicted; GOLD stage II, ≥ 50% to < 80% predicted; GOLD stage III, ≥ 30% to < 50% predicted; and GOLD stage IV, < 30% predicted) [19]. On the day of the spirometry test, 10 ml of peripheral venous blood was collected for blood cell counting, including total white blood cells (WBC), neutrophils (NEU), and eosinophils (EOS), using a hematology analyzer. Counts of WBC, NEU, and EOS were used as outcomes indicating systemic inflammation.
Exposure assessment
Daily (24 h) mean air pollution concentration data, including PM2.5, PM10, NO2, SO2, and CO, from January 1, 2013 to December 31, 2014 were extracted from the Shanghai Environmental Monitoring Center (SEMC) database [20]. The daily concentrations for each pollutant of the nearest monitoring sites to the residential address were assigned to the corresponding participant. The locations of six fixed-site monitoring sites (Hongkou, Jing’an, Huangpu, Putuo, Xuhui, and Yangpu) were designed to reflect the general level of the study area. The units of concentrations for air pollutants were μg/m3 for PM2.5, PM10, NO2, and SO2 and mg/m3 for CO. In order to adjust for the potential confounding effects of weather, we also obtained daily meteorological data (i.e., ambient temperature and relative humidity) from the Shanghai Meteorological Bureau. Weather data were measured at a fixed-site meteorological station located at Hongqiao Airport in Shanghai.
Statistical analysis
Multivariable linear regression models were utilized to explore the associations of short-term exposure to air pollutants with lung function measures and blood cell counts. We adjusted for potential confounding covariates based on known associations with lung function or air pollution, including age (years), sex, body mass index (BMI), smoking history (ever-smoker or never-smoker), pack per year, marriage [12, 21](unmarried, married, divorced, or widowed), biomass use (yes or no), occupational exposure (yes or no), relative humidity, temperature, day of the week, season (winter, spring, summer and autumn), and school vacation (yes or no). Use of biomass is defined as using biomass (i.e. charcoal, coal, or coke, or burning of wood, dung, or crop residue) for more than 6 months for cooking or heating. Detailed definitions of smoking history, biomass use and occupational exposure can be found in Supplementary materials - Methods. Average exposure levels of each air pollutant were calculated at three time points: on the day of (Lag0, 0–24 h), the day before (Lag1, 24–48 h), and two days before (Lag2, 48–72 h) the spirometry test were estimated separately. A priori hypothesis of this test was that acute exposure to each air pollutant on Lag0 would have the strongest associations with lung function and blood cell counts. The results were presented as estimated changes and their 95% confidence intervals (CIs) in each lung function parameter for per interquartile range (IQR) increase in PM2.5, PM10, SO2, NO2, and CO. A sensitivity analysis was conducted by excluding COPD patients to test the results’ robustness in non-COPD population.
We also conducted stratified analyses to explore the potential effect modification by age (≥ 55 vs. < 55 years old), sex (male vs. female), and smoking history (ever-smoker vs. never-smoker) on the associations of air pollutants at Lag 0 with lung function. To test the statistical significance of the differences between stratified outcomes, we calculated the 95% CIs between subgroups as shown below: \(\hat {{{{{{{\mathcal{Q}}}}}}}}_1-\hat {{{{{{{\mathcal{Q}}}}}}}}_2 \pm 1.96 \sqrt{S\hat E^2_1 -S\hat E^2_2}.\) \(\hat {{{{{{{\mathcal{Q}}}}}}}}\)1 and \(\hat {{{{{{{\mathcal{Q}}}}}}}}\)2 are the estimates for the 2 categories, and S\({{{\hat{\mathrm E}}}}\)1 and S\({{{\hat{\mathrm E}}}}\)2 are their respective standard errors [22].
R software (version 3.4.2, R Foundation for Statistical Computing, http://cran.r-project.org/) was used in this analysis, and p < 0.05 (two-tailed) was considered statistically significant.
