Abstract
Objectives
We hypothesised that maternal diet-induced-obesity has adverse consequences for offspring energy expenditure and susceptibility to obesity in adulthood, and that the prebiotic polydextrose (PDX) would prevent the consequences of programming by maternal obesity.
Methods
Female mice were fed a control (Con) or obesogenic diet (Ob) for 6 weeks prior to mating and throughout pregnancy and lactation. Half the obese dams were supplemented with 5% PDX (ObPDX) in drinking water throughout pregnancy and lactation. Offspring were weaned onto standard chow. At 3 and 6 months, offspring energy intake (EI) and energy expenditure (EE by indirect calorimetry) were measured, and a glucose-tolerance test performed. Offspring of control (OffCon), obese (OffOb) and PDX supplemented (OffObP) dams were subsequently challenged for 3 weeks with Ob, and energy balanced reassessed. Potential modifiers of offspring energy balance including gut microbiota and biomarkers of mitochondrial activity were also evaluated.
Results
Six-month-old male OffOb demonstrated increased bodyweight (BW, P < 0.001) and white adipose tissue mass (P < 0.05), decreased brown adipose tissue mass (BAT, P < 0.01), lower night-time EE (P < 0.001) versus OffCon, which were prevented in OffObP. Both male and female OffOb showed abnormal glucose-tolerance test (peak [Glucose] P < 0.001; AUC, P < 0.05) which was prevented by PDX. The Ob challenge resulted in greater BW gain in both male and female OffOb versus OffCon (P < 0.05), also associated with increased EI (P < 0.05) and reduced EE in females (P < 0.01). OffObP were protected from accelerated BW gain on the OB diet compared with controls, associated with increased night-time EE in both male (P < 0.05) and female OffObP (P < 0.001). PDX also prevented an increase in skeletal muscle mtDNA copy number in OffOb versus OffCon (P < 0.01) and increased the percentage of Bacteroides cells in faecal samples from male OffObP relative to controls.
Conclusions
Maternal obesity adversely influences adult offspring energy balance and propensity for obesity, which is ameliorated by maternal PDX treatment with associated changes in gut microbiota composition and skeletal muscle mitochondrial function.
Similar content being viewed by others
Introduction
Maternal obesity constitutes the most common obstetric risk factor in developed countries with direct implications not only for maternal and neonatal morbidity and mortality but also for increased risk of obesity in the next generation [1,2,3]. Mother–child cohort studies suggest the acquisition of obesogenic traits from mother via an undefined association between maternal body mass index (BMI) in pregnancy and risk of obesity in childhood and beyond [2]. Increasing experimental evidence suggests that exposure to maternal obesity in utero and during lactation, especially maternal hyperglycaemia and insulin resistance [4] associated inflammation and metabolic dysfunction, may contribute to this relationship [5], impacting Global Sustainable Development Goals, in terms of health and wellbeing of current and future generations [6]. Interventions are therefore urgently sought.
In view of this unmet clinical need, we have investigated the potential of a dietary supplement to improve the maternal metabolic profile in obese pregnant mice and thereby prevent deleterious effects on offspring metabolism, inflammation and energy balance. Polydextrose (PDX) is a low calorie, neutral tasting, condensation polymer of D-glucose, sorbitol, and citric acid, which is water soluble, resistant to digestion in the small intestine, but partially fermented by endogenous microbiota in the large intestine, leading to its classification as a soluble dietary fibre [7]. Randomised placebo-controlled trials and two recent meta-analysis of studies in adult humans have reported increased satiety, and improved glucose homoeostasis and lipid profiles with PDX supplementation [7,8,9,10,11]. Therefore, PDX supplementation in obese women offers the potential to improve metabolic profile and inflammation during pregnancy to positively impact on the developing offspring [12, 13].
We have previously reported cardiometabolic dysfunction in the offspring of mice with diet-induced obesity [14,15,16,17]. In this study we have addressed the effect of obesity and PDX supplementation on offspring metabolic function, with a focus on energy balance, both intake and expenditure. Energy expenditure has been relatively under-explored, in models of maternal/offspring obesity. A recent meta-analysis addressing the effect of maternal obesogenic diets in rodents on offspring food intake and body mass concluded that, overall, effects on appetite are modest, whereas the increase in offspring bodyweight are consistent with permanent alterations in metabolism [18].
Materials and methods
Animal husbandry
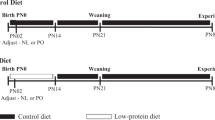
All studies were approved locally by the Animal Welfare and Ethics Committee (AWERB) and were conducted under UK Home Office License (Taylor, PPL 70/7090). Power calculations were performed based on previous in vivo data to estimate sample size. Female C57BL/6J mice were fed either a standard chow diet (RM1, Special Dietary Services, UK) or a semisynthetic obesogenic diet (approx. 16% fat, 33% simple sugars, 15% protein, total energy 16.7 kJ/g (4.0 kcal/g), as previously described [14] (Supplementary methods and Supplementary Table 1). Following successful mating, a subgroup of obesogenic diet-fed dams were randomly assigned to supplementation with PDX (5% w/v) in the drinking water throughout gestation and lactation (n = 34), generating three experimental groups; control (Con); obese (Ob) and obese + PDX (ObP, Fig. 1). This concentration of PDX has previously proven efficacious in reducing insulin resistance in adult non-pregnant rats, without adverse effects or alteration in calorific intake [19].
