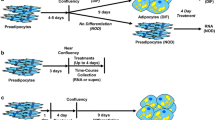
Abstract
Background/objectives
Polybrominated diphenyl ethers (PBDEs) are chemicals that were added to consumer products to reduce flammability but were deemed toxic and bioaccumulative and were phased out of commerce. Flame retardants (FRs) such as Dechlorane Plus (DP) were introduced as replacements. DP is being produced in high volumes and is detected in the environment, human milk, and human serum. Although human exposure to DP is evident, little is known about its potential effects on human health. We and others have shown that some FRs are potential obesogens, i.e., promote adipogenesis. However, the effects of DP on adipogenesis are not known.
Methods
Murine 3T3-L1 and human primary subcutaneous (Sc) and omental (Om) preadipocytes were differentiated in the presence of DP (0.001–10 µM) and adipogenic effects were measured. Further, the ability of DP to activate the adipogenic transcription factor peroxisome proliferator-activated receptor γ (PPARγ) was also assessed.
Results
We show that treatment of murine preadipocytes with DP significantly (p < 0.05) increased lipid accumulation (2.5-fold) and the mRNA expression of adipogenic markers: fatty acid binding protein 4 (Fabp4), lipoprotein lipase (Lpl), perilipin (Plin), adipsin, and adiponectin. DP also significantly (p < 0.05) increased the protein levels of selected mature adipocyte markers. We further show using luciferase reporter assays that DP increased PPARγ transcriptional activity by threefold (p < 0.05). When the PPARγ agonist was replaced by DP in the human preadipocyte differentiation cocktail, DP significantly (p < 0.05) increased the mRNA levels of adipogenic markers, PPARγ, FABP4, and PLIN in human Sc as well as Om cultures. Finally, PPARγ antagonist studies revealed that DP-mediated upregulation of adipogenic markers Fabp4 and Lpl did not occur via PPARγ activation.
Conclusion
The current study shows that DP can induce adipogenesis of murine and human preadipocytes. We show that, although DP can directly activate PPARγ, its adipogenic effects may be mediated via other pathways.
Similar content being viewed by others
Introduction
Stringent fire safety regulations set forth by the state of California led to the mass production and application of flame retardants (FRs), such as polybrominated diphenyl ethers (PBDEs), which are organic molecules that delay the spread of fire [1]. PBDEs were incorporated in various consumer products such as plastics, textiles, electronic equipment, and construction materials. However, due to their persistent, toxic, and bioaccumulative properties PBDEs were phased out of commerce [2]. As such, alternative FRs were introduced to replace PBDEs. These replacements include organophosphate FRs and polychlorinated FRs such as Dechlorane Plus (DP) [3, 4].
Since its introduction in the 1960s, DP has been used in products such as cable coatings, electrical wires, hard connectors in computers/televisions, and plastic roofing materials [5]. The annual production of DP is estimated to be 4500–5000 tonnes worldwide [6, 7]. Despite its high level of production over several decades, little attention has been given to DP and its potential effects on the environment and human health.
DP was first detected in the fish, air, and sediment in the Great Lakes of North America, and since then, other studies have reported its presence in biotic and abiotic samples [8,9,10,11]. DP levels have also been detected in human milk, serum, and hair [12,13,14]. These studies show that DP is ubiquitous in the environment and is detected in human samples.
It is well established that chemicals such as FRs, which are additives in consumer products, may act as endocrine-disrupting compounds (EDCs). EDCs are defined as chemicals that can interfere with the endocrine system [15]. We and others have previously shown that some FRs are potential obesogens, defined as chemicals that can promote the differentiation of preadipocytes into mature adipocytes—a process that is known as adipogenesis [16,17,18,19]. Evaluating whether FRs can potentially dysregulate preadipocyte differentiation in vitro and understanding the mechanisms by which they may do so can shed light on their potential role in promoting obesity and the metabolic syndrome. To the best of our knowledge, the effects of DP on the endocrine systems or on adipogenesis have not been previously studied.
Murine and human in vitro model systems have been used extensively to study adipogenesis. The process of adipogenesis is regulated by a tightly coordinated network of transcriptional activities that give rise to the expression of various proteins involved in establishing a mature adipocyte phenotype. At the core of this elaborate network are two key adipogenic transcription factors, CCAAT/enhancer-binding proteins (C/EBPs) and peroxisome proliferator-activated receptor γ (PPARγ), that oversee the process of terminal differentiation [20].
In this study, we used both murine 3T3-L1 and human primary preadipocytes to investigate the effects of DP on adipogenesis. Here we show for the first time that DP has obesogenic properties in murine and human primary preadipocytes. Further, we show that DP in luciferase reporter assays can activate the transcriptional activity of PPARγ. We also show that, despite DP’s ability to transactivate PPARγ, the transcriptional activity of this nuclear receptor was not necessary for the ability of DP to upregulate mRNA levels of adipogenic markers Fabp4 and Lpl.