Results
Population and spirometry tests
The baseline characteristics of all participants are shown in Table 1. The mean (± standard deviation, SD) age of analyzed participants was 54.4 (± 12.7) years. Among them, 42.0% were male, 5.1% were current smokers, and 20.5% were former smokers. 532 participants were spirometry-defined COPD patients in this population, of whom 60.2% were at GOLD stage Ι, 32.0% at GOLD stage II, and 7.9% at GOLD stage III or IV. Among spirometry-defined COPD patients, 31 participants (5.8%) had a previous diagnosis of COPD and 252 participants (47.4%) were defined as ever smokers.
Exposure to air pollutants
Daily average concentrations of air pollutants are summarized in Table S1. The concentrations of PM2.5 at the six fixed-site monitoring stations ranged from 10 μg/m3 to 244 μg/m3. The mean (± SD) concentrations at Lag0 were 65.2 (± 47.2) μg/m3, 96.6 (± 73.2) μg/m3, 54.5 (± 26.6) μg/m3, 29.7 (± 21.6) μg/m3, and 0.90 (± 0.39) mg/m3 for PM2.5, PM10, NO2, SO2, and CO, respectively. Strong correlations (Pearson rho > 0.75) were observed between the concentrations of each pair of pollutants for Lag 0.
Associations between air pollutants and lung function
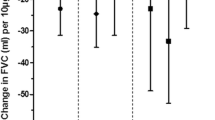
The associations between air pollutants exposure and parameters of lung function are shown in Fig. 1. For FEV1/FVC, statistically negative associations were observed with SO2 at Lag0, Lag1, and Lag2, with both PM2.5 and NO2 at Lag0 and Lag 1, with PM10 at Lag0, and with CO at Lag1. For per IQR increase in concentrations, the largest reductions on FEV1/FVC (%) were 0.510 (95% CI: 0.052, 0.969) for PM2.5 at Lag1, 0.436 (95% CI: 0.045, 0.819) for PM10 at Lag0, 0.812 (95% CI: 0.236, 1.392) for NO2 at Lag1, 1.046 (95% CI: 0.274, 1.818) for SO2 at Lag1, and 1.003 (95% CI: 0.371, 1.634) for CO at Lag1. Our results suggested that the acute associations were predominantly presented on the day of and the day before spirometry test, and diminished after 2 days. However, no associations were observed for exposure to air pollutants and FEV1 and FVC in all lags we examined.
Data were presented as estimated values ± 95% CI. The Y-axis indicate estimated changes in lung function parameters for an increase of per interquartile range (IQR) of the daily average PM2.5, PM10, NO2, SO2, and CO measured on the day of (Lag0), the day before (Lag1) and two days before (Lag2) the spirometry test.
For SAD indicators, we found PM2.5 (−38.34 mL/s [95% CI: −76.59, −0.09]), SO2 (−103.01 mL/s [95% CI: −171.74, −34.29]), and CO (−56.98 mL/s [95% CI: −108.11, −5.85]) at Lag0 were associated with reduced FEF25–75% (Fig. 1). In addition, increases in all air pollutants except for CO were associated with decreased FEV3/FVC (%) at Lag0 (−0.331 [95% CI: −0.603, −0.052] for PM2.5, −0.365 [95% CI: −0.641, −0.089] for PM10, −0.443 [95% CI: −0.784, −0.105] for NO2, and −0.564 [95% CI: −1.066, −0.066] for SO2). The negative effects of air pollutants on FEV3/FVC were also found for SO2 at Lag1 and Lag2, and for NO2 and CO at Lag1. For FEF75%, statistically negative associations were observed with SO2 at Lag2. In the sensitivity analyses, we found similar associations of air pollutants with lung function parameters among non-COPD participants (Fig. S1). None of the air pollutants were associated with lung function parameters in patient subgroups with COPD or asthma, possibly due to the limited subgroup sizes (Data not shown).
Figures 2–4 present the associations between each pollutant and lung function parameters stratified by sex, smoking history, and age. With regard to sex, negative effects were found for all pollutants on FEF75%, and PM2.5, PM10, and CO on FEF25–75% and FEV3/FVC only in males. The difference between males and females were not significant, except for in the associations of SO2 on FEF75% between males and females (p = 0.013) (Fig. 2). PM2.5, PM10, and SO2 were associated with lower FEV1/FVC in ever-smokers (Fig. 3). In the age-specific analysis, the modifications of age on associations between air pollutants and lung function were inconsistent, but it is notable that FEF25–75% in the younger patients (< 55 years old) was more susceptible to all pollutants than in the older patients (≥ 55 years old) (p < 0.05) (Fig. 4).