Female dams were fed either an obesogenic (n = 34) or a control diet (n = 18). Following successful mating a subgroup of obese dams were supplemented with 5% PDX (n = 12) in the drinking water. All offspring were weaned onto control diet and were followed up to 6 months. I male and 1 female was studied at each time point: 30 days, 3 months and 6 months. At 3 months after recording baseline characteristics, 1 male and 1 female from each litter (n = 10) were exposed to the obesogenic diet for 3 weeks and reassessed.
Offspring of control dams (OffCon), obese dams (OffOb) and obese PDX supplemented dams (OffObP) were weaned and maintained on standard chow, and one male and one female from each litter studied at time points 30 days, 3 and 6 months of age. Therefore, no evaluation included more than one subject of each sex from each litter.
Indirect calorimetry
Energy expenditure (EE), respiratory exchange ratio (RER) and food intake in the offspring, were measured using LabMaster® Automated Home Cage Phenotyping (TSE Systems, Bad Homburg, Germany).
Organ collection
At each time point, animals were killed by rising concentration of CO2 or cervical dislocation, in accordance with Schedule 1 of UK Home Office guidelines. All animals were sacrificed mid-morning, blood was taken by cardiac puncture, organs were removed and immediately snap frozen in liquid nitrogen for deoxyribonucleic acid (DNA) extraction and the fat pads (perineal, gonadal, inguinal and subcutaneous) and the skeletal muscle tibialis anterior were weighed.
Glucose-tolerance test
PDX has been shown to improve glucose tolerance in mice [20]. To determine whole body glucose tolerance, an intra-peritoneal glucose-tolerance test (i.p.GTT) was performed in the dams at gestational day 16 (GD16) and in the offspring at 30, 90 and 180 days of age. Animals were injected (i.p.) with a glucose load (1 g/kg; 10% glucose solution). Blood glucose was measured at 15, 30, 60 and 120 min after glucose injection using an AlphaTRAK® Glucose meter (Abbott Animal Health).
Cytokine profile
To assess the impact of PDX on inflammatory cytokines in obese pregnancy, a subgroup of dams (n = 5) were killed at gestational day 16, by a rising concentration of CO2 and maternal blood samples were taken by cardiac puncture and serum stored at −80 °C. Twenty-four adipocytokines were measured from pooled serum samples, using a Proteome Profiler Mouse Adipokine Array kit (R&D Systems) (see Supplementary methods for details).
Obesogenic dietary challenge
In a separate cohort of offspring (OffCon, and OffObP) at 3 months of age, 1 male and 1 female from each litter were provided ad libitum access to the maternal obesogenic diet (see above) for 3 weeks, to assess the impact of an obesogenic dietary challenge on the adult phenotype.
Quantitative real-time PCR
Greater brown fat distribution and activation may influence energy expenditure due to increased metabolic activity. We therefore evaluated expression of relevant brown fat genes (see Supplementary Table S3 for primers and sequences). Total RNA was extracted from brown adipose tissue (BAT) samples with the RNeasy mini kit (QIAGEN). RNA (1 μg) was reverse transcribed into cDNA with the Superscript II kit (Invitrogen). Semi-quantitative real-time PCR with SYBR Green JumpStart Taq ReadyMix (Sigma-Aldrich) was used to detect and amplify target cDNA. Relative gene expression was calculated using the ∆∆ Ct method. Genes of interest were normalised to the housekeeping gene Cyclophilin B.
Mitochondrial DNA copy number in offspring skeletal muscle
MtDNA content varies between different cell types depending on the bioenergetic needs, but can also change in response to physiological stimuli, leading to alterations of mtDNA being employed as a biomarker of mitochondrial dysfunction [21, 22]. Total genomic DNA was isolated from skeletal muscle using the DNeasy blood and tissue kit (Qiagen, UK) according to the manufacturer’s guidelines, and treated by sonication to minimise effects of dilution bias. Absolute mtDNA copy number was determined by real-time qPCR. The primers (see Supplementary Table 2 for sequences) used were specific to mouse mitochondrial and nuclear genome targets (mMitoF1/R1 and mB2MF1/R1, respectively), as detailed previously [22].
Analysis of offspring faecal microbiota
Since PDX is hypothesised to influence the maternal microbiome [13] with vertical transfer to neonates, we investigated broad-spectrum faecal microbiota profiles in offspring at weaning, 3 months and 6 months of age. Offspring faecal samples were snap frozen and stored at −80 °C. Samples were quantified for broad-spectrum gut bacterial species using probes targeting six phylogenetic groups (for detailed methods, targets and specific probes see Supplementary methods). Phylogenetic characterisation was performed using 16S rRNA in situ hybridisation and whole cell fluorescence in situ hybridisation (FISH) combined with flow cytometry as described by Rigottier-Gois and colleagues [23] employing 16S rRNA-targeted oligonucleotide probes, and targets for rRNA dot-blot hybridisation (Panel of group- and species-specific 16S rRNA-targeted oligonucleotide probes, Supplementary Table S4).
Statistical analysis
Data are expressed as means ± SEM. Statistical analysis was performed with GraphPad Prism 5 (GraphPad Software Inc., San Diego, California, USA). When comparing more than two groups, one-way ANOVA followed by Bonferroni post hoc test was employed. When comparing two groups, Student’s t-test was used. Normal distributions and equality of variance between groups were checked by visual inspection of scatter plots. Statistical significance was considered when P value < 0.05. χ2 test was used to test differences in reproductive outcomes between experimental groups.