Materials and methods
Murine cell culture and preadipocyte differentiation
3T3-L1 mouse embryonic fibroblasts were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 5.6 mM glucose (Hyclone, Mississauga, ON, Canada), supplemented with 10% bovine calf serum (ATCC, Manassas, VA, USA) and 1% penicillin/streptomycin (P/S) (Life Technologies, Burlington, ON, Canada). At 70% confluence, cells were passaged to 6-well plates and left to reach confluence. Two days post-confluence (day 0), cells were induced to differentiate in DMEM containing 5.6 mM glucose supplemented with 10% fetal bovine serum (FBS; Wisent, Montreal, QC, Canada) and 1% P/S (Life Technologies, Burlington, ON, Canada). Differentiation was induced with 500 µM 3-isobutyl-1-methylxanthine (IBMX) and 100 nM insulin (Roche Diagnostics, Laval, QC, Canada) (MI) with either DP (0.001–10 µM; AccuStandard, New Haven, CT, USA), dimethyl sulfoxide (DMSO (0.1% for 0.001–1 µM DP and 0.65% for 10 µM DP); solvent control), 250 nM dexamethasone (SigmaAldrich), or 5 µM rosiglitazone, a PPARγ agonist (SigmaAldrich). Two days post-differentiation, medium was replaced with 100 nM of insulin with either DP (0.001–10 µM) or solvent control (DMSO (0.1% for 0.001–1 µM DP and 0.65% for 10 µM DP). Medium was subsequently replaced every 2 days until the indicated time points (2, 4, 6, or 8 days). For the PPARγ antagonist studies, 5 μM of the irreversible antagonist GW9662 (SigmaAldrich) was added to the differentiation medium and replaced twice daily due to its short half-life [21].
Isolation of human primary preadipocytes and differentiation into adipocytes
Human primary preadipocytes were isolated from donor matched subcutaneous abdominal (Sc) and omental (Om) adipose tissue samples obtained from 4 weight-stable donors (2 females and 2 males) undergoing elective abdominal surgery, as approved by the Ottawa Health Science Network Research Ethics Board # 1995023-01 H. Mean age ( ± SD) of patients was 55 ± 12 years and mean body mass index ( ± SD) was 32 ± 8 kg/m2. Preadipocytes were isolated as described previously [22]. Briefly, adipose tissue was dissected to remove connective tissue and blood vessels and then digested with collagenase CLS type 1 (600 U/g of tissue; Worthington Biochemical, Lakewood, New Jersey, USA). The digested tissue was processed by progressive size filtration and centrifugation, and cells were exposed to erythrocyte lysis buffer. Preadipocytes were grown in DMEM containing 5.6 mM glucose supplemented with 10% FBS (Wisent, Montreal, QC, Canada), 1% P/S (Life Technologies, Burlington, ON, Canada), and 0.1% fungizone (Thermo Fisher Scientific, Burlington, ON, Canada) (designated growth medium).
For differentiation, preadipocytes were seeded in six-well plates and left to reach confluence. Differentiation was induced in growth medium containing 5.6 mM glucose and supplemented with adipogenic inducers (500 µM IBMX, (Sigma-Aldrich), 100 nM insulin, and 1 µM dexamethasone) supplemented with DP (10 µM), solvent control (0.65% DMSO), or positive control (200 nM rosiglitazone). Every 3 days, 50% of the medium was replaced for a total of 12 days.
Lipid staining and quantification
Preadipocytes were seeded and differentiated in 96-well plates following the differentiation protocols described above. At the indicated time points (day 8 for 3T3-L1 and day 15 for human primary preadipocytes), cells were fixed with paraformaldehyde (4%; Electron Microscopy Sciences, Hatfield, PA, USA) and stained with Nile Red (stains cytoplasmic lipid droplets; Sigma Aldrich) and 4,6-diamidino-2-phenylindole (DAPI; stains cell nuclei; Sigma Aldrich, Oakville, ON, Canada) as previously described [23]. For quantification of lipids in the 3T3-L1 cultures, Nile Red fluorescence was quantified at 485/528 nm (excitation/emission) and normalized to DAPI staining measured at 360/460 (excitation/emission). All data were then normalized to solvent control (data reported as fold change over solvent control). Fluorescence was measured using the Synergy 2 Microplate Reader (BioTek Instruments Inc., Winooski, VT, USA). Images of Nile Red and DAPI staining were taken using the Leica TCD SP8 confocal microscope (Leica Microsystems, Toronto, ON, Canada) at 10× or 20× magnification.