Fifty-five years old was the median age among the whole population. Data were presented as estimated values ± 95% CI. The Y-axis indicate estimated changes in lung function parameters for an increase of per interquartile range (IQR) of the daily average PM2.5, PM10, NO2, SO2, and CO measured on the day (Lag0) of the spirometry test.
Associations between air pollutants and inflammatory cell counts
Figure 5 shows the associations of exposure to air pollutants with blood cell counts. Increases in PM2.5, PM10, NO2, SO2, and CO at Lag0 were significantly associated with lower neutrophil counts (/µl) (−0.094 [95% CI: −0.161, −0.028] for PM2.5, −0.082 [95% CI: −0.144, −0.021] for PM10, −0.085 [95% CI: −0.166, −0.004] for NO2, −0.130 [95% CI: −0.243, −0.017] for SO2, and −0.124 [95% CI: −0.215, −0.034] for CO, respectively). In addition, increases of per IQR in PM2.5 and PM10 at Lag2 were associated with 0.136 (95% CI: 0.029,0.242) and 0.233 (95% CI: 0.105, 0.363) higher WBC counts (/µl), respectively. We did not observe any significant associations with the eosinophil percentage.
Discussion
In this study, we found that short-term exposure to PM2.5, PM10, NO2, SO2, and CO were associated with decreased FEV1/FVC, FEF 25–75%, FEF75%, and FEV3/FVC, indicating air pollution-related airflow limitation and small airway dysfunction. In subgroup analysis, significant negative associations between the five pollutants and SAD parameters were found only in males but not in females. We also found that exposure to all analyzed air pollutants on the day of the determination of lung function was correlated to lower neutrophil counts, while PM2.5 and PM10 on two days before the spirometry test were associated with higher white blood cell counts. These results added to the evidence on the adverse effects of acute exposure to multiple high-level air pollutants on lung function, especially small airway dysfunction and activation of systemic immune response.
Previous studies have aimed at the associations between air pollutants and markers of large and middle airway impairment, such as FEV1 and FVC. Previous studies demonstrated that an increase in short-term PM2.5 and PM10 was correlated to decreases in FEV1 and FVC [11]. An FEV1/FVC less than 0.7 is to evaluate individuals at risk of COPD, predicting COPD-related hospitalization and mortality. Acute exposure to NO2 but not PM10 was correlated with a decrease of FEV1/FVC ratio among nonsmoking, healthy adults [12]. In this study, we also found adverse relationships between acute exposure to air contaminants, including PM2.5, PM10, NO2, SO2, and CO, and FEV1/FVC, but not FEV1 or FVC alone. FEV1 and FVC are insensitive parameter for small airway changes and more related with restricted ventilation. In contrast, FEV1/FVC is an indicator of airflow obstruction. An FEV1/FVC less than 0.7 is to evaluate individuals at risk of COPD, predicting COPD-related hospitalization and mortality. Thus, our results suggests that the acute detrimental effects of air pollutants are related to airflow obstruction in proximal and distal airways without distinct restriction.
Evidence for the effects of acute exposure to air pollution on peripheral airways is still scarce. FEF25–75%, FEF75% and FEV3/FVC have been the most commonly cited spirometric variables as indicators of small airway obstruction [17, 23, 24]. Dauchet et al. reported that acute exposure to NO2 was correlated with a lower FEF25–75% and FEF25% and that PM10 was correlated to decrease of FEF75% in nonsmoking, healthy adults [12]. Consistently, NO2 and PM10 showed a similar albeit insignificant association with FEF25–75% and FEF75% in our study. In addition, we assessed the acute exposure to PM2.5, SO2 and CO and found that they were negatively associated with FEF25–75% and FEV3/FVC. First, for per IQR increase in concentrations of the five pollutants, the largest reductions on FEF25–75% and FEF75% were −171 ml/s and 79 ml/s, respectively, which were 6% and 8% for the mean FEF25–75% and FEF75% of 3.07 L/s and 0.98 L/s in this population. Thus, the acute exposure causes significant changes in small airways. For patients with COPD or asthma, in whom the obstruction has already exist, such acute reductions may result in exacerbation of respiratory symptoms. Second, as reported in previous longitudinal studies, development of asthma and COPD might begin with SAD without obvious symptoms in the early stage. Thus, our findings alert protecting vulnerable population from short-term extensive exposure to air pollutants to avoid related SAD.