Results
Maternal characteristics
Bodyweight, food intake in pregnancy
There was no difference in gestational weight gain or calorific intake during gestation between the obese dams and the obese dams supplemented with PDX (Fig. 2a, b).
a Gestational bodyweights and b calorific intake during gestation in obese (Ob) and obese supplemented with PDX (ObP) dams (n = 6–7). c Response to a glucose-tolerance test (GTT) and the respective area under the curve (AUC) on GD16. d Litter size and e birth weight in control (Con), obese (Ob) and obese supplemented with PDX (ObP) dams (n = 8–16). f Cytokine profile from late gestation obtained by two samples of pooled serum samples (n = 5 per pool) from dams at day 16. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, comparison with the obese group.
Glucose tolerance in pregnancy
The obese dams receiving PDX demonstrated improved glucose tolerance (Fig. 2c) and a reduced area under the glucose curve (AUC) 2 h after the i.p. glucose load compared to the obese dams GD16 (Fig. 2c).
Reproductive success
Maternal obesity affected both fertility and pup survival rates and was associated with increased rates of cannibalism in the obese dams. Control dams had 89% successful pregnancies and only 6% cannibalization compared to 44% and 18%, respectively, for obese dams. Administration of PDX in obese pregnant and lactating dams improved fertility rates by 14% and reduced cannibalization of the newborn pups (P < 0.05, Chi-squared test, data not shown).
Maternal cytokine profile at gestational day 16
Inflammatory markers were decreased in obese dams following PDX dietary supplementation; notably, TNF-α and CSF-1 showed a 4-and 3-fold decrease, respectively (Fig. 2f).
Offspring characteristics
Birth weight and litter size
There was no influence of maternal obesity or PDX on the birth weight of offspring. There was a reduction in the litter size due to maternal obesity, which was partially reversed by maternal dietary supplementation with PDX during pregnancy (Fig. 2d, e).
Body composition, energy balance and glucose tolerance at 30 days and 3 months
At 30 days of age there was no difference in bodyweight, calorific intake, EE or glucose tolerance between offspring of obese and lean dams (data not shown).
At 3-months-of age, offspring did not differ between groups in bodyweight or body composition (fat pad mass) or in calorific intake (data not shown).
Following i.p.GTT at 3 months, male OffOb showed an increase in the peak blood glucose concentration compared to OffCon although the area under the glucose curve was not different from OffCon (Fig. 3a, inset). In female OffOb, both peak blood glucose concentration after 15 min and AUC were elevated compared to OffCon. Maternal dietary supplementation of PDX resulted in lower peak blood glucose concentration in female OffObP at 15 min compared with OffOb (Fig. 3a).
a Response to a glucose-tolerance test (GTT) and the respective area under the curve (AUC, inset). Average energy expenditure (EE) and RER during daytime or nighttime (as indicated) in male (b) and female (c) offspring of control (OffCon), obese (OffOb) and obese dams supplemented with PDX (OffObP) at 3 months of age, n = 6–15. Data are expressed as mean ± SEM. *P < 0.05; **P < 0.01 versus OffCon. ♯P < 0.05 male OffObP vs. OffOb.
There was no effect of maternal obesity on male or female 3-month-old offspring EE compared to controls. However, maternal dietary PDX supplementation in obese dams was associated with an increase in EE in male OffObP compared to OffOb during both day and nighttime (Fig. 3b). There was no effect of maternal PDX supplementation on EE in female OffOb (Fig. 3c).
Both male and female OffOb showed a significant reduction in respiratory exchange ratio compared to OffCon, which was not observed in female OffObP (Fig. 3b, c).
Body composition, energy balance and glucose tolerance at 6 months of age
Bodyweight of 6-month-old male OffOb was increased compared to OffCon. Maternal dietary supplementation with PDX was associated with a reduction in bodyweight in male OffObP only (P < 0.001, Fig. 4a).
Average a bodyweight, b weight of white adipose tissue (WAT) and c brown adipose tissue corrected for bodyweight. d GTT with AUC inset. e Day-time energy expenditure and f night-time energy expenditure in male and female offspring of control (OffCon), obese (OffOb) and obese dams supplemented with PDX (OffObP) at 6 months of age (n = 6–7). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
The increase in male OffOb bodyweight was reflected in greater white adipose tissue (WAT) mass (Fig. 4b) compared to OffCon, with an increase in the visceral fat pad mass (mesenteric fat mass [g]: OffOb: 0.92 ± 0.08, n = 6 versus, OffCon 0.65 ± 0.03, n = 7, P < 0.05). Maternal PDX supplementation prevented the rise in male offspring WAT mass and mesenteric fat mass secondary to maternal obesity (Fig. 4b, mesenteric fat mass [g]: OffOb 0.65 ± 0.03, n = 7, versus OffObP 0.51 ± 0.06, n = 7, P < 0.05).
Male OffOb had decreased BAT compared with OffCon (Fig. 4c) when corrected for bodyweight. Maternal dietary PDX supplementation normalised BAT weight relative to controls and resulted in male offspring with higher BAT weight compared to OffOb.
Both male and female OffOb demonstrated a greater peak glucose concentration in response to a glucose load (i.p.GTT, Fig. 4d) and a greater AUC compared with OffCon. Maternal dietary PDX supplementation normalised offspring glucose profiles following the GTT (Fig. 4d).