RNA extraction and real-time quantitative PCR (RT-qPCR)
Total RNA was extracted from differentiating preadipocytes (treated as described above) at the indicated time points using the RNeasy Mini Kit in which genomic DNA was removed using the RNase-Free DNase Kit (Qiagen, Mississauga, ON, Canada). RNA (0.5 µg) was reverse transcribed using iScript cDNA Synthesis Kit (Bio-Rad, Mississauga, ON, Canada) following the manufacturer’s recommendations. Sample cDNA was amplified and quantified in a CFX96-PCR Detection System using the iQSYBR SsoFast EvaGreen Supermix (Bio-Rad, Mississauga, ON, Canada). The primer pairs for each gene target are summarized in Table S1. All genes were normalized to β-actin levels and analyzed using the comparative CT (ΔΔCT) method.
Western blot analysis
Preadipocytes (3T3-L1) were differentiated as described above. On day 8 of differentiation, whole-cell extracts were prepared using RIPA (20 mM TRIS pH 7.5, 150 mM NaCl, 1% Sodium Deoxycholate, 2% IGEPAL CA-630 (aka NP-40), 0.4% sodium dodecyl sulfate (SDS), 1 mM EDTA, and 10% Glycerol; all from Thermo Fisher Scientific, Burlington, ON, Canada) buffer in the presence of protease inhibitors (Roche Diagnostics, Mississauga, ON, Canada). Total protein (20 µg) was resolved by SDS polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. Nonspecific binding sites were blocked by 5% non-fat milk, and the membranes were probed with the following antibodies directed against: FABP4 (AF3150; R&D Systems, Minneapolis, MN, USA), LPL (AF7197; R&D Systems, Minneapolis, MN, USA), and β-actin (13E5; Cell Signalling, Danvers, MA, USA). The membranes were then incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies and were visualized using Clarity Western ECL Substrate. Bands were detected using the ChemiDoc System and then quantified using the Image Lab software and normalized to β-actin levels (all from Bio-Rad, Mississauga, ON, Canada unless otherwise indicated).
Reporter gene assay
COS-7 cells were seeded in phenol red-free DMEM (Wisent, Montreal, QC, Canada) supplemented with 5% dextran-coated charcoal-stripped serum (Sigma-Aldrich). Twenty four hours post-seeding, cells were transfected with plasmid DNA using Fugene6 (Promega, Madison, WI, USA) according to the manufacturer’s recommendations. For PPARγ transcriptional assays, cells were transfected with 5 ng of pRL-CMV (renilla luciferase; internal control), 25 ng of pcDNA mPPARγ, and 125 ng of 3× PPARγ response element (PPRE)-luciferase (PPRE-luc). All plasmids were generous gifts from Dr. Jae Bum Kim [24]. Seven hours after transfection, cells were treated with vehicle control (0.1–0.65% DMSO), indicated concentration of DP, or 200 nM rosiglitazone. Twenty-four hours after treatment, cells were lysed using 1× Passive Lysis Buffer (Promega). Luciferase activity was quantified with the Dual Luciferase Assay Kit (Promega) using the Glomax96 Luminometer (Promega). Luciferase activity was normalized to renilla levels and then to vehicle control.
Statistical analysis
One-way or two-way analysis of variance, followed by a Dunnett’s or Tukey’s post-hoc test, were used when comparing multiple means within an experiment. Student’s t-test was used when comparing two means. Significance was defined as p < 0.05. Statistical analyses were performed using the SigmaPlot software 12.5 (San Jose, CA, USA).
Results
DP induces adipogenesis in murine preadipocytes
To investigate whether DP has obesogenic properties, we induced murine 3T3-L1 preadipocytes to differentiate in the presence of insulin and IBMX supplemented with either solvent control or varying concentrations of DP. At day 6 of differentiation, we assessed the mRNA levels of adipocyte markers by RT-qPCR. We found that treatment with 10 μM DP significantly increased the mRNA levels of mature adipocyte markers fatty acid binding protein 4 (Fabp4), lipoprotein lipase (Lpl), and perilipin (Plin) by 11-, 2.3-, and 11-fold, respectively, compared to solvent control (Fig. 1a–c). Further, 10 μM DP treatment significantly increased the mRNA levels of adipokines adiponectin and adipsin by 5.7- and 2.9-fold, respectively, compared to solvent control (Fig. 1d, e). At day 8 of differentiation, we assessed lipid accumulation in these cultures and found that treatment with 10 μM DP also significantly increased lipid accumulation by 2.5-fold compared to solvent control (Fig. 2a, b). In addition, to validate our mRNA findings at the protein level, we assessed the protein expression levels of select adipogenic markers at day 8 of differentiation. We found that, as expected, and consistent with our mRNA data, the protein levels of adipogenic markers were also increased (Fig. 2c–g). We show that the protein levels of PPARγ and LPL were significantly increased with 10 μM DP by 5.3- and 7.9-fold, respectively, compared to solvent control (Fig. 2d, e). The protein levels of PLIN and FABP4 were also increased by 19.4- and 17.5-fold with 10 μM DP, respectively, compared to solvent control, although these increases did not reach statistical significance (Fig. 2f, g). These results suggest that DP has adipogenic properties in murine cultures.