Although the co-linearity among pollutants cannot be ruled out, most associations were found between gaseous pollutants (i.e., SO2, NO2, and CO) and lung function parameters. Substantial evidence has shown that acute exposure to these gaseous pollutants was closely associated with airway inflammation [25, 26], airway hyperresponsiveness, and bronchoconstriction in both larger and smaller airways [27], and the development and adverse outcomes of COPD [28, 29] and asthma [30,31,32]. Gaseous pollutants are smaller than particulate pollutants and therefore easily diffuse into the peripheral airways and might alter the pH of the airway mucosa, which may lead to an immediate immune inflammatory response [33, 34]. Our results highlight the need for closely monitoring and rigorously controlling of the concentrations of gaseous air pollutants to prevent chronic airway inflammatory diseases, such as COPD and asthma.
The pro-inflammatory role of air contaminants might be one of the mechanisms underlying its effects [35, 36]. A meta-analysis found that acute exposure to multiple air pollutants (PM10, PM2.5, NO2, and SO2) at high concentrations was correlated with increased fractional exhaled nitric oxide (FeNO), suggesting that the exposure of air contaminants may induce respiratory diseases via airway inflammation [37]. A recent study also reported decreased neutrophil counts after 24-hour diesel exhaust exposure, accompanied by higher levels of an activated peripheral neutrophil phenotype and increased neutrophil extracellular trap formation in the lung [38]. Blomberg et al. found increased neutrophil counts and levels of myeloperoxidase (MPO) in bronchial wash samples of healthy individuals after NO2 exposure [39]. In our analysis, we found associations between acute exposure to air contaminants and decreased peripheral neutrophils at Lag0. This may be due to recruitment of activated neutrophils to the respiratory tract upon acute exposure, leading to a decrease in neutrophil counts in peripheral blood. Consistent with previous studies, we also found that PM2.5 and PM10 were correlated with elevated leukocytes at Lag 2 [12, 40]. A lagged increase in the total white blood cell count at Lag 2 might indicate subsequent systemic inflammation and the release of white blood cells into the peripheral blood from the bone marrow [41, 42]. Acute airway inflammation and peripheral neutropenia caused by air pollutants may be an underlying mechanism of airflow limitation [43]. In our study, the blood cell counts suggested a disturbance of immune system associated with acute exposure, but were not directly related with decreased lung function parameters. Additional insights into the direct and indirect mechanistic effects of different air pollutants, especially gaseous pollutants, on neutrophil activation may be useful.
It is crucial for public health intervention to identify participants who are vulnerable to air pollution. In the Framingham study, previous-day air pollution exposure and FEV1 did not vary by smoking status [11]. In our study, we found that ever-smokers seem to be more susceptible to air contaminants with regard to FEV1/FVC, FEF25–75%, FEF75%, and FEV3/FVC than never-smokers. Our result was in line with a previous study, which found that the negative correlations between PM10, NO2, and SO2 and lung function were generally weaker among never-smokers than among smokers [44]. It is mechanically plausible given that both air pollutants and cigarette smoke could induce acute epithelial injury and neutrophilic inflammation, which further results in chronic airway inflammation [26, 45]. The susceptibility of ever-smokers may result from overlapping pro-inflammatory effects of the two detrimental factors. Males were more vulnerable to detrimental effects of acute exposure of air contaminants than females. These findings should be paid attention and the differential susceptibility to air contaminants-related SAD needs further study.
Our study has many strengths. First, this study adds to the limited evidence that multiple pollutants may reduce lung function at high exposure levels. Second, most previous studies have focused on spirometric indicators of large and middle airway dysfunction. Our results provide a more comprehensive view of the possible acute effects of air pollutants on lung function by exploring the associations between acute exposure to various air pollutants and peripheral small airways. Third, we also found an immediate decrease in peripheral neutrophils and a lagged increase in white blood cells after acute exposure to air pollutants, suggesting that acute inflammatory response may play a role in air pollutant-related airway obstruction. Finally, using the multistage randomized sampling strategy, we included participants from urban and rural areas, males and females, and adults of all ages, which may contribute to minimizing investigator bias.
