Abstract
Background/objective
Biliopancreatic diversion with duodenal switch (BPD-DS) is the most effective bariatric intervention to treat morbid obesity and related disorders. Single-anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) is a new bariatric procedure devised with the purpose of simplifying the complexity of the BPD-DS technique while maintaining its efficacy. However, whether BPD-DS and SADI-S result in similar fasting and post-prandial hormone profiles has not yet been studied. Therefore, the purpose of this study was to assess and compare the hormone response to a standardized mixed meal in subjects operated with BPD-DS or SADI-S.
Subjects/methods
Subjects submitted to BPD-DS (n = 9) or SADI-S (n = 9) 1.5 years earlier on average, with no past nor current diabetes diagnosis underwent a liquid mixed-meal tolerance test (MMTT) to assess the baseline and post-prandial profile of glucose, enteropancreatic hormones and total bile acids.
Results
Fasting glucose, enteropancreatic hormones and total bile acids levels after BPD-DS and SADI-S were similar. After the MMTT, the response of subjects who underwent SADI-S was characterized by higher glucose (t = 30 min: p < 0.05; iAUC: 156.1 ± 46.2 vs. 103.4 ± 35.8 mmol/L × min, p = 0.02), GLP-1 (t = 30 min: p < 0.05; iAUC: 5388 ± 3010 vs. 2959.0 ± 2146 pmol/L × min, p = 0.02), glucagon (t = 30 min: p < 0.05; iAUC: 678.7 ± 295.2 vs. 376.9 ± 215.7 pmol/L × min, p = 0.02), insulin (t = 30 and 45 min: p < 0.05); and C-peptide levels (t = 30 and 45 min: p < 0.05), when compared to BPD-DS.
Conclusions
The post-prandial hormone secretion profile after SADI-S is characterized by increased GLP-1, glucagon and insulin secretion, when compared to BPD-DS, which suggests the existence of different endocrine driven mechanisms leading to weight loss and metabolic improvement after the two procedures.
Similar content being viewed by others
Introduction
Biliopancreatic diversion (BPD) and biliopancreatic diversion with duodenal switch (BPD-DS) are the most effective bariatric interventions for the treatment of morbid obesity and related metabolic disorders [1,2,3]. Still, BPD and BPD-DS are technically demanding procedures, associated with long operative times and high rates of postoperative complications and malnutrition [2]. The single-anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) technique was introduced in 2007 by Sánchez-Pernaute and Antonio Torres in order to simplify the BPD-DS surgical technique by performing a “one-anastomosis” BPD-DS, while retaining the principles and the outcomes of BPD-DS [4].
The technical feasibility of the SADI-S procedure, the post-operative weight loss, the impact in nutritional status and resolution of obesity comorbidities were the focus of several studies over the last decade. Non randomized patient series with a relatively small number of individuals that underwent BPD-DS or SADI-S for a period of time spanning up to 2 years showed that the two procedures resulted in similar rates of weight loss, diabetes remission and overall metabolic improvement, without increased incidence of nutritional deficiencies [5,6,7]. In addition, diabetes remission rates for the first 3 years after surgery were higher after SADI-S when compared with the Roux-en-Y gastric bypass (RYGB) [5, 8]. Besides achieving similar outcomes when compared with BPD-DS, SADI-S requires a shorter operative time and is associated with fewer perioperative and postoperative complications [6, 7, 9].
However, whether BPD-DS and SADI-S result in similar fasting and post-prandial hormone profiles has not yet been studied. Therefore, the purpose of this study was to assess and compare the hormone response to a standardized mixed meal in subjects operated with BPD-DS or SADI-S.
Materials and methods
Participants
The study was approved by the Institutional Ethical Review Board and written informed consents were obtained from all participants before any intervention was initiated.
From the cohort of patients submitted to BPD-DS (n = 23) and SADI-S (n = 19) at our center, subjects meeting the enrollment criteria were consecutively invited to participate in the study.
Patient groups were paired according to age, pre-operative body mass index (BMI) and follow-up time after surgery (1.6 ± 0.3 years for BPD-DS and 1.5 ± 0.3 years for SADI-S). Exclusion criteria included medical history of diabetes prior or after surgery (Hb1Ac > 6.5% and fasting plasma glucose >7.0 mmol/L) and pregnancy.
