Abstract
Primary effusion lymphoma (PEL) is an aggressive type of non-Hodgkin lymphoma localized predominantly in body cavities. Kaposi’s sarcoma-associated herpes virus (KSHV) is the causative agent of PEL. PEL is an incurable malignancy and has extremely poor prognosis when treated with conventional chemotherapy. Immunomodulatory drugs (IMiDs) lenalidomide and pomalidomide are Food and Drug Administration-approved drugs for the treatment of various ailments. IMiDs display pronounced antiproliferative effect against majority of PEL cell lines within their clinically achievable concentrations, by arresting cells at G0/G1 phase of cell cycle and without any induction of KSHV lytic cycle reactivation. Although microarray examination of PEL cells treated with lenalidomide revealed activation of interferon (IFN) signaling, blocking the IFN pathway did not block the anti-PEL activity of IMiDs. The anti-PEL effects of IMiDs involved cereblon-dependent suppression of IRF4 and rapid degradation of IKZF1, but not IKZF3. Small hairpin RNA-mediated knockdown of MYC enhanced the cytotoxicity of IMiDs. Bromodomain (BRD) and extra-terminal domain (BET) proteins are epigenetic readers, which perform a vital role in chromatin remodeling and transcriptional regulation. BRD4, a widely expressed transcriptional coactivator, belongs to the BET family of proteins, which has been shown to co-occupy the super enhancers associated with MYC. Specific BRD4 inhibitors were developed, which suppress MYC transcriptionally. Lenalidomide displayed synergistic cytotoxicity with several structurally distinct BRD4 inhibitors (JQ-1, IBET151 and PFI-1). Furthermore, combined administration of lenalidomide and BRD4 inhibitor JQ-1 significantly increased the survival of PEL bearing NOD–SCID mice in an orthotopic xenograft model as compared with either agent alone. These results provide compelling evidence for clinical testing of IMiDs alone and in combination with BRD4 inhibitors for PEL.
Similar content being viewed by others
Introduction
Primary effusion lymphoma (PEL) is an aggressive type of non-Hodgkin lymphoma localized predominantly in body cavities, which is observed primarily in patients with HIV infection1 and is associated with infection by Kaposi’s sarcoma-associated herpesvirus (KSHV).2 The prognosis of PEL is extremely poor with a median survival of 4 and 6 months in HIV-positive and -negative patients, respectively.3 Thus, there is an urgent need to develop new treatment regimens for PEL.
Thalidomide and its analogues, lenalidomide and pomalidomide, are collectively known as immunomodulatory drugs (IMiDs).4 Thalidomide was originally introduced as a sedative but was later withdrawn from the market due to birth defects and neuropathy.5 Subsequently, thalidomide was found to significantly improve the response rate and survival of patients with multiple myeloma (MM).6 The second-generation IMiDs, lenalidomide and pomalidomide, possess more potent anti-myeloma, anti-inflammatory and immunomodulatory activities than thalidomide.5 Thalidomide directly binds to and inhibits the cereblon (CRBN) ubiquitin ligase7 and CRBN has been shown to be required for the anti-myeloma activity of IMiDs.8, 9 Lenalidomide-bound CRBN acquires the ability to degrade Ikaros family zinc finger proteins 1 and 3 (IKZF1 and IKZF3), two specific B-cell transcription factors. The loss of IKZF1 and IKZF3 was shown to be both necessary and sufficient for the anti-myeloma effect of lenalidomide.10, 11
Bromodomain (BRD)-containing proteins regulate lysine acetylation,12 an important mechanism to regulate chromatin structure. BRD and extra-terminal (BET) subfamily has four members, BRD2, BRD3, BRD4 and BRDT; all of them share a common domain structure. In recent times, potent and highly specific BRD4 inhibitors were developed.13, 14, 15 These inhibitors were shown to suppress MYC transcriptionally and demonstrate promising preclinical activity against MYC-driven cancers.16, 17, 18
Here we report that PEL cells are highly sensitive to IMiDs, lenalidomide and pomalidomide within their physiologically achievable concentrations. Furthermore, we discovered that low-dose combinations of IMiDs with BRD4 inhibitors displayed synergistic antiproliferative activity against PEL.