DP increases the mRNA levels of adipogenic markers in differentiated 3T3-L1 adipocytes. Murine 3T3-L1 preadipocytes were induced to differentiate in the presence of 500 µM IBMX and 100 nM insulin and supplemented with either solvent control (DMSO) or Dechlorane Plus (DP; 0.001–10 µM). At day 6 of differentiation, total RNA was isolated and a–e the expression levels of the indicated adipogenic markers were quantified by real-time qPCR. Levels were normalized to endogenous β-actin and expressed as fold over solvent control. Results from five separate experiments are graphically represented as mean ± S.E.M. *p < 0.05 compared to solvent control as assessed by one-way ANOVA with Dunnett’s post-hoc tests
DP increases lipid accumulation and protein expression levels of adipogenic markers in differentiated 3T3-L1 adipocytes. Murine 3T3-L1 preadipocytes were induced to differentiate in the presence of 500 µM IBMX and 100 nM insulin and supplemented with either solvent control (DMSO) or Dechlorane Plus (DP; 0.001–10 µM). At day 8 of differentiation, lipid accumulation was visualized using Nile Red (green) staining (a) and then quantified (b). Lipid accumulation, Nile Red fluorescence, was normalized to DAPI (blue) fluorescence and expressed as fold over solvent control from four separate experiments. At day 8 of differentiation, cellular proteins were extracted and equal amounts of solubilized cellular proteins were separated by SDS-PAGE and immunoblotted with antibodies against PPARγ, LPL, FABP4, and β-actin as a loading control (c). Densitometric data from three separate experiments, normalized to loading control, are graphically presented as means ± S.E.M (d–g). *p < 0.05 and ***p < 0.001 compared to solvent control as assessed by one-way ANOVA with Dunnett’s post-hoc tests
To further characterise the effects of DP on adipogenesis, we performed a time-course analysis in which we measured the mRNA levels of adipogenic transcription factors and adipogenic markers in 3T3-L1 preadipocytes differentiating in the presence of insulin and IBMX supplemented with either solvent control or 10 μM DP by RT-qPCR at days 2, 4, and 6. We found that DP treatment significantly increased the mRNA level of adipogenic transcription factor C/ebpα by twofold at day 2 of differentiation compared to solvent control and that this increase was maintained at days 4 and 6 by 2.2- and 3.1-fold, respectively, compared to solvent control (Fig. 3a). DP significantly increased the mRNA levels of other transcription factors Pparγ and sterol regulatory element binding protein 1 (Srebp1) only at day 4 of differentiation by 1.9- and 1.4-fold, respectively, compared to solvent control (Fig. 3b, c). The mRNA levels of Plin and Fabp4 increased at day 4 (4.6- and 4.2-fold, respectively) and this increase was maintained by day 6 (3.5- and 3.9-fold, respectively) compared to solvent control (Fig. 3d, e). Finally, the mRNA levels of adiponectin significantly increased at day 4 of differentiation with DP treatment compared to solvent control (Fig. 3f).
Evaluation of DP-mediated temporal gene expression profiles in differentiating 3T3-L1 preadipocytes. Murine 3T3-L1 preadipocytes were induced to differentiate in the presence of 500 µM IBMX and 100 nM insulin and supplemented with either solvent control (DMSO) or Dechlorane Plus (DP; 10 µM). At days 2, 4, and 6 of differentiation, total RNA was isolated and the expression levels of the indicated adipogenic markers were quantified by real-time qPCR (a–f). Levels were normalized to endogenous β-actin and expressed as fold over solvent control for respective time point. Results from four separate experiments are graphically represented as mean ± S.E.M. *p < 0.05, **p < 0.01, and ***p < 0.0001 compared to solvent control for respective time point as assessed by one-way ANOVA with Dunnett’s post-hoc tests
Lipid accumulation and mRNA levels of adipogenic markers during adipogenesis were also assessed in 3T3-L1 preadipocytes, which were induced to differentiate in standard differentiation conditions (in the presence of IMBX and insulin supplemented with dexamethasone, a glucocorticoid receptor (GR) agonist, or rosiglitazone, a PPARγ agonist. As expected, the levels of adipogenic markers and lipid accumulation were increased with dexamethasone and rosiglitazone compared to solvent control (Figure S1). Taken together, these data suggest that DP is adipogenic and a potential obesogenic compound.
DP-mediated adipogenesis may be via PPARγ transactivation
Since 3T3-L1 preadipocytes are able to differentiate with IBMX and insulin with dexamethasone or rosiglitazone (Figure S1), it was possible that DP may mediate its adipogenic effects via promoting GR activation, PPARγ activation, or both. To address this, we transfected COS-7 cells with constructs containing PPARγ cDNA and a 3× PPRE-luciferase reporter plasmid and treated with DP or rosiglitazone, serving as positive control. We found that 10 μM DP significantly increased reporter gene activity by threefold compared to solvent control (Fig. 4a). As expected, rosiglitazone also increased reporter gene activity by 16-fold compared to solvent control (Fig. 4b). We also transfected COS-7 cells with a GR expression vector and MMTV- or GRE-luciferase reporter plasmids; however, we found that DP had no effect on reporter gene activity for either condition (data not shown). Therefore, the data indicate that DP-mediated effects may be via PPARγ, but not GR, activation.