Study design
Subjects (n = 9/group) underwent a mixed-meal tolerance test (MMTT), in which a standardized commercially available liquid meal (Fresubin Energy Drink, 200 mL, 300 kcal [50E% carbohydrate, 15E% protein and 35E% fat]; Fresenius Kabi Deutschland, Bad Homburg, Germany) was ingested over a maximum period of 15 minutes after a 12 h overnight fast. Venous blood samples were collected into EDTA tubes (S-Monovette® 7.5 ml, K2 EDTA Gel, 1.6 mg/mL, Sarstedt), before the meal (−15 and 0 min) and at 15, 30, 45, 60, 90, and 120 min after the start of meal intake. Venous sampling was performed through an indwelling antebrachial vein cannula with saline flushing of the catheter in between each blood collection. The plasma was then separated and stored at −20 °C until assayed.
Surgical procedures
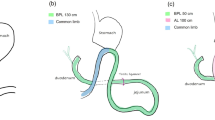
All surgeries were performed laparoscopically by the same surgical team at a single public and academic bariatric center. The BPD-DS was performed with a common limb of 100 cm and an alimentary limb of 200 cm, while the SADI-S was performed with a 300 cm common channel (Fig. 1). Small intestine limbs were measured by counting 10 cm intervals after stretching the anti-mesenteric border.
For the BPD-DS, a sleeve gastrectomy was performed over a 36 French Boogie, starting 2 cm proximal to the pylorus. After right gastric artery ligation a duodenal division was made 3 cm distally to the pylorus. Afterwards, the small intestine was marked with sutures set at 100 cm and 300 cm of the ileocecal valve, respectively. Then, the small intestinal loop carrying the 300 cm mark was moved cranially to complete an end-to-side hand-made duodenal-ileal anastomosis in ante-colic position, while at 100 cm mark a side-to-side anastomosis was made between the biliopancreatic and alimentary limb, thus creating a 100 cm common channel. To conclude, the small intestine was divided between the two anastomoses to restore the intestinal transit in a Roux-en-Y fashion.
For the SADI-S procedure, a sleeve gastrectomy procedure was performed as previously described for BPD-DS that was followed by an unique end-to-side hand-made duodenal-ileal anastomosis at 300 cm from the ileocecal valve. Methylene blue was used for testing the anastomosis and mesenteric defects were closed as standard procedures in both surgical techniques.
After surgery, patients are assessed by the multidisciplinary clinical team and underwent routine biochemical measurements for metabolic and nutritional parameters every 3 months for the first year after surgery, every 6 months on the second year after surgery and yearly thereafter.
The mortality rate in our patient cohort was 0% for both surgeries. The rate of major complications in the first 90 days after surgery was 0% in patients submitted to BPD-DS and 15.8% (3/19) in the overall patient cohort submitted to SADI-S [N = 19; duodenal fistula (n = 1), hemoperitoneum (n = 1) and gastric tube fistula (n = 1)].
Biochemical measurements
Blood glucose levels were measured during the blood sample collection using a glucometer (Freestyle Precision Neo Glucose meter, Abbott, USA).
For analysis of glucose-dependent insulinotropic polypeptide (GIP), glucagon like peptide-1 (GLP-1), glucagon, peptide YY (PYY) and neurotensin (NT) plasma samples were extracted using 70% ethanol prior to analysis. Total GLP-1, total GIP, and pancreatic glucagon levels were measured by radioimmunoassay (RIA) specifically targeting the C-terminal of GLP-1 (antiserum 89390) or GIP (antiserum 867) or glucagon (antiserum 4305), as described before [10,11,12,13]. RIA for total PYY was performed using a monoclonal antibody MAB8500 (Abnova, clone RPY-B12), which reacts equally with PY1-36 and PYY3-36 [11, 14]. An in-house developed assay was used to assess total NT levels (antibody code 3D97), as previously described [15, 16]. The free and bound hormone moieties were separated with plasma-coated charcoal for all the described assays (E. Merck, Darmstadt, Germany). Sensitivity for all assays was between 1–5 pmol/L and the intra-assay coefficients of variation were below 10% at 20 pmol/L. To minimize inter-assay variation, all samples were run consecutively, using identical reagents and protocols
Insulin and C-peptide levels were measured by an electrochemiluminescence sandwich immunoassay (ECLIA) on a Cobas 8000 e602 module (Roche Diagnostics, Mannheim, GmbH). The coefficient of variation was below 5% and 8% for insulin and C-peptide, respectively, using liquid human serum-based controls (Liquichek™ Immunoassay Plus Control, Bio-Rad).