Maternal obesity resulted in lower EE in male OffOb during day and night compared with OffCon. Maternal PDX supplementation prevented the reduced EE associated with maternal obesity during both the active night-phase and the day-time rest-phase. There was no difference in EE between the female offspring at 6 months (Fig. 4e, f).
Obesogenic dietary challenge
Bodyweight
Male and female OffOb had greater bodyweight after 3 weeks’ exposure to the obesogenic dietary challenge than similarly challenged OffCon. The exaggerated weight gain in both male and female OffOb on the obesogenic diet was prevented by maternal dietary PDX supplementation (Fig. 5a).
Offspring phenotype at 3 months after 3 weeks on the obesogenic diet. a Bodyweights and b average daily calorific intake. c Energy expenditure and d RER in male and female offspring of control (OffCon), obese (OffOb) and obese dams supplemented with PDX (OffObP) at 3 months of age and after 3-weeks exposure to obesogenic diet, n = 7–10. Exposure to obesogenic diet (OD) and maternal diet significantly accounted for variation (two-way ANOVA). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
Energy intake
Calorific intake increased across all offspring groups following the obesogenic dietary challenge. Female, but not male, OffOb (Fig. 5b) increased calorific intake by 25% compared with OffCon fed the same hyper-calorific diet. Maternal dietary supplementation with PDX in the obese dams prevented the increased food intake in females on the high calorie diet.
Energy expenditure
Male and female offspring, in all experimental groups, showed decreased EE during their active (nighttime) phase following the high fat dietary challenge. An observed reduction in EE after dietary challenge in adult male and female OffOb offspring compared to control was prevented by maternal PDX (Fig. 5c) such that OffObP was similar to control.
Respiratory exchange ratio
The dietary challenge normalised respiratory exchange ratio across all groups, such that male and female OffOb no longer showed the reduction in RER observed at baseline (Fig. 5d).
Skeletal muscle mitochondrial DNA copy number
Mitochondrial DNA copy number was investigated as a potential determinant of the observed reduction in energy expenditure and glucose tolerance in OffOb. At 30 days of age, prior to any phenotypic change in the OffOb, MtDNA copy number ratio in male OffOb skeletal muscle was markedly increased compared to controls (Fig. 6a). This was prevented by maternal PDX supplementation, such that OffObP males were similar to control. There was no significant effect of maternal diet on mitochondrial copy number ratio in female OffObP, although when sexes were combined there was a highly significant effect of maternal obesity on offspring skeletal muscle Mt/N ratio at 30 days, which was prevented by PDX.
a MtDNA copy number in skeletal muscle from male and female offspring of control (OffCon), obese (OffOb) and obese dams supplemented with PDX (OffObP) at 30 days of age (n = 5–6). b mRNA expression of brown adipose tissue biomarkers of BAT activity at at 30 days of age. c Percentage of bacteroides (Bac+) and d Eubacterium rectale–Clostridium coccoides (Erec+) in bacteria cells (EUB+) identified in faecal samples from male and female offspring of control (OffCon), obese (OffOb) and obese dams supplemented with PDX (OffObP) at c weaning and d 6 months of age (n = 6). e Representative FACS plot. EUB+ and gated cells. FL1 histogram, green fluorescence is the total number of bacteria hybridising with the EUB 338-FITC probe. FL4 histogram, red fluorescence, shows the proportion of cells targeted by the group Cy5-probe in the sample. Data are expressed as mean ± SEM. *P < 0.05; **P < 0.01, ***P < 0.001.
Biomarkers of brown fat activation
An increase in Dio2 mRNA expression in 6-month-old male OffOb, a gene encoding Type 2 iodothyronine deiodinase involved in thermogenesis, was prevented by maternal dietary PDX supplementation. Pgc-1a mRNA expression (Peroxisome proliferator-activated receptor gamma coactivator 1-alpha) was increased in female OffOb and was similarly prevented by maternal PDX (Fig. 6b). Mitochondrial UCP-1 involved in non-shivering thermogenesis was upregulated in male OffObP compared to OffCon (Fig. 6b) but unaffected by maternal obesity alone.
Gut microbiota—faecal analysis of broad-spectrum gut bacterial species
Maternal dietary PDX supplementation increased the percentage of Bacteroides in the male offspring bacterial population compared to OffCon at weaning (Fig. 6c).
In 6-month-old offspring of obese dams the microbiota showed marked differences compated with controls (Fig. 6c). Male and female OffOb demonstrated a higher percentage of Eubacterium rectale–Clostridium coccoides group compared with OffCon. There was no apparent influence of maternal PDX treatment on OffObP at 6 months.
Discussion
Here we report, in a mouse model, the influence of maternal obesity on offspring energy expenditure and the potential therapeutic benefit of maternal dietary intervention with the prebiotic polydextrose. Our main findings were firstly, that PDX improves glycaemic control and reproductive function in obese pregnancy, without affecting calorific intake or gestational weight gain; secondly, that maternal PDX treatment improves glucose homoeostasis in both male and female offspring; and thirdly, that maternal PDX treatment prevents offspring weight gain, via sex specific changes in energy intake and energy expenditure. Lastly, maternal PDX supplementation provided protection against the effects of an obesogenic diet in adulthood.
Maternal phenotype
Polydextrose has been shown to improve adult glucose metabolism [24] but not previously in pregnant women or obese pregnant animals. In the present study obese dams showed greatly improved glucose tolerance after supplementation with PDX. This was associated with an improvement in inflammatory cytokine profile in late gestation. Maternal glycaemia (and foetal hyperinsulinaemia) together with inflammatory mediators have been implicated in life-long obesity risk through the altered foetal hypothalamic neurodeveopment leading to disturbance of anabolic, adipogenic and neurotrophic pathways and permanent influences on metabolic and physiological development [25,26,27].