Results
IMiDs show selective cytotoxicity towards PEL
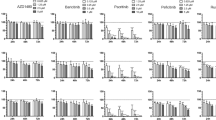
To examine the cytotoxicity of IMiDs against PEL, we treated a panel of 35 logarithmically growing hematopoietic cell lines (Supplementary Table 1) for 5 days with increasing concentrations of IMiDs. At concentrations that are achievable clinically (2.2 μM for lenalidomide19 and 179 nM for pomalidomide20), six out of the nine PEL cell lines (BC-3, BCBL-1, JSC-1, VG-1, UMPEL-1 and UMPEL-3) were sensitive to IMiDs with IC50 (50% inhibitory concentration) ranging from 0.2 to 1.2 μM and from 32 to 111 nM for lenalidomide and pomalidomide, respectively (Figure 1a and Table 1), whereas BC-1, BCP-1 and APK-1 were sensitive to only higher doses of IMiDs with IC50 ranging from 2.6 to 10 μM and from 226 to 744 nM for lenalidomide and pomalidomide, respectively (Figure 1a and Table 1). MM.1S (Myeloma), Daudi (Burkitt’s lymphoma) and TMD8 (activated B-cell diffuse large B-cell lymphoma (ABC-DLBCL)) were also sensitive to both IMiDs with IC50 ranging from 0.2 to 2.1 μM and from 38 to 113 nM for lenalidomide and pomalidomide, respectively (Supplementary Figure S1). All the remaining cell lines were either resistant to IMiDs or required higher doses for a moderate effect (Supplementary Figure S1). Consistent with its known requirement for in vivo metabolism,8 thalidomide did not have any major effect on the growth of any of the cell lines tested or required a high dose for moderate effect (Figure 1a and Supplementary Figure S1). Treatment of PEL cells with IMiDs resulted in G1 cell cyle arrest (Figure 1b and Supplementary Figure S2A). In contrast, IMiDs had no major effect on cell cycle progression in DG-75 (Burkitt lymphoma) and OCILY-8 (germinal center B-cell diffuse large B-cell lymphoma) cells that were resistant to their antiproliferative effect (Figure 1b and Supplementary Figure S2A).
IMiDs are effective against PEL. (a) PEL cell lines were treated with increasing concentrations of lenalidomide, pomalidomide and thalidomide for 5 days, and cell viability was measured using an MTS (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. The values shown are mean±s.e. (n=3) of a representative experiment performed in triplicate for three times. (b) Cell cycle analysis of BC-3, BCBL-1, JSC-1 and DG-75 cells treated with indicated doses of lenalidomide (Len) and pomalidomide (Pom) for 48 h. Cells were stained with propidium iodide and analyzed by flow cytometry. Data are representative of more than three individual experiments. (c) Heat map representation of 992 genes that are up- or downregulated (P<0.05) in BC-3 and BCBL-1 cells following 24 h treatment with lenalidomide (5 μM). (d) Gene Set Enrichment Analysis showing enrichment of gene sets, which are involved in IFN signaling among genes affected by lenalidomide treatment in PEL. NES, normalized enrichment score; q, false discovery rate.
Gene Set Enrichment Analysis identifies activation of IFN signaling in PEL by lenalidomide
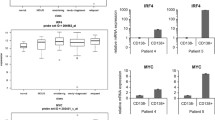
To delineate the gene network affected by lenalidomide, BC-3 and BCBL-1 cells were treated with lenalidomide (5 μM) for 24 h followed by genome-wide microarray analysis. Unsupervised hierarchical clustering separated samples according to their treatment group, indicating a common transcriptional response to treatment with lenalidomide in PEL (Figure 1c). Rather than inducing nonspecific changes in gene expression, lenalidomide induced significant changes in a limited number of genes. Thus, there were 992 genes (390 down- and 602 upregulated genes) whose expressions were changed significantly (P<0.05) in both the cell lines. We used a Gene Set Enrichment Analysis program to identify functional gene sets that were enriched in PEL cells on treatment with lenalidomide.21 Among the gene signatures identified by this analysis were gene sets containing genes that are known targets of interferon (IFN) and MYC signaling pathways (Figure 1d and Supplementary Figure S2B). We used quantitative reverse transcriptase–PCR to confirm upregulation of IFNs and IFN-specific genes by lenalidomide in PEL (Supplementary Figure S2C).
IFNs α, β and γ are cytotoxic to PEL but are not essential for the antiproliferative effect of IMiDs
In the case, IMiDs block the proliferation of PEL by activating the IFN pathway, then treatment with recombinant IFNs should mimic the effect of IMiDs. To test this hypothesis, we treated a panel of cell lines with increasing concentrations of recombinant IFNs α, β and γ. All the PEL cell lines were sensitive to recombinant IFNs α, β or γ (Figure 2a). In particular, BC-3, BCBL-1 and JSC-1 were highly sensitivity to IFNs α and β. Although BC-1 and VG-1 cells were relatively resistant to IFNs α and β, they were sensitive to IFN-γ. In contrast, DG-75 and BJAB, the two IMiD-resistant cell lines, showed little or no inhibitory effect on treatment with any IFN (Figure 2a).