The total bile acid (TBA) levels were measured using a commercial assay kit (Total Bile Acid Assay Kit, STA-631, Cell BioLabs, Inc, San Diego, CA, USA).
Statistical analysis and calculations
Insulin resistance was assessed using the homeostasis model assessment of insulin resistance (HOMA-IR) and calculated according to the formula: [fasting glucose (mg/dL) × fasting insulin (mU/L) ÷ 405], while the pancreatic β-cell function was measured by the HOMA of β-cell function (HOMA-β), which was calculated according to the formula [(100 × 360 × Insulin (mU/L)) ÷ (Fasting glucose (mg/dL)−63)] [17]. The percentage of excess BMI loss (EBMIL) was calculated as [(preoperative BMI−postoperative BMI) ÷ (preoperative BMI−25) × 100], and the percentage of total weight loss TWL was calculated as [(preoperative weight−postoperative weight) ÷ (preoperative weight) × 100]. Total area under the curve (tAUC) was calculated using the trapezoidal rule and incremental area under the curve (iAUC) was calculated subtracting the basal values to the tAUC. Prehepatic insulin secretion rate (ISR) was calculated from C-peptide plasmatic levels, using the ISEC software (ISEC, Version 3.4a, Hovorka, 1994), as described before [18]. Insulin clearance was then calculated according to the formula [tAUCISR ÷ tAUCinsulin.].
Nominal variables are expressed as number of cases and percentage (%), and the continuous variables are expressed as mean ± standard SD) unless stated otherwise. Normality was evaluated using the D’Agostinho and Pearson test. For continuous variables that passed this test, an unpaired two-tailed t-test was used to compare the means of the two groups. For variables that did not pass the normality test, a Mann Whitney test was used. Comparisons between timepoints during the MMTT were performed using a two-way analysis of variance (ANOVA) with Sidak’s post hoc test. Fisher’s exact test was used to compare nominal variables. Statistical analysis was performed using the GraphPad Prism version 6.01 for Windows (GraphPad Software, La Jolla California USA). A p < 0.05 was considered statistically significant.
Results
Demographic data
There were no significant differences in the anthropometric and metabolic features between the two patient groups both at the time of the surgery (pre-operative) and at the time of the MMTT (post-operative) (Table 1). Besides that, there were no differences between the two surgical groups in the rate of micronutrient and protein deficits. In addition, iron, vitamin B12 and total protein levels were within the normal limits in every patient at the time of MMTT (data not shown).
Fasting state
Fasting levels of glucose and enteropancreatic hormones were not significantly different between the groups (Table 2). No significant differences were also observed for fasting TBA levels.
Hormone response to the mixed meal
Glucose and enteropancreatic hormone response to the mixed meal differed significantly between the subjects that underwent BPD-DS and SADI-S (Fig. 2 and Table 2). In particular, the iAUC of glucose, glucagon and GLP-1 were significantly higher in SADI-S group as compared to the BPD-DS (Table 2).
Peripheral levels of glucose (a), insulin (b), C-peptide (c), glucagon (d), GLP-1 (e), GIP (f), PYY (g), NT (h) and TBA (i), and ISR (j) in subjects without prior or current diagnosis of diabetes, previously submitted to BPD-DS (n = 9) or SADI-S (n = 9) after ingestion of a standard mixed-meal served at t = 0 min. Data are present as mean ± standard error of the mean. *p < 0.05, **p < 0.01, and ***p < 0.001. BPD-DS biliopancreatic diversion with duodenal switch, SADI-S single-anastomosis duodeno-ileal bypass with sleeve gastrectomy, GIP glucose-dependent insulinotropic polypeptide, GLP-1 glucagon like peptide-1, NT Neurotensin, PYY Peptide YY, TBA Total bile acids, ISR insulin secretion rate
Thirty minutes after the mixed meal intake, significantly higher levels of glucose, insulin, C-peptide, glucagon and GLP-1 were observed in the SADI-S group (Fig. 2a–e). At 45 min of MMTT, the insulin and C-peptide levels remained significantly higher in the SADI-S group (Fig. 2b, c).