PDX also improved reproductive success in obese pregnant mice, with beneficial effects on fertility and litter size. Obesity perturbs the hypothalamic–pituitary–gonadal axis and ovarian cycle, reducing FSH and LH in the follicular and ovulatory phase while also shortening the luteal phase to reduce progesterone levels. It is possible, therefore, that PDX, either directly or indirectly, may influence reproductive hormones in gestation to improve reproductive capacity [28].
Effect of maternal obesity on offspring body composition and glucose tolerance
Human cohort studies demonstrate that maternal overweight and obesity is associated with greater adiposity in offspring [5, 29]. We found that male offspring were heavier with increased WAT mass and reduced BAT mass at 6 months of age. The impaired glucose tolerance observed in both males and females, at 3 months, antedates any observed changes in body composition (BIA) suggesting an alternative cause, potentially pancreatic beta cell dysfunction previously implicated in this model [30] or the early changes in mitochondrial function observed.
Effect of maternal obesity and PDX on offspring energy expenditure
In this study we present novel evidence for the developmental programming of altered EE secondary to maternal diet-induced obesity, and prevention by maternal PDX supplementation. PDX influenced EE from as early as 3 months of age, preceding the subsequent changes in body composition, without affecting energy intake. Previously the scant literature in this area includes demonstration of reduced EE in 6-month-old infants born to overweight and obese mothers [31], in genetically altered mice following intrauterine exposure to gestational diabetes [32], and in 30-day-old offspring of severely obesity rats [33]. Changes in both RER and EE were associated with hepatic mitochondrial dysfunction, with reduced PGC-1α mRNA expression, and impaired fatty acid oxidation [33]. Taken together, these findings suggest impaired nutrient sensing and fuel switching in offspring of obese dams. Compromised fatty acid oxidation would be consistent with the development of a fatty liver phenotype which we have previously described in this rodent model [15, 17].
Response to an obesogenic environment in adulthood: energy intake on the obesogenic diet
Female offspring of obese dams demonstrated hyperphagia secondary to maternal obesity only when exposed to obesogenic dietary challenge, suggesting programming of sex specific effects on food preference, and implicating mesolimbic reward pathways [34]. Perinatal ‘junk food’ exposure similarly increases the preference for palatable diets in juvenile and adult rat offspring, and we previously reported reduced Mυ-opioid receptor expression in the ventral tegmental area (VTA) of female ‘junk-food’ offspring only [35, 36]. Moreover, we have previously reported in the offspring of obese rats, structural and functional deficits in neuronal development in the hypothalamic arcuate and paraventricular nucleus associated with leptin resistance and hyperphagia [37]. Prevention of female hyperphagia by maternal PDX supplementation, therefore, could imply protection of central neurotrophic development in the neonatal brain.
Male offspring of obese mice had lower energy expenditure than controls. In man, a blunted glucose-induced thermogenesis has been observed in obese individuals, increasing susceptibility to obesity when consuming diets rich in sugars [38,39,40]. Since OffOb males were not obese at 3 months, a programmed deficit in diet-induced thermogenesis or central insulin resistance at the level of the hypothalamus could underlie the reduction in night-time EE during the obesogenic dietary challenge. Reduced physical activity can also play a role in reduced EE, however, this is unlikely in the murine model employed here, since we have previously reported that male offspring of obese mice have a hyperactive ADHD-like phenotype [41].
Mitochondrial biogenesis and activation
The observed increase in mitochondrial DNA copy number in skeletal muscle at 30 days of age in OffOb males is consistent with early developmental exposure to maternal high glucose-induced ROS, secondary to maternal obesity, and could reflect compensatory mitochondrial biogenesis in response to a decline in mitochondrial function [21, 42, 43]. Alternatively, the increase in MtDNA may be non-functional and a maladaptive response to oxidative stress [44] or hyperglycaemia [45], which can lead to an increase in tissue MtDNA and inflammation through activating of mTOR pathways and induction of TNFα [46]. Either way, the data suggest an independent influence of maternal obesity on skeletal muscle mtDNA levels and hence mitochondrial function prior to the development of other metabolic defects, which might suggest a primary mechanism in the developmental programming due to maternal obesity.
Biomarkers of brown fat activation
The increased expression Type 2 iodothyronine deiodinase (D2) which mediates adaptive thermogenesis in BAT may reflect the increased sympathetic drive [47] previously described in this model [14, 48, 49]. PDX may theoretically prevent this increased Dio2 gene expression by normalising hypothalamic development and sympathetic drive in OffOb [37]. Indeed, others have shown that probiotics rescue neurogenesis and behavioural deficits in dysbiotic mice treated with antibiotics [50].
Mitochondrial UCP-1 expression was upregulated in BAT of male offspring of obese dams treated with PDX compared to control offspring and may contribute to the increased energy expenditure observed. Prebiotics may increase thermogenic capacity in BAT by increasing UCP-1 expression [51] through altering microbiota and their by-products, short chain fatty acids, which can act as both energy source and receptor-mediated metabolic regulators of host energy metabolism involving processes such as hepatic gluconeogenesis and lipid metabolism via AMPK and PGC-1a activation [52].