DP directly transactivates PPARγ. COS-7 cells were transfected as described in the Materials and Methods section with pcDNA-mPPARγ, 3× PPRE-luciferase, and pCMV-RL and treated with solvent control (DMSO), increasing amounts of DP (a), or 200 nM rosiglitazone (b). Twenty-four hours after treatment, reporter gene activity was determined by luminescence. Results from three separate experiments are graphically represented as mean ± S.E.M. *p < 0.05 and **p < 0.01 compared to solvent control as assessed by one-way ANOVA with Dunnett’s post-hoc tests
DP induces adipogenesis in Sc and Om human primary preadipocytes
Given that 10 μM DP increases adipogenesis in a murine model system, we next asked whether DP can also increase human adipogenesis. In addition, since we showed that DP can directly activate PPARγ, we replaced the PPARγ agonist in the human differentiation cocktail with DP. We induced donor matched human primary Sc and Om preadipocytes to differentiate in the presence of insulin, IBMX, and dexamethasone supplemented with either DP or solvent control. At day 12 of differentiation, we measured the mRNA levels of adipogenic markers by RT-qPCR. We found that, in Sc cultures, treatment with 10 μM DP significantly increased the mRNA levels of adipogenic transcription factors PPARγ and SREBP-1 by 1.8- and 3-fold, respectively, compared to solvent control (Fig. 5b, c). In the Om cultures, PPARγ mRNA levels were significantly increased by 1.5-fold, whereas SREBP-1 mRNA levels only trended to increase with DP treatment compared to solvent control (Fig. 5h, i). Furthermore, we assessed the mRNA levels of adipocyte markers and found that in Sc cultures DP treatment significantly increased the levels of FABP4, LPL, and PLIN by 7.4-, 2.7-, and 3.6-fold, respectively, compared to solvent control (Fig. 5d–f). In Om cultures, DP significantly increased FABP4 and PLIN mRNA levels by 6.1- and 2.8-fold, respectively, compared to solvent control but had no effect on LPL levels (Fig. 5j–l). As expected, in the positive control treatments the mRNA levels of adipogenic markers in Sc and Om cultures were increased in the presence of rosiglitazone compared to solvent control (Figure S2). Taken together, these results show that DP can induce adipogenesis in human cultures and that DP may act as a PPARγ agonist in this model system.
DP increases the mRNA levels of adipogenic markers in differentiating human primary subcutaneous and omental preadipocytes. Human primary subcutaneous abdominal and omental preadipocytes were induced to differentiate in the presence of 500 µM IBMX, 100 nM insulin, and 1 µM dexamethasone and supplemented with either solvent control (DMSO) or 10 µM DP. At day 15 of differentiation, cultures were photographed and lipids were visualized using Nile Red (green) staining (a and g). At day 12 of differentiation, total RNA was isolated and the expression levels of the indicated adipogenic markers were quantified by real-time qPCR (b–f) and (h–l). Levels were normalized to endogenous β-actin and expressed as fold over solvent control. Results from four separate experiments are graphically represented as mean ± S.E.M. *p < 0.05 and **p < 0.01 compared to solvent control as assessed by paired t-test
DP-mediated increase in Fabp4 and Lpl in 3T3-L1 cells are independent of PPARγ transactivation
Since we showed that DP can directly activate PPARγ using a PPRE-dependent luciferase assay and was able to replace rosiglitazone in the differentiation cocktails used for primary human cells, we asked whether inhibiting PPARγ activity would completely or partially abolish the adipogenic effects of DP. To test this, we induced murine 3T3-L1 preadipocytes to differentiate in the presence of insulin and IBMX supplemented with either solvent control, DP, or rosiglitazone with or without the PPARγ antagonist, GW9662. At day 6 of differentiation, we measured the mRNA levels of the adipogenic markers, Fabp4 and Lpl, which have PPREs at the promoter and therefore are targets of PPARγ activation [25, 26]. As expected, GW9662 significantly inhibited the rosiglitazone-mediated Fabp4 and Lpl mRNA expression by 35% and 47%, respectively (Fig. 6b, c). However, contrary to our expectations, GW9662 had no effect on the DP-mediated increases in Fabp4 and Lpl, suggesting that, although DP can directly activate PPARγ in a luciferase reporter assay, its adipogenic effects may also be exerted through other mechanisms.