Contrarily, at 120 min NT decreased back to fasting levels after the post-prandial increase in the SADI-S group, while NT levels remained significantly higher and did not decrease to fasting levels in the BPD-DS group (Fig. 2h).
No significant differences in GIP, PYY, and TBA post-prandial levels were observed between the two groups (Fig. 2f, g and i).
ISR was higher at 15 to 30 and 30 to 45 min of the MMTT in patients submitted to SADI-S when compared to the BPD-DS group (Fig. 2j). Insulin clearance was similar in the two groups (Table 2).
Discussion
In the present study the enteropancreatic hormone responses to a standardized liquid mixed meal in patients without prior or current diagnosis of diabetes previously submitted to BPD-DS or SADI-S were investigated.
After the surgical procedures, patients that underwent SADI-S and BPD-DS achieved similar weight loss rates, as well as levels of insulin resistance and pancreatic β-cell function, as assessed by HOMA-IR and HOMA-β. Previously, similar findings were reported by Torres A et al. in a 3-years follow-up study of a cohort that included patients submitted to SADI-S (n = 97), BPD-DS (n = 77) and RYGB (n = 97) [5].
Our study shows that patients submitted to BPD-DS or SADI-S depict similar fasting enteropancreatic hormones and TBA profiles.
By comparing the anatomic rearrangement produced by the two surgical interventions, both result in identical gastric sleeves (36 French Bougie) and intestinal “absorptive” lengths (300 cm) (Fig. 1). Since after BPS-DS nutrients transit pass a 200 cm alimentary limb, followed by a 100 cm common limb, while after the one anastomosis performed on the SADI-S procedure changes the original configuration of BPD-DS with three intestinal limbs (alimentary, biliopancreatic and common limb) into two limbs, the biliopancreatic and the “absorptive channel”. Thus, from an anatomical point of view the differences observed in the post-prandial hormonal response to a standardized test could be attributed to the exposure to bile acids and pancreatic juices of an additional 200 cm of small intestine of the BPD-DS alimentary limb converted into “absorptive channel” in the SADI-S. As most gut hormones are known to be secreted in response to absorption and therefore it is reasonable that for responses to be higher in those patients that have higher common absorptive limbs.
The post-prandial glucose excursion of subjects submitted to SADI-S was found to be higher when compared to the patients submitted to BPD-DS, the difference in the glycemic profile is likely to result from the anatomical differences induced by the two surgical procedures. The main sources of carbohydrates of the liquid meal used in the MMTT are maltodextrin (69.4% to 75.0%) and sucrose (25.0% to 30.6%). Only a small fraction of those carbohydrates is digested in the mouth trough the salivary α-amylase, while the majority is digested by the pancreatic carbohydrases (mainly α-amylase) and absorbed in the proximal small intestine [19]. Since, the subjects that underwent SADI-S have a longer intestinal length, where the carbohydrates can contact the pancreatic juices to be digested and then absorbed, a greater post-prandial glucose excursion would be predicted to occur in those patients when compared to the subjects that underwent BPD-DS.
Despite not being directly comparable with our data, post-prandial insulin levels were reported to be lower in non-operated subjects with similar BMI ranges, than those found in our patients, both submitted to SADI-S and BPD-DS, while fasting and post-prandial GLP-1 levels are higher in SADI-S and BPD-DS compared with those controls [20, 21]. Findings that are in agreement with the anatomical modification induced by these surgeries, since both result in early arrival of nutrients to the distal small intestine, where GLP-1-producing cells are known to predominate [22, 23]. For same reasons, higher NT and PYY levels were expected to occur after SADI-S and BPD-DS.