Pgc1α is the master regulator of mitochondrial biogenesis and linked to adaptive thermogenesis, following ‘BAT activation’. Increased Pgc1α expression in skeletal muscle of offspring of obese dams which was prevented by maternal PDX treatment appears counter-intuitive, as BAT activation would be expected to contribute to greater energy expenditure, if the observed increase in mtDNA were indeed functional. However, in addition to stimulating mitochondrial proliferation in skeletal muscle, PGC‐1α activation favours enhanced lipid- over carbohydrate-mediated mitochondrial respiration in skeletal muscle in mice, and leads to intrinsic mitochondrial adaptations in fatty acid-induced uncoupling and a reduction in mitochondrial superoxide production [53]. This ‘fuel switching’ is consistent with the observed reduction in RER, and thus increased lipid oxidation, in offspring of obese mice and may represent a compensatory response to reduce ROS production, or a direct influence of the gut microbiota [52].
Offspring microbiota profile
Inheritable microbiota, passed from an obese mother to offspring during labour, may contribute to the modern patterns of human health and disease affecting gut barrier integrity and energy provision [54] but also maturation of the immune system [55], insulin sensitivity, energy expenditure and visceral adiposity [56]. Indeed, we have previously implicated impaired innate immunity in offspring liver together with an increase in pro-inflammatory markers associated with NAFLD in offspring of obese mice [17]. A recent landmark study demonstrated that transplanted gut microbiota from stool microbes of 2-week-old infants born to obese mothers increases inflammation and susceptibility to NAFLD in recipient germ-free mice [57].
Prebiotic effects of polydextrose on offspring microbiota
Maternal supplementation with PDX in obese pregnant mice resulted in increased abundance of Bacteroides compared to controls. Administration of prebiotics has previously been shown to improve pregnancy outcomes [58] and influence maternal transfer of microbiota and initial establishment of bifidobacteria in the infant [59]. In obese humans an increase in bacteroides relative abundance is associated with weight-loss [60]. We report a similar effect here, with maternal PDX intervention, in which obesity traits in the offspring were reduced associated with an increase in bacteroides relative abundance.
Conclusions
In this study, evidence has been presented that diet-induced maternal obesity in the mouse results in reduced EE, glucose intolerance and increased bodyweight in 6-month male offspring compared to controls. Moreover, following a 3-week obesogenic dietary challenge, offspring of obese dams had reduced energy expenditure, increased calorific intake an increased weight gain compared to controls. The offspring obesogenic phenotype is preceded by evidence of early mitochondrial damage and changes in the gut microbiota, which are prevented by maternal polydextrose. Polydextrose is a synthetic indigestible glucose polymer, classified as a dietary fibre and therefore, safe for use in pregnancy. However, there is currently a lack of high-quality scientific data on the use of polydextrose, or indeed other prebiotics, in pregnant or breastfeeding women. The present study supports the safety and efficacy of polydextrose supplementation in obese pregnancy.
References
Poston L, Caleyachetty R, Cnattingius S, Corvalan C, Uauy R, Herring S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016;4:1025–36.
Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VW, Eriksson JG, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017;5:53–64.
Heslehurst N, Vieira R, Akhter Z, Bailey H, Slack E, Ngongalah L, et al. The association between maternal body mass index and child obesity: a systematic review and meta-analysis. PLoS Med. 2019;16:e1002817.
Scholtens DM, Kuang A, Lowe LP, Hamilton J, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal glycemia and childhood glucose metabolism. Diabetes Care. 2019;42:381–92.
Dalrymple K, Thompson J, Begum S, Godfrey KM, Poston L, Seed, PT. et al. Relationships of maternal body mass index and plasma biomarkers with childhood body mass index and adiposity at 6 years; the Children of SCOPE study. Int J Pead Ob. 2019;14:e12537.
Hanson M, Barker M, Dodd JM, Kumanyika S, Norris S, Steegers E, et al. Interventions to prevent maternal obesity before conception, during pregnancy, and post partum. Lancet Diabetes Endocrinol. 2017;5:65–76.
do Carmo MM, Walker JC, Novello D, Caselato VM, Sgarbieri VC, Ouwehand AC, et al. Polydextrose: physiological function, and effects on health. Nutrients. 2016;8. https://doi.org/10.3390/nu809055300.
Ibarra A, Astbury NM, Olli K, Alhoniemi E, Tiihonen K. Effects of polydextrose on different levels of energy intake. A systematic review and meta-analysis. Appetite. 2015;87:30–7.
Ibarra A, Astbury NM, Olli K, Alhoniemi E, Tiihonen K. Effect of polydextrose on subjective feelings of appetite during the satiation and satiety periods: a systematic review and meta-analysis. Nutrients. 2016;8. https://doi.org/10.3390/nu8010045.
Ibarra A, Olli K, Pasman W, Hendriks H, Alhoniemi E, Raza GS, et al. Effects of polydextrose with breakfast or with a midmorning preload on food intake and other appetite-related parameters in healthy normal-weight and overweight females: An acute, randomized, double-blind, placebo-controlled, and crossover study. Appetite. 2017;110:15–24.
Olli K, Salli K, Alhoniemi E, Saarinen M, Ibarra A, Vasankari T, et al. Postprandial effects of polydextrose on satiety hormone responses and subjective feelings of appetite in obese participants. Nutr J. 2015;14:2.
Airaksinen K, Jokkala J, Ahonen I, Auriola S, Kolehmainen M, Hanhineva K. et al. High-fat diet, betaine, and polydextrose induce changes in adipose tissue inflammation and metabolism in C57BL/6J mice. Mol Nutr Food Res. 2018;62:e1800455. https://doi.org/10.1002/mnfr.201800455.