PEL cells are sensitive to IFNs α, β and γ. (a) BC-3, BCBL-1, JSC-1, BC-1, VG-1, BJAB and DG-75 cells were treated with indicated concentrations of recombinant IFNs for 5 days and cell viability was measured using an MTS (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. The values shown are mean±s.e. (n=3). (b) Blocking of IFNs α, β and γ (IFNs αβγ) together did not block the antiproliferative activity of IMiDs in PEL. BC-3 and BCBL-1 were treated with indicated concentrations of IMiDs, IFNs αβγ and IFNs αβγ-blocking antibodies combined (Block Abs Combi) for 4 days. IFN-α-blocking antibody was used at a concentration, which blocks 450 U/ml of IFN-α by 50%; IFN-β-blocking antibody was used at a concentration, which blocks 350 U/ml of IFN-α by 50%; and IFN-γ-blocking antibody was used at a concentration, which blocks 1090 U/ml of IFN-γ by 50%. Isotype antibodies (Iso Ab) corresponding to same species was used as control. The values shown are mean±s.e. (n=3). (c) IFNs are not secreted into the supernatants of PEL cells on treatment with IMiDs. BC-3 and BCBL-1 cells were treated with dimethyl sulfoxide (DMSO, vehicle control), lenalidomide 5 μM (Len) and pomalidomide 500 nM (Pom) for 48 h. Recombinant IFNs (rIFNs)—α, β and γ—were used at a concentration of 100, 200 and 1000 pg/ml, respectively, as positive controls.
Interestingly, the neutralizing antibodies against IFNs α, β and γ, when used singly (Supplementary Figure S3) or in combination (Figure 2b), did not block the antiproliferative effect of IMiDs against PEL, although they effectively blocked the antiproliferative effect of their respective recombinant IFNs. Further none of the IFNs were secreted into the supernatants of the PEL cells treated with IMiDs (Figure 2c). Collectively, these results suggest that activation of the IFN pathway is not solely responsible for the antiproliferative effect of IMiDs against PEL.
IMiDs have no effect on KSHV lytic replication in PEL
It was conceivable that induction of KSHV lytic replication contributed to the cell death and activation of IFN signaling observed following treatment with IMiDs. KSHV replication and transcription activator is a master regulator and marker for lytic reactivation.22 Treatment of PEL cell lines with IMiDs failed to induce replication and transcription activator expression, as determined by immunoblotting (Supplementary Figure S4A). In addition, we failed to detect infectious virions in the supernatant from IMiD-treated PEL cells when assayed on 293-PAN-Luc reporter cell line (Supplementary Figure S4B).23 Thus, IMiDs do not induce lytic reactivation of KSHV.
IMiDs downregulate IRF4 expression in PEL
MM cells are addicted to IRF4 (Shaffer et al.24) and the antiproliferative activity of lenalidomide and pomalidomide in myeloma and ABC-DLBCL is associated with downregulation of IRF4.25, 26 To delineate the role of IRF4 in the survival of PEL and in their response to IMiDs, we used western blotting to compare its expression in a panel of 35 cell lines comprising 11 hematologic malignancies, including 9 PEL cell lines. Expression of IRF4 was robust in all cell lines derived from PEL, MM, ABC-DLBCL, Waldenstrom macroglobulinemia and Hodgkin lymphoma, but was weak to absent in the cell lines derived from other hematologic malignancies (Figure 3a). Treatment of BC-3, BCBL-1 and JSC-1 with IMiDs resulted in significant decrease in the expression of IRF4 and its downstream target MYC, thus suggesting that IMiDs exert their cytotoxicity toward PEL by downregulating IRF4 (Figure 3b). In contrast, IMiDs had no significant effect on the levels of IRF4 and MYC in DG-75 cells (Figure 3b).
Uniform expression of IRF4 in PEL. (a) Expression of IRF4 in a panel of 35 hematological cancer cell lines. Cell lysates were prepared from logarithmically growing cell lines and blotted for IRF4 and GAPDH (glyceraldehyde 3-phosphate dehydrogenase). Blots are representative of three individual experiments. PEL, primary effusion lymphoma; CML, chronic myelogenous leukemia; T-ALL, T-cell acute lymphoblastic leukemia; AML, acute myelogenous leukemia; ABC-DLBCL, activated B-cell diffuse large B-cell lymphoma; GCB-DLBCL, germinal center B-cell diffuse large B-cell lymphoma; MCL, mantle cell lymphoma; WM, Waldenstrom macroglobulinemia; MW, molecular weight. (b) Immunoblot analysis showing the effect of lenalidomide (Len) and pomalidomide (Pom) at the indicated doses for 48 h on the expression of IRF4, MYC and TUBA (Tubulin, loading control) in BC-3, BCBL-1, JSC-1 and DG-75 cells. Blots are representative of three individual experiments.