Interestingly, the TBA response to the mixed meal was similar after both bariatric surgery procedures. Bile acids facilitate intestinal fat absorption and stimulate the secretion of gastrointestinal hormones (such as GLP-1, PYY, and GIP) through activation of TGR5 receptors, located at the basolateral membranes of the enterocytes [24,25,26,27]. So, the higher post-prandial levels of GLP-1 observed in subjects that underwent SADI-S could be attributed to the bile acid interactions across a longer intestinal length (300 cm in SADI-S vs. 100 cm in BPD-DS), leading to a greater stimulation of GLP-1 producing cells. Following the same rationale, it could be expected that post-prandial GIP and PYY levels should also be higher in the SADIS-S group. Indeed, although GIP and PYY levels were numerically higher in the SADIS-S group, no significantly differences were observed when compared to the BPD-DS group.
Counterintuitively, pancreatic glucagon increased after the mixed meal in the SADI-S group. Similar observations in post-bariatric patients were made in previous studies [11, 28]. The explanation for this observation is not yet fully understood, although it has been suggested that post-operatively some glucagon might be derived from “pancreatic type processing” of proglucagon in the gut cells [29, 30]. According to our results, the glucagon secretion could also be triggered by bile acids stimulation, as already described for GLP-1, since BPD-DS group have almost no increase of glucagon after the mixed meal, in contrast to what occurs in the SADI-S group.
Higher post-prandial insulin and C-peptide levels were observed in SADI-S patients when compared to the BPD-DS group, likely driven by increased insulin secretion and not due to a difference in the insulin clearance rate between the two surgical procedures. Since both glucose and GLP-1 stimulate insulin and C-peptide secretion by pancreatic β-cells [31, 32], the higher insulin secretion could be justified by the significantly higher post-prandial levels of glucose and GLP-1 observed in the subjects submitted to SADI-S (Fig. 3).
Schematic representation of the hormonal response to the mixed meal in subjects that underwent SADI-S when compared to the BPD-DS. A longer common limb results in a higher exposure of the enteric cells to bile acids leading to an increase of the release of GLP-1 by the neuroendocrine intestinal cells to the blood stream. Besides that, a higher exposure of the carbohydrates to the pancreatic juices leads to a higher glucose absorption and consequently higher levels of glucose. Both GLP-1 and glucose lead to the increase of insulin secretion by the pancreatic β-cell
Post-prandial NT response to the mixed meal lasted markedly longer in the BPD-DS group when compared with the SADI-S group. NT is predominantly produced in the gastrointestinal tract and has a well-established effect on intestinal lipid absorption [33, 34]. Post-bariatric levels of NT were found increase particularly after malabsorptive bariatric surgeries [35, 36]. Loeffelholz et al. suggested that patients submitted to BPD-DS may have higher NT levels to mediate intestinal transportation of fatty acids and so, to overcome deficiency of essential fatty acids [35]. Following this rationale, since BPD-DS results in a shorter common limb when compared with SADI-S group, this anatomical difference could have an impact on lower intestinal lipid absorption. So, a longer lasting NT response after the mixed meal in patients submitted to BPD-DS could represent an adaptive response to counteract the limited lipid absorption.
Despite the fact ghrelin levels were not measured, surgical induced modification of ghrelin secretion dynamics could provide an alternative explanation for the differences observed. Ghrelin is predominantly secreted in the gastric fundus and has a major role in energy and glucose balance regulation both under normal physiological conditions and after bariatric surgical interventions [37, 38]. However, given the fact that similar stomach resections were performed in both surgical procedures and fasting ghrelin levels are usually low in obese individuals being further suppressed by food intake [38, 39], no major differences were anticipated to occur between the two study groups.
Overall, in an integrative manner the post-prandial hormone profiles observed after the procedures suggest that SADI-S as compared to BPD-DS, which leads to higher GLP-1 and glucagon excursions, could promote an enhanced “anti-obesity” hormone response, as these hormones are known to induce weight loss by decreasing appetite or increasing energy expenditure. Yet, the clinical outcomes observed after the two surgical techniques are comparable [5, 6], therefore the decrease in absorption capacity induced by BPD-DS could be sufficient to allow similar weight loss despite not being achieved by the same mechanisms.