Raza GS, Putaala H, Hibberd AA, Alhoniemi E, Tiihonen K, Makela KA, et al. Polydextrose changes the gut microbiome and attenuates fasting triglyceride and cholesterol levels in Western diet fed mice. Sci Rep. 2017;7:5294.
Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–92.
Oben JA, Mouralidarane A, Samuelsson AM, Matthews PJ, Morgan ML, McKee C, et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol. 2010;52:913–20.
Oben JA, Patel T, Mouralidarane A, Samuelsson AM, Matthews P, Pombo J, et al. Maternal obesity programmes offspring development of non-alcoholic fatty pancreas disease. Biochem Biophys Res Commun. 2010;394:24–8.
Mouralidarane A, Soeda J, Visconti-Pugmire C, Samuelsson AM, Pombo J, Maragkoudaki X, et al. Maternal obesity programs offspring nonalcoholic fatty liver disease by innate immune dysfunction in mice. Hepatology. 2013;58:128–38.
Lagisz M, Blair H, Kenyon P, Uller T, Raubenheimer D, Nakagawa S. Little appetite for obesity: meta-analysis of the effects of maternal obesogenic diets on offspring food intake and body mass in rodents. Int J Obes. 2015;39:1669–78.
Witaicenis A, Fruet AC, Salem L, Di, Stasi LC. Dietary polydextrose prevents inflammatory bowel disease in trinitrobenzenesulfonic acid model of rat colitis. J Med Food. 2010;13:1391–6.
Stenman LK, Waget A, Garret C, Briand F, Burcelin R, Sulpice T, et al. Probiotic B420 and prebiotic polydextrose improve efficacy of antidiabetic drugs in mice. Diabetol Metab Syndr. 2015;7:75.
Malik AN, Czajka A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion. 2013;13:481–92.
Czajka A, Malik AN. Hyperglycemia induced damage to mitochondrial respiration in renal mesangial and tubular cells: Implications for diabetic nephropathy. Redox Biol. 2016;10:100–7.
Rigottier-Gois L, Bourhis AG, Gramet G, Rochet V, Dore J. Fluorescent hybridisation combined with flow cytometry and hybridisation of total RNA to analyse the composition of microbial communities in human faeces using 16S rRNA probes. FEMS Microbiol Ecol. 2003;43:237–45.
Shimomura Y, Maeda K, Nagasaki M, Matsuo Y, Murakami T, Bajotto G, et al. Attenuated response of the serum triglyceride concentration to ingestion of a chocolate containing polydextrose and lactitol in place of sugar. Biosci Biotechnol Biochem. 2005;69:1819–23.
Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017;356:j1.
Roman AS, Rebarber A, Fox NS, Klauser CK, Istwan N, Rhea D, et al. The effect of maternal obesity on pregnancy outcomes in women with gestational diabetes. J Matern Fetal Neonatal Med. 2011;24:723–7.
Bo S, Menato G, Signorile A, Bardelli C, Lezo A, Gallo ML, et al. Obesity or diabetes: what is worse for the mother and for the baby? Diabetes Metab. 2003;29:175–8.
Al-Sobayil K, Zeitoun M, Abdel-Salam A. Effectiveness of a functional synbiotic syrup on pregnancy rate, neonatal birth weight and progesterone profile of oestrous-synchronized Najdi ewes. J Food Agric Environ. 2010;8:80–5.
Castillo-Laura H, Santos IS, Quadros LC, Matijasevich A. Maternal obesity and offspring body composition by indirect methods: a systematic review and meta-analysis. Cad Saude Publica. 2015;31:2073–92.
Taylor PD, McConnell J, Khan IY, Holemans K, Lawrence KM, Asare-Anane H, et al. Impaired glucose homeostasis and mitochondrial abnormalities in offspring of rats fed a fat-rich diet in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2005;288:R134–9.
Rising R, Lifshitz F. Lower energy expenditures in infants from obese biological mothers. Nutr J. 2008;7:15.
Lau SM, Lin S, Stokes RA, Cheng K, Baldock PA, Enriquez RF, et al. Synergistic effects of genetic beta cell dysfunction and maternal glucose intolerance on offspring metabolic phenotype in mice. Diabetologia. 2011;54:910–21.
Borengasser SJ, Lau F, Kang P, Blackburn ML, Ronis MJ, Badger TM, et al. Maternal obesity during gestation impairs fatty acid oxidation and mitochondrial SIRT3 expression in rat offspring at weaning. PLoS ONE. 2011;6:e24068.
Ong ZY, Muhlhausler BS. Maternal “junk-food” feeding of rat dams alters food choices and development of the mesolimbic reward pathway in the offspring. FASEB J. 2011;25:2167–79.
Gugusheff JR, Bae SE, Rao A, Clarke IJ, Poston L, Taylor PD, et al. Sex and age-dependent effects of a maternal junk food diet on the mu-opioid receptor in rat offspring. Behav Brain Res. 2016;301:124–31.
Bayol SA, Farrington SJ, Stickland NC. A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. Br J Nutr. 2007;98:843–51.
Kirk SL, Samuelsson AM, Argenton M, Dhonye H, Kalamatianos T, Poston L, et al. Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS ONE. 2009;4:e5870.