This study also has several limitations. First, daily concentrations of each pollutant were measured at the nearest monitoring site to the residential address for each subject. Therefore, exposure misclassification is possible. Second, information on micro-environmental air pollution exposure and activity patterns was not collected in our study, which may have contributed to exposure measurement error. Third, air pollutants were highly correlated in this study, so it was difficult to evaluate the contribution of a single pollutant separately. In addition, the potential confounders that were not collected initially could not be adjusted for in the statistical models, such as diets and exercise. Forth, airway resistance was not measured in this study. Finally, whether the inflammatory response relates temporally to specific alterations in lung function would been elucidated in the following study.
Conclusions
In conclusion, our findings suggested that acute exposure to high-level air pollution was correlated with impaired lung function, including dysfunction in small airways. The peripheral neutrophil count was also negatively associated with air pollutants, indicating inflammation caused by air pollution exposure. Our results underline the acute effects of air pollutants on small airways. Further investigation is needed to understand the role of air pollution-induced SAD in chronic lung diseases, including COPD and asthma.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Macklem PT. The physiology of small airways. Am J Respir Crit Care Med. 1998;157:S181–3.
Usmani OS, Singh D, Spinola M, Bizzi A, Barnes PJ. The prevalence of small airways disease in adult asthma: A systematic literature review. Respir Med. 2016;116:19–27.
Boeck L, Gensmer A, Nyilas S, Stieltjes B, Re TJ, Tamm M, et al. Single-Breath Washout Tests to Assess Small Airway Disease in COPD. Chest 2016;150:1091–100.
Incorvaia C, Riario-Sforza GG, Pravettoni C, Yacoub MR, Frati F. Impairment of small airways in COPD patients with frequent exacerbations and effects of treatment with tiotropium. Int J Chron Obstruct Pulmon Dis. 2008;3:123–6.
Stockley JA, Ismail AM, Hughes SM, Edgar R, Stockley RA, Sapey E. Maximal mid-expiratory flow detects early lung disease in α(1)-antitrypsin deficiency. Eur Respir J. 2017;49:1602055.
Skylogianni E, Triga M, Douros K, Bolis K, Priftis KN, Fouzas S, et al. Small-airway dysfunction precedes the development of asthma in children with allergic rhinitis. Allergol Immunopathol (Madr). 2018;46:313–21.
Xiao D, Chen Z, Wu S, Huang K, Xu J, Yang L, et al. Prevalence and risk factors of small airway dysfunction, and association with smoking, in China: findings from a national cross-sectional study. Lancet Respir Med. 2020;8:1081–93.
Lepeule J, Litonjua AA, Coull B, Koutrakis P, Sparrow D, Vokonas PS, et al. Long-term effects of traffic particles on lung function decline in the elderly. Am J Respir Crit Care Med. 2014;190:542–8.
Rice MB, Ljungman PL, Wilker EH, Dorans KS, Gold DR, Schwartz J, et al. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham heart study. Am J Respir Crit Care Med. 2015;191:656–64.
Hou D, Ge Y, Chen C, Tan Q, Chen R, Yang Y, et al. Associations of long-term exposure to ambient fine particulate matter and nitrogen dioxide with lung function: A cross-sectional study in China. Environ Int. 2020;144:105977.
Rice MB, Ljungman PL, Wilker EH, Gold DR, Schwartz JD, Koutrakis P, et al. Short-term exposure to air pollution and lung function in the Framingham Heart Study. Am J Respir Crit Care Med. 2013;188:1351–7.
Dauchet L, Hulo S, Cherot-Kornobis N, Matran R, Amouyel P, Edmé JL, et al. Short-term exposure to air pollution: Associations with lung function and inflammatory markers in non-smoking, healthy adults. Environ Int. 2018;121:610–9.
Glencross DA, Ho TR, Camiña N, Hawrylowicz CM, Pfeffer PE. Air pollution and its effects on the immune system. Free Radic Biol Med. 2020;151:56–68.