Furthermore, from a clinical perspective it is tempting to extrapolate whether the different glycaemic excursion curve and parallel hormone responses could be translated into improved glycaemic outcomes after bariatric surgery interventions. Type 2 diabetes is well-known to be a progressive disease, starting far before the clinical diagnosis that typically progresses from insulin resistance with preserved glycaemic homeostasis, to pre-diabetes and then to established disease with impaired fasting and postprandial glucose control, as the pancreatic reserve declines [40, 41]. The post-prandial response observed after BPD-DS and SADI-S may suggest that the later procedure could be advantageous in patients with insulin resistance, pre-diabetes and diabetes in earlier stages of the disease with reasonable pancreatic reserve, while BPB-DS is likely to perform better in those patients with more advanced stages of the disease. However, this is a speculative working hypothesis that needs to be formally confirmed by a randomized clinical trial to compare the long-term outcomes of BPD-DS and SADI-S in patients with different degrees of pancreatic functional reserve. In this study the fasting and postprandial hormone profiles of subjects submitted to SADI-S and BPD-DS were assessed for the very first time in a patient population without prior or current diabetes diagnosis. These preliminary findings provide important clues into the endocrine mechanisms underlying the weight loss and metabolic effects of these procedures. Yet, before these findings can be extrapolated into a broad patient population, these results need to be validated in patients characterized in further detail, as we must acknowledge the limitation of relying on crude measurement tools, such as HOMA-IR and HOMA-B that were used to assess insulin sensitivity and pancreatic function when compared to the gold standard hyperinsulinaemic-euglycaemic clamp [42, 43]. Besides that, a comprehensive evaluation of these hormonal dynamics not only at a single time point after surgery, both also before surgery and at different time-points after surgery along the weight loss process until stabilization would unquestionably be further.
Conclusion
The post-prandial hormone secretion profiles of subjects who underwent SADI-S are characterized by higher levels of glucose, GLP-1, glucagon, insulin and C-peptide, when compared to BPD-DS. These data show that despite having apparently similar clinical outcomes, the endocrine mechanisms underlying the weight loss and metabolic effects of the two surgical procedures are potentially different. Moreover, in future the hormonal profile likely to be achieved after BPD-DS or SADI-S could be taken into account to obtain personalized post-bariatric outcomes depending of the patient glycaemic status and pancreatic reserve to improve clinical outcomes.
References
Skogar ML, Sundbom M. Duodenal switch is superior to gastric bypass in patients with super obesity when evaluated with the Bariatric Analysis and Reporting Outcome System (BAROS). Obes Surg. 2017;27:2308–16.
Strain GW, Torghabeh MH, Gagner M, Ebel F, Dakin GF, Abelson JS, et al. The Impact of Biliopancreatic Diversion with Duodenal Switch (BPD/DS) over 9 years. Obes Surg. 2017;27:787–94.
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37.
Sanchez-Pernaute A, Rubio Herrera MA, Perez-Aguirre E, Garcia Perez JC, Cabrerizo L, Diez Valladares L, et al. Proximal duodenal-ileal end-to-side bypass with sleeve gastrectomy: proposed technique. Obes Surg. 2007;17:1614–8.
Torres A, Rubio MA, Ramos-Levi AM, Sanchez-Pernaute A. Cardiovascular risk factors after Single Anastomosis Duodeno-Ileal Bypass with Sleeve Gastrectomy (SADI-S): a New Effective Therapeutic Approach? Curr Atheroscler Rep. 2017;19:58.
Cottam A, Cottam D, Portenier D, Zaveri H, Surve A, Cottam S, et al. A matched cohort analysis of Stomach Intestinal Pylorus Saving (SIPS) surgery versus biliopancreatic diversion with duodenal switch with two-year follow-up. Obes Surg. 2017;27:454–61.
Surve A, Zaveri H, Cottam D, Belnap L, Cottam A, Cottam S. A retrospective comparison of biliopancreatic diversion with duodenal switch with single anastomosis duodenal switch (SIPS-stomach intestinal pylorus sparing surgery) at a single institution with two year follow-up. Surg Obes Relat Dis. 2017;13:415–22.
Cottam A, Cottam D, Zaveri H, Cottam S, Surve A, Medlin W, et al. An analysis of mid-term complications, weight loss, and type 2 diabetes resolution of Stomach Intestinal Pylorus-Sparing Surgery (SIPS) Versus Roux-En-Y Gastric Bypass (RYGB) with Three-Year Follow-Up. Obes Surg. 2018;28:2894–902.