DP-mediated increase in adipogenic markers Fabp4 and Lpl gene expression is independent of PPARγ activity. Murine 3T3-L1 preadipocytes were induced to differentiate in the presence of 500 µM IBMX and 100 nM insulin and supplemented with either solvent control (DMSO), 10 µM DP, or 5 µM rosiglitazone. All samples were treated with either PPARγ inhibitor GW9662 (5 µM) or Veh (DMSO). At day 8 of differentiation, lipids and nuclei were visualized using Nile Red (green) and DAPI (blue) staining, respectively (a). At day 6 of differentiation, total RNA was isolated and the expression levels of Fabp4 (b) and Lpl (c) were quantified by real-time qPCR. Levels were normalized to endogenous β-actin and expressed as fold over respective solvent control. Results from three separate experiments are graphically represented as mean ± S.E.M. ##p < 0.01 and ###p< 0.001 compared to respective solvent control and *p < 0.05 between indicated pairs as assessed by one-way ANOVA with Tukey post-hoc tests
Discussion
DP is one of the replacement FRs for the toxic and bioaccumulative PBDEs [2, 4]. However, even though DP has been used commercially for several decades and has been detected in the environment and in humans, not much is known about its effects on human health. In this study, we show for the first time that DP has adipogenic properties. We show this not only in a murine model system but also in human primary cells (Figs. 1, 2, and 5). Our findings are in line with previous work by our group and others which show that other FRs can have adipogenic effects [16,17,18]. The other class of replacement FRs, such as the organophosphate mixture, Firemaster 550® (FM550) and its components triphenyl phosphate (TPP) and isopropylated triphenyl phosphates (IPTP), induced adipogenesis in 3T3-L1 cultures, where, similar to the present study, these FRs increased the lipid accumulation and gene expression of adipogenic markers [16]. Also, replacing PPARγ agonist with FM550, TPP, or IPTP in the human preadipocyte differentiation cocktail increased adipogenesis in human primary Sc preadipocytes [17]. As such, our data contribute to the growing concern that environmental pollutants, including FRs, may act as obesogens.
In this study, we show that DP can directly activate PPARγ, a transcription factor considered as the master regulator of adipogenesis [20] (Fig. 4a). Our group and others have previously shown that FRs including FM550 and its components TPP and IPTP as well as plasticizers including Bisphenol A (BPA) and its analog BPS can activate PPARγ and increase adipogenesis [16,17,18, 27]. In the present study, we show that the mRNA levels of C/ebpα were increased by day 2 of differentiation with DP treatment compared to background conditions that included insulin, IBMX, and solvent control (Fig. 3a). At early stages of adipogenesis, increased levels of cyclic adenosine monophosphate (increased by IBMX treatment) raise C/EBPβ expression, which in turn induces the expression of both C/EBPα and PPARγ [20, 28]. It may be possible that DP treatment led to the activation of C/EBPβ through yet unknown mechanisms or perhaps it activated existing PPARγ, which in turn may have induced the mRNA expression of C/ebpα.
The environmental obesogen hypothesis suggests that environmental pollutants can lead to differentiation of preadipocytes into adipocytes via a variety of mechanisms [19, 29]. Interestingly, although in this study we showed that DP can directly activate PPARγ, we found that the DP-mediated increase in the adipogenic markers Fabp4 and Lpl mRNA were independent of PPARγ activation (Fig. 6). We have previously shown that BPA and BPS directly activated PPARγ and that concomitantly BPA- and BPS-mediated increase in Fabp4 and Lpl mRNA were in fact PPARγ dependent [27]. Similarly, TPP- and IPTP-mediated increase in Fabp4 mRNA levels measured in differentiated 3T3-L1 adipocytes were also PPARγ dependent [16]. However, in the latter study, although TPP and IPTP could activate PPARγ, TPP- and IPTP-mediated lipid accumulation was not PPARγ dependent [16]. These data agree with growing evidence which illustrates that, although these environmental pollutants are generally referred to as obesogens, they have various and distinct modes of action.
The data also suggest that the DP-mediated adipogenic effects may involve more than one mechanism of action and may be gene specific. For example, since Fabp4 and Lpl possess response elements other than PPREs at their promoter regions such as CCAAT/enhancer-binding protein alpha-response elements (recognized by C/EBPα) and sterol regulatory elements (recognized by SREBP-1), respectively, it is possible that by day 6 of differentiation other transcription factors such as C/EBPα and SREBP-1 could compensate for the antagonistic effects of GW9662 on PPARγ activation in the DP-treated cells [30, 31]. Meaning that, although we never tested whether DP can activate C/EBPα or SREBP-1, it is possibly that, in addition to direct PPARγ activation, DP may mediate induction of Fabp4 and Lpl via directly or indirectly activating C/EBPα or SREBP-1.