Salvi S, Blomberg A, Rudell B, Kelly F, Sandström T, Holgate ST, et al. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med. 1999;159:702–9.
Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391:1706–17.
Ferguson GT, Enright PL, Buist AS, Higgins MW. Office spirometry for lung health assessment in adults: a consensus statement from the National Lung Health Education Program. Respir Care. 2000;45:513–30.
Morris ZQ, Coz A, Starosta D. An isolated reduction of the FEV3/FVC ratio is an indicator of mild lung injury. Chest 2013;144:1117–23.
Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65.
Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87.
Cai J, Zhao A, Zhao J, Chen R, Wang W, Ha S, et al. Acute effects of air pollution on asthma hospitalization in Shanghai, China. Environ Pollut. 2014;191:139–44.
Ploubidis GB, Silverwood RJ, DeStavola B, Grundy E. Life-Course Partnership Status and Biomarkers in Midlife: Evidence From the 1958 British Birth Cohort. Am J Public Health. 2015;105:1596–603.
Chen R, Kan H, Chen B, Huang W, Bai Z, Song G, et al. Association of particulate air pollution with daily mortality: the China Air Pollution and Health Effects Study. Am J Epidemiol. 2012;175:1173–81.
Cirillo I, Klersy C, Marseglia GL, Vizzaccaro A, Pallestrini E, Tosca M, et al. Role of FEF25%-75% as a predictor of bronchial hyperreactivity in allergic patients. Ann Allergy, Asthma Immunol: Off Publ Am Coll Allergy, Asthma, Immunol. 2006;96:692–700.
Xiao D, Chen Z, Wu S, Huang K, Xu J, Yang L, et al. Prevalence and risk factors of small airway dysfunction, and association with smoking, in China: findings from a national cross-sectional study. Lancet Respir Med. 2020;8:1081–1093.
Ji X, Han M, Yun Y, Li G, Sang N. Acute nitrogen dioxide (NO2) exposure enhances airway inflammation via modulating Th1/Th2 differentiation and activating JAK-STAT pathway. Chemosphere 2015;120:722–8.
Wigenstam E, Elfsmark L, Bucht A, Jonasson S. Inhaled sulfur dioxide causes pulmonary and systemic inflammation leading to fibrotic respiratory disease in a rat model of chemical-induced lung injury. Toxicology 2016;368-369:28–36.
Lazarus SC, Wong HH, Watts MJ, Boushey HA, Lavins BJ, Minkwitz MC. The leukotriene receptor antagonist zafirlukast inhibits sulfur dioxide-induced bronchoconstriction in patients with asthma. Am J Respir Crit Care Med. 1997;156:1725–30.
Santus P, Russo A, Madonini E, Allegra L, Blasi F, Centanni S, et al. How air pollution influences clinical management of respiratory diseases. A case-crossover study in Milan. Respir Res. 2012;13:95.
Doiron D, de Hoogh K, Probst-Hensch N, Fortier I, Cai Y, De Matteis S, et al. Air pollution, lung function and COPD: results from the population-based UK Biobank study. Eur Respir J. 2019;54:1802140.
Sunyer J, Basagaña X, Belmonte J, Antó JM. Effect of nitrogen dioxide and ozone on the risk of dying in patients with severe asthma. Thorax 2002;57:687–93.
Poynter ME, Persinger RL, Irvin CG, Butnor KJ, van Hirtum H, Blay W, et al. Nitrogen dioxide enhances allergic airway inflammation and hyperresponsiveness in the mouse. Am J Physiol Lung Cell Mol Physiol. 2006;290:L144–52.
Canova C, Torresan S, Simonato L, Scapellato ML, Tessari R, Visentin A, et al. Carbon monoxide pollution is associated with decreased lung function in asthmatic adults. Eur Respir J. 2010;35:266–72.
Sekiguchi K, Yasui F, Fujii E. Capturing of gaseous and particulate pollutants into liquid phase by a water/oil column using microbubbles. Chemosphere 2020;256:126996.
Nováková Z, Novák J, Kitanovski Z, Kukučka P, Smutná M, Wietzoreck M, et al. Toxic potentials of particulate and gaseous air pollutant mixtures and the role of PAHs and their derivatives. Environ Int. 2020;139:105634.