Surve A, Cottam D, Sanchez-Pernaute A, Torres A, Roller J, Kwon Y, et al. The incidence of complications associated with loop duodeno-ileostomy after single-anastomosis duodenal switch procedures among 1328 patients: a multicenter experience. Surg Obes Relat Dis. 2018;14:594–601.
Orskov C, Rabenhoj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes. 1994;43:535–9.
Patricio BG, Morais T, Guimaraes M, Veedfald S, Hartmann B, Hilsted L, et al. Gut hormone release after gastric bypass depends on the length of the biliopancreatic limb. Int J Obes. 2018.
Krarup T, Holst JJ. The heterogeneity of gastric inhibitory polypeptide in porcine and human gastrointestinal mucosa evaluated with five different antisera. Regul Pept. 1984;9:35–46.
Orskov C, Jeppesen J, Madsbad S, Holst JJ. Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest. 1991;87:415–23.
Toräng S, Bojsen-Moller KN, Svane MS, Hartmann B, Rosenkilde MM, Madsbad S, et al. In vivo and in vitro degradation of peptide YY3-36 to inactive peptide YY3-34 in humans. Am J Physiol Regul Integr Comp Physiol. 2016;310:R866–74.
Kuhre RE, Bechmann LE, Wewer Albrechtsen NJ, Hartmann B, Holst JJ. Glucose stimulates neurotensin secretion from the rat small intestine by mechanisms involving SGLT1 and GLUT2, leading to cell depolarization and calcium influx. Am J Physiol Endocrinol Metab. 2015;308:E1123–30.
Pedersen JH, Stadil F, Fahrenkrug J. Preparation of 125I-(Tyr 3)- and 125I-(Tyr 11)- neurotensin for radioimmunoassay. Scand J Clin Lab Invest. 1983;43:483–91.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Hovorka R, Soons PA, Young MA. ISEC: a program to calculate insulin secretion. Comput Methods Prog Biomed. 1996;50:253–64.
Gropper SS, Smith JL. Advanced nutrition and human metabolism: Cengage Learning; 2012.
Torekov SS, Iepsen E, Christiansen M, Linneberg A, Pedersen O, Holst JJ, et al. KCNQ1 long QT syndrome patients have hyperinsulinemia and symptomatic hypoglycemia. Diabetes. 2014;63:1315–25.
Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601.
Palha AM, Pereira SS, Costa MM, Morais T, Maia AF, Guimaraes M, et al. Differential GIP/GLP-1 intestinal cell distribution in diabetics’ yields distinctive rearrangements depending on Roux-en-Y biliopancreatic limb length. J Cell Biochem. 2018;119:7506–14.
Guedes TP, Martins S, Costa M, Pereira SS, Morais T, Santos A, et al. Detailed characterization of incretin cell distribution along the human small intestine. Surg Obes Relat Dis. 2015;11:1323–31.
Kuhre RE, Wewer Albrechtsen NJ, Larsen O, Jepsen SL, Balk-Moller E, Andersen DB, et al. Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Mol Metab. 2018;11:84–95.
Ullmer C, Alvarez Sanchez R, Sprecher U, Raab S, Mattei P, Dehmlow H, et al. Systemic bile acid sensing by G protein-coupled bile acid receptor 1 (GPBAR1) promotes PYY and GLP-1 release. Br J Pharmacol. 2013;169:671–84.
Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–77.
Adrian TE, Ballantyne GH, Longo WE, Bilchik AJ, Graham S, Basson MD, et al. Deoxycholate is an important releaser of peptide YY and enteroglucagon from the human colon. Gut. 1993;34:1219–24.
Falken Y, Hellstrom PM, Holst JJ, Naslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96:2227–35.
Lund A, Bagger JI, Wewer Albrechtsen NJ, Christensen M, Grondahl M, Hartmann B, et al. Evidence of Extrapancreatic Glucagon Secretion in Man. Diabetes. 2016;65:585–97.
Holst JJ, Pedersen JH, Baldissera F, Stadil F. Circulating glucagon after total pancreatectomy in man. Diabetologia. 1983;25:396–9.
Vilsboll T. The effects of glucagon-like peptide-1 on the beta cell. Diabetes Obes Metab. 2009;11(Suppl 3):11–8.
MacDonald PE, El-kholy W, Riedel MJ, Salapatek AMF, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002;51(suppl 3):S434–S42.