Nevertheless, replacement of the PPARγ agonist in the human preadipocyte differentiation cocktail with DP increased human adipogenesis. These findings are consistent with our previous studies where we showed that TPP and IPTP could replace PPARγ agonist and mediate Sc preadipocyte differentiation [17]. Although we did not assess whether DP could replace other adipogenic inducers, such as dexamethasone, a GR agonist, its indirect or direct effects on other transcription factors cannot be ruled out in this model system. For example, we have shown previously that BPA and BPS induced human Sc adipogenesis when they were substituted for the GR agonist in the differentiation cocktail, suggesting that BPA and BPS could mediate adipogenesis via GR-related pathways, when concomitantly they could also directly activate PPARγ [32].
Finally, to the best of our knowledge, we are the first to assess the effects of environmental pollutants on human Om differentiation. Om (visceral) adipose tissue expansion in obesity is associated with metabolic syndrome such as insulin resistance and diabetes [33]. In this study, we show that DP treatment increased adipogenesis of both the Sc and Om preadipocytes. In addition, most adipogenic genes were upregulated by DP treatment in both Sc and Om preadipocyte, with the exception of LPL mRNA levels, where DP had no effect on LPL mRNA expression in Om cultures. This indicates that DP may have gene-specific effects in different depots. Future studies may investigate the relevance of the in vitro findings by performing in vivo studies using animal models exposed to the chemical either in utero and through lactation or as mature animals. Such studies have been previously performed for other obesogenic compounds, such as bisphenols and other FRs [34]. One caveat of this study is the relatively high concentration of DP that was needed to achieve adipogenesis. Even though the concentrations needed for adipogenesis are higher than the ones observed in the population, it is widely accepted that it is difficult to extrapolate in vitro doses to human exposure. In addition, the inducers used in vitro for optimal differentiation often exceed the physiological concentrations in vivo, and therefore, it is also expected that chemicals may be required to be used at higher concentrations in in vitro model systems. Furthermore, human exposure to chemicals is chronic and some of the chemicals may have lipophilic properties and may be accumulative in tissues, including the fat tissue. In fact, DP was deemed bioaccumulative in several species [35], showing that it may accumulate in the adipose tissue. In addition, chemicals may adhere to the plastic or media components in vitro, and therefore, the intracellular concentrations may be different than the ones applied. More knowledge is needed on the pharmacokinetics of DP in humans and in cell culture in order to be able to model how the doses used in vitro correlate to the in vivo exposure. Further, the relevance to humans remains to be seen in epidemiological studies in which exposure levels to DP may correlate with development of obesity and metabolic disease.
In summary, we have shown for the first time that the replacement FR DP has potential obesogenic effects. We show that DP induces adipogenesis in murine and human cultures. DP can directly activate the master regulator of adipogenesis, PPARγ. However, some DP-mediated adipogenic end points were independent of PPARγ activation, suggesting that other potential modes of actions of DP may be involved. Future work should attempt to delineate the scope of potential DP-mediated mechanisms of action regulating adipogenesis.
References
Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, et al. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ Sci Technol. 2012;46:13056–66.
Siddiqi MA, Laessig RH, Reed KD. Polybrominated diphenyl ethers (PBDEs): new pollutants-old diseases. Clin Med Res. 2003;1:281–90.
Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, et al. Alternate and new brominated flame retardants detected in U.S. house dust. Environ Sci Technol. 2008;42:6910–6.
Fan X, Kubwabo C, Rasmussen PE, Wu F. Non-PBDE halogenated flame retardants in Canadian indoor house dust: sampling, analysis, and occurrence. Environ Sci Pollut Res Int. 2016;23:7998–8007.
Zheng XB, Luo XJ, Zeng YH, Wu JP, Mai BX. Sources, gastrointestinal absorption and stereo-selective and tissue-specific accumulation of Dechlorane Plus (DP) in chicken. Chemosphere. 2014;114:241–6.
Ren N, Sverko E, Li YF, Zhang Z, Harner T, Wang D, et al. Levels and isomer profiles of dechlorane plus in Chinese air. Environ Sci Technol. 2008;42:6476–80.
Wang DG, Yang M, Qi H, Sverko E, Ma WL, Li YF, et al. An Asia-specific source of dechlorane plus: concentration, isomer profiles, and other related compounds. Environ Sci Technol. 2010;44:6608–13.
Hoh E, Zhu L, Hites RA. Dechlorane plus, a chlorinated flame retardant, in the Great Lakes. Environ Sci Technol. 2006;40:1184–9.
Sverko E, Tomy GT, Reiner EJ, Li YF, McCarry BE, Arnot JA, et al. Dechlorane plus and related compounds in the environment: a review. Environ Sci Technol. 2011;45:5088–98.
Sverko E, Tomy GT, Marvin CH, Zaruk D, Reiner E, Helm PA, et al. Dechlorane plus levels in sediment of the lower Great Lakes. Environ Sci Technol. 2008;42:361–6.
Xian Q, Siddique S, Li T, Feng YL, Takser L, Zhu J. Sources and environmental behavior of dechlorane plus--a review. Environ Int. 2011;37:1273–84.