Brunekreef B, Holgate ST. Air pollution and health. Lancet 2002;360:1233–42.
Li Y, Rittenhouse-Olson K, Scheider WL, Mu L. Effect of particulate matter air pollution on C-reactive protein: a review of epidemiologic studies. Rev Environ Health. 2012;27:133–49.
Chen X, Liu F, Niu Z, Mao S, Tang H, Li N, et al. The association between short-term exposure to ambient air pollution and fractional exhaled nitric oxide level: A systematic review and meta-analysis of panel studies. Environ Pollut. 2020;265:114833.
Wooding DJ, Ryu MH, Li H, Alexis NE, Pena O, Carlsten C. Acute air pollution exposure alters neutrophils in never-smokers and at-risk humans. Eur Respir J. 2020;55:1901495.
Blomberg A, Krishna MT, Helleday R, Söderberg M, Ledin MC, Kelly FJ, et al. Persistent airway inflammation but accommodated antioxidant and lung function responses after repeated daily exposure to nitrogen dioxide. Am J Respir Crit Care Med. 1999;159:536–43.
Green R, Broadwin R, Malig B, Basu R, Gold EB, Qi L, et al. Long- and short-term exposure to air pollution and inflammatory/hemostatic markers in midlife women. Epidemiology 2016;27:211–20.
Sakai M, Sato Y, Sato S, Ihara S, Onizuka M, Sakakibara Y, et al. Effect of relocating to areas of reduced atmospheric particulate matter levels on the human circulating leukocyte count. J Appl Physiol. 2004;97:1774–80.
Swiston JR, Davidson W, Attridge S, Li GT, Brauer M, van Eeden SF. Wood smoke exposure induces a pulmonary and systemic inflammatory response in firefighters. Eur Respir J. 2008;32:129–38.
Brightling C, Greening N. Airway inflammation in COPD: Progress to precision medicine. Eur Respir J. 2019;54:1900651.
Havet A, Hulo S, Cuny D, Riant M, Occelli F, Cherot-Kornobis N, et al. Residential exposure to outdoor air pollution and adult lung function, with focus on small airway obstruction. Environ Res. 2020;183:109161.
Pouwels SD, Zijlstra GJ, van der Toorn M, Hesse L, Gras R, Ten Hacken NH, et al. Cigarette smoke-induced necroptosis and DAMP release trigger neutrophilic airway inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2016;310:L377–86.
Funding
This study was supported by National key R&D plan (2020YFC2003700, 2022YFC3703000), The National Natural Science Foundation of China (81800008, 82041003), Science and Technology Commission of Shanghai Municipality (20DZ2261200, 20Z11901000, 20XD1401200), Shanghai Municipal Health Commission and Shanghai Municipal Administrator of Traditional Chinese Medicine(ZY(2021-2023)-0207-01), and Shanghai Municipal Key Clinical Specialty (shslczdzk02201).
Author information
Authors and Affiliations
Contributions
TW contributed substantially to data collection, patient management, statistical analysis and interpretation, and the writing of the manuscript. CC performed guided the design and contributed substantially to data collection, patient management, statistical analysis, and interpretation. YY, LL, JW, and MY contributed to the acquisition, analysis, or interpretation of data; HK contributed to study design, exposure assessment, and statistical analysis and interpretation. DY contributed to study design, statistical analysis and interpretation, and the writing of the manuscript. YS contributed to administrative, technical, and material support and to critical revision of the manuscript for important intellectual content. JC contributed to study design, exposure assessment, statistical analysis and interpretation and the writing of the manuscript. DH contributed substantially to the study design, data collection, statistical analysis and interpretation, and the writing of the manuscript. All authors read and approved the final manuscript. The corresponding authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The ethics committee of Beijing Capital Medical University approved the study (No. 11-ke-42), and all participants signed informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, T., Chen, C., Yang, Y. et al. Associations between short-term exposure to ambient air pollution and lung function in adults. J Expo Sci Environ Epidemiol (2023). https://doi.org/10.1038/s41370-023-00550-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41370-023-00550-0
- Springer Nature America, Inc.