Bogardus C, Lillioja S, Mott D, Zawadzki J, Young A, Abbott W. Evidence for reduced thermic effect of insulin and glucose infusions in Pima Indians. J Clin Invest. 1985;75:1264–9.
Schutz Y, Bessard T, Jequier E. Diet-induced thermogenesis measured over a whole day in obese and nonobese women. Am J Clin Nutr. 1984;40:542–52.
Golay A, Schutz Y, Felber JP, Jallut D, Jequier E. Blunted glucose-induced thermogenesis in ‘overweight’ patients: a factor contributing to relapse of obesity. Int J Obes. 1989;13:767–75.
Fernandes C, Grayton H, Poston L, Samuelsson AM, Taylor PD, Collier DA, et al. Prenatal exposure to maternal obesity leads to hyperactivity in offspring. Mol Psychiatry. 2012;17:1159–60.
Rooney JP, Ryde IT, Sanders LH, Howlett EH, Colton MD, Germ KE, et al. PCR based determination of mitochondrial DNA copy number in multiple species. Methods Mol Biol. 2015;1241:23–38.
Al-Kafaji G, Sabry MA, Bakhiet M. Increased expression of mitochondrial DNA-encoded genes in human renal mesangial cells in response to high glucose-induced reactive oxygen species. Mol Med Rep. 2016;13:1774–80.
Masser DR, Clark NW, Van Remmen H, Freeman WM. Loss of the antioxidant enzyme CuZnSOD (Sod1) mimics an age-related increase in absolute mitochondrial DNA copy number in the skeletal muscle. Age. 2016;38:323–33.
Malik AN, Parsade CK, Ajaz S, Crosby-Nwaobi R, Gnudi L, Czajka A, et al. Altered circulating mitochondrial DNA and increased inflammation in patients with diabetic retinopathy. Diabetes Res Clin Pract. 2015;110:257–65.
Picca A, Lezza AMS, Leeuwenburgh C, Pesce V, Calvani R, Bossola M, et al. Circulating mitochondrial DNA at the crossroads of mitochondrial dysfunction and inflammation during aging and muscle wasting disorders. Rejuvenation Res. 2018;21:350–9.
de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest. 2001;108:1379–85.
Samuelsson AM, Clark J, Rudyk O, Shattock MJ, Bae SE, South T, et al. Experimental hyperleptinemia in neonatal rats leads to selective leptin responsiveness, hypertension, and altered myocardial function. Hypertension. 2013;62:627–33.
Samuelsson AM, Morris A, Igosheva N, Kirk SL, Pombo JM, Coen CW, et al. Evidence for sympathetic origins of hypertension in juvenile offspring of obese rats. Hypertension. 2010;55:76–82.
Mohle L, Mattei D, Heimesaat MM, Bereswill S, Fischer A, Alutis M, et al. Ly6C(hi) monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep. 2016;15:1945–56.
Rodriguez VM, Portillo MP, Pico C, Macarulla MT, Palou A. Olive oil feeding up-regulates uncoupling protein genes in rat brown adipose tissue and skeletal muscle. Am J Clin Nutr. 2002;75:213–20.
den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–40.
Hoeks J, Arany Z, Phielix E, Moonen-Kornips E, Hesselink MK, Schrauwen P. Enhanced lipid-but not carbohydrate-supported mitochondrial respiration in skeletal muscle of PGC-1alpha overexpressing mice. J Cell Physiol. 2012;227:1026–33.
Zhu MJ, Du M, Ford SP. Impacts of maternal obesity on placental and gut inflammation and health. J Anim Sci. 2013;92:1840–9. https://doi.org/10.2527/jas.2013-7106.
Myles IA, Fontecilla NM, Janelsins BM, Vithayathil PJ, Segre JA, Datta SK. Parental dietary fat intake alters offspring microbiome and immunity. J Immunol. 2013;191:3200–9.
Stolarczyk E, Vong CT, Perucha E, Jackson I, Cawthorne MA, Wargent ET, et al. Improved insulin sensitivity despite increased visceral adiposity in mice deficient for the immune cell transcription factor T-bet. Cell Metab. 2013;17:520–33.
Soderborg TK, Clark SE, Mulligan CE, Janssen RC, Babcock L, Ir D, et al. The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat Commun. 2018;9:4462.
Laitinen K, Poussa T, Isolauri E, Nutrition AMI, Intestinal Microbiota G. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. Br J Nutr. 2009;101:1679–87.
Collado MC, Cernada M, Bauerl C, Vento M, Perez-Martinez G. Microbial ecology and host-microbiota interactions during early life stages. Gut Microbes. 2012;3:352–65.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3.
Acknowledgements
This work was supported by a BBSRC industrial CASE studentship (BB/G017093/1) with Tate & Lyle PLC; Tommy’s Charity; European Union 7th Framework Programme (FP7/2007–2013) project EarlyNutrition under grant agreement No. 289346, Medical Research Council UK; Newton Fund (Partnership Project grant) RCUK-CONACYT Research Partnerships MR/N029259/1. Polydextrose was provided by Tate & Lyle. This study also received support from the NIHR Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed in this publication are those of the author and not necessarily those of the NHS, the NIHR, or the Department of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Maragkoudaki, X., Naylor, M., Papacleovoulou, G. et al. Supplementation with a prebiotic (polydextrose) in obese mouse pregnancy improves maternal glucose homeostasis and protects against offspring obesity. Int J Obes 44, 2382–2393 (2020). https://doi.org/10.1038/s41366-020-00682-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-020-00682-5
- Springer Nature Limited