Li J, Song J, Zaytseva YY, Liu Y, Rychahou P, Jiang K, et al. An obligatory role for neurotensin in high-fat-diet-induced obesity. Nature. 2016;533:411–5.
Ratner C, Hundahl C, Holst B. The metabolic actions of neurotensin secreted from the gut. Cardiovasc Endocrinol & Metab. 2016;5:102–11.
von Loeffelholz C, Gissey LC, Schumann T, Henke C, Kurzbach A, Struck J, et al. The anorexigenic peptide neurotensin relates to insulin sensitivity in obese patients after BPD or RYGB metabolic surgery. Int J Obes. 2018.
Christ-Crain M, Stoeckli R, Ernst A, Morgenthaler NG, Bilz S, Korbonits M, et al. Effect of gastric bypass and gastric banding on proneurotensin levels in morbidly obese patients. J Clin Endocrinol Metab. 2006;91:3544–7.
Tymitz K, Engel A, McDonough S, Hendy MP, Kerlakian G. Changes in ghrelin levels following bariatric surgery: review of the literature. Obes Surg. 2011;21:125–30.
Gil-Campos M, Aguilera CM, Canete R, Gil A. Ghrelin: a hormone regulating food intake and energy homeostasis. Br J Nutr. 2006;96:201–26.
Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–9.
Festa A, Williams K, D’Agostino R Jr., Wagenknecht LE, Haffner SM. The natural course of beta-cell function in nondiabetic and diabetic individuals: the insulin resistance atherosclerosis study. Diabetes. 2006;55:1114–20.
Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S151–6.
Tam CS, Xie W, Johnson WD, Cefalu WT, Redman LM, Ravussin E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care. 2012;35:1605–10.
Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95.
Acknowledgements
Authors would like to thank the Senior Nurse Sandra Tavares (Centro Hospitalar de Entre o Douro e Vouga, Santa Maria da Feira, Portugal) for her technical assistance during the MMTT; and Lene Brus Albaek (Department of Biomedical Sciences, University of Copenhagen, Copenhagen, Denmark), and Lene Ravn (Department of Clinical Biochemistry, Rigshospitalet, Copenhagen, Denmark) for their technical assistance with assays.
Funding:
UMIB is funded by grants from Foundation for Science and Technology (FCT) Portugal (UID/ Multi/00215/2013 and UID/MULTI/0215/2016). JJH holds an unrestricted grant from the NNF Center for Basic Metabolic Research, Copenhagen, Denmark. The NNF Center for Basic Metabolic Research is an independent research institution at the University of Copenhagen, Denmark.
Author contributions:
Conceived and designed the experiments: MG, MN, JJH, MPM; performed the experiments: SSP, MG, RA, AMP, CBL, BH, MN, LH; analyzed data: SSP, CBL, BH; contributed reagents/materials/analysis tools: LH, JJH, MN, MMP; discussed the results and implications: SSP, MG, MN, JJH, MPM; wrote or reviewed the paper: SSP, MG, RA, AMP, CBL, BH, LH, JJH, MN, MPM. All authors have approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pereira, S.S., Guimarães, M., Almeida, R. et al. Biliopancreatic diversion with duodenal switch (BPD-DS) and single-anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) result in distinct post-prandial hormone profiles. Int J Obes 43, 2518–2527 (2019). https://doi.org/10.1038/s41366-018-0282-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0282-z
- Springer Nature Limited
This article is cited by
-
One-Stage Vs. Two-Step One Anastomosis Duodenal Switch (OADS/SADI-S): A Safety and Efficacy Single-Center Propensity-Score Matched Analysis
Obesity Surgery (2024)
-
Spanish Experience with Latero-Lateral Duodeno-Ileostomy + Sleeve Gastrectomy with Magnet Anastomosis System
Obesity Surgery (2024)
-
Single Anastomosis Duodeno-Ileostomy with Sleeve Gastrectomy/Single Anastomosis Duodenal Switch (SADI-S/SADS) IFSO Position Statement—Update 2023
Obesity Surgery (2024)
-
Remission of Type II Diabetes Mellitus after Duodenal Switch: the Contribution of Common Channel Length
Obesity Surgery (2023)
-
Towards precision medicine in bariatric surgery prescription
Reviews in Endocrine and Metabolic Disorders (2023)