Siddique S, Xian Q, Abdelouahab N, Takser L, Phillips SP, Feng YL, et al. Levels of dechlorane plus and polybrominated diphenylethers in human milk in two Canadian cities. Environ Int. 2012;39:50–5.
Ren G, Yu Z, Ma S, Li H, Peng P, Sheng G, et al. Determination of Dechlorane Plus in serum from electronics dismantling workers in South China. Environ Sci Technol. 2009;43:9453–7.
Zheng J, Wang J, Luo XJ, Tian M, He LY, Yuan JG, et al. Dechlorane Plus in human hair from an e-waste recycling area in South China: comparison with dust. Environ Sci Technol. 2010;44:9298–303.
Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology. 2012;153:4097–110.
Tung EWY, Ahmed S, Peshdary V, Atlas E. Firemaster(R) 550 and its components isopropylated triphenyl phosphate and triphenyl phosphate enhance adipogenesis and transcriptional activity of peroxisome proliferator activated receptor (Ppargamma) on the adipocyte protein 2 (aP2) promoter. PLoS ONE. 2017;12:e0175855.
Tung EWY, Peshdary V, Gagne R, Rowan-Carroll A, Yauk CL, Boudreau A, et al. Adipogenic effects and gene expression profiling of Firemaster(R) 550 components in human primary preadipocytes. Environ Health Perspect. 2017;125:097013.
Pillai HK, Fang M, Beglov D, Kozakov D, Vajda S, Stapleton HM, et al. Ligand binding and activation of PPARgamma by Firemaster(R) 550: effects on adipogenesis and osteogenesis in vitro. Environ Health Perspect. 2014;122:1225–32.
Grun F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147(6 Suppl):S50–5.
Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–73.
Li X, Ycaza J, Blumberg B. The environmental obesogen tributyltin chloride acts via peroxisome proliferator activated receptor gamma to induce adipogenesis in murine 3T3-L1 preadipocytes. J Steroid Biochem Mol Biol. 2011;127:9–15.
Peshdary V, Gagnon A, Sorisky A. Effect of high glucose concentration on human preadipocytes and their response to macrophage-conditioned medium. Can J Diabetes. 2016;40:411–8.
Greenspan P, Mayer EP, Fowler SD. Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol. 1985;100:965–73.
Kim JB, Wright HM, Wright M, Spiegelman BM. ADD1/SREBP1 activates PPARgamma through the production of endogenous ligand. Proc Natl Acad Sci USA. 1998;95:4333–7.
Frohnert BI, Hui TY, Bernlohr DA. Identification of a functional peroxisome proliferator-responsive element in the murine fatty acid transport protein gene. J Biol Chem. 1999;274:3970–7.
Fan C, Yan J, Qian Y, Wo X, Gao L. Regulation of lipoprotein lipase expression by effect of hawthorn flavonoids on peroxisome proliferator response element pathway. J Pharmacol Sci. 2006;100:51–8.
Ahmed S, Atlas E. Bisphenol S- and bisphenol A-induced adipogenesis of murine preadipocytes occurs through direct peroxisome proliferator-activated receptor gamma activation. Int J Obes (Lond). 2016;40:1566–73.
Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–96.
Holtcamp W. Obesogens: an environmental link to obesity. Environ Health Perspect. 2012;120:a62–8.
Shin J, Li B, Davis ME, Suh Y, Lee K. Comparative analysis of fatty acid-binding protein 4 promoters: conservation of peroxisome proliferator-activated receptor binding sites. J Anim Sci. 2009;87:3923–34.
Schoonjans K, Gelman L, Haby C, Briggs M, Auwerx J. Induction of LPL gene expression by sterols is mediated by a sterol regulatory element and is independent of the presence of multiple E boxes. J Mol Biol. 2000;304:323–34.
Boucher JG, Ahmed S, Atlas E. Bisphenol S induces adipogenesis in primary human preadipocytes from female donors. Endocrinology. 2016;157:1397–407.
Patel P, Abate N. Body fat distribution and insulin resistance. Nutrients. 2013;5:2019–27.
Heindel JJ, Newbold R, Schug TT. Endocrine disruptors and obesity. Nat Rev Endocrinol. 2015;11:653–61.
Feo ML, Baron E, Eljarrat E, Barcelo D. Dechlorane Plus and related compounds in aquatic and terrestrial biota: a review. Anal Bioanal Chem. 2012;404:2625–37.
Acknowledgements
Thank you to the patients and surgeons of The Ottawa Hospital for human adipose tissue samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Peshdary, V., Calzadilla, G., Landry, A. et al. Dechlorane Plus increases adipogenesis in 3T3-L1 and human primary preadipocytes independent of peroxisome proliferator-activated receptor γ transcriptional activity. Int J Obes 43, 545–555 (2019). https://doi.org/10.1038/s41366-018-0072-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0072-7
- Springer Nature Limited