PEL cells have constitutive nuclear factor-κB (NF-κB) activity due to the presence of KSHV viral proteins27, 28, 29 and aberrant NF-κB activity has been shown to upregulate the expression of IRF4.30 To test whether IMiDs repress IRF4 expression by inhibiting NF-κB pathway, BC-3 and BCBL-1 cells stably expressing an NF-κB promoter-driven luciferase reporter construct were treated with increasing concentrations of IMiDs. As shown in Supplementary Figure S5A, IMiDs failed to block NF-κB promoter-driven luciferase activity. Further, no change in the secretion of interleukin-6, a known target of classical NF-κB pathway,31 processing of p100 into p52 and expression of NF-κB pathway proteins were observed in IMiD-treated PEL cells (Supplementary Figures S5B and C). Taken together, these results demonstrate that IMiDs have no significant effect on the constitutive NF-κB activity present in PEL cells.
PEL cells are addicted to IRF4 for survival
To provide genetic evidence in support of the hypothesis that IMiDs exert their cytotoxic effect against PEL through downregulation of IRF4, we studied the effect of IRF4 knockdown in BC-3 cells. For this purpose, we generated a polyclonal population of BC-3 cells expressing a tetracycline-inducible-H1 (TO/H1) promoter-driven small hairpin RNA (shRNA) targeting IRF4 (shIRF4) (Supplementary Figures S6A and B) followed by generation of single cell clones (Supplementary Figure S6C). On treatment with doxycycline (Dox), significant downregulation of IRF4 was observed in a number of clones (Supplementary Figure S6C). Downregulation of MYC, a target of IRF4,24 and cleavage of poly (ADP-ribose) polymerase on Dox treatment was observed only in those clones where IRF4 was downregulated (Figure 4a and Supplementary Figure S6C). Strikingly, cellular proliferation was decreased rapidly only in clones were IRF4 was downregulated on treatment with Dox (Figure 4b and Supplementary Figure S6D). Treatment of a clone, BC-3-shIRF4-F11, with Dox resulted in G1 cell cycle arrest (Figure 4c) and the appearance of cells with condensed and fragmented nuclei suggestive of apoptosis (Figure 4d), a finding further confirmed by staining with annexinV/propidium iodide (Figure 4e). In contrast, Dox treatment had no significant effects on cell cycle progression and apoptosis in BC-3-shSCR cells (Figures 4c–e). Collectively, the above results suggest that downregulation of IRF4 is toxic to BC-3 cells by inhibiting cell cycle progression and through induction of apoptosis.
PEL cells are addicted to IRF4. (a) BC-3 cells stably expressing tetracycline-inducible H1 promoter (H1/TO)-driven shRNA targeting IRF4, clone F11 (shIRF4-F11) and shRNA targeting scrambled sequence (shSCR) were treated with Dox (500 ng/ml) for 3 days and immunoblotted for the expression of IRF4, MYC, poly (ADP-ribose) polymerase (PARP) and TUBA. (b) BC-3 cells stably expressing shSCR and shIRF4-F11 were treated with Dox for indicated time points and cell viability was measured by MTS (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. The values shown are mean±s.e. of a representative experiment performed in triplicate for three times. (c) Cell cycle analysis of BC-3 cells stably expressing shSCR and shIRF4-F11 treated with and without Dox for 48 h. Cells were stained with propidium iodide (PI) and analyzed by flow cytometry. Data are representative of two individual experiments. (d) BC-3 cells stably expressing shSCR and shIRF4-F11 were treated with Dox for 72 h. Cells were then stained with Hoescht 33342 (50 μg/ml) and photographed. (e) BC-3 cells stably expressing shSCR and shIRF4-F11 were treated with Dox for 48 h, stained with annexinV-FITC/PI and analyzed for apoptosis by flow cytometry. Data are representative of two individual experiments.
IMiDs rapidly downregulate IKZF1 and silencing of IKZF1 is toxic to PEL
Ikaros family proteins IKZF1 and IKZF3 are B-cell transcription factors that have crucial roles in immunity and cell-fate decisions.32 Recently, it was shown that IMiDs selectively degrade these transcription factors in MM cells.10, 11 In PEL, both IMiDs led to significant and near-complete downregulation of IKZF1 in all the three PEL cell lines even at the lowest concentration (that is, 0.5 μM lenalidomide and 50 nM pomalidomide) tested, but had only a modest effect in the DG-75 cell line (Figure 5a). In contrast, the effect of IMiDs on the level of expression of IKZF3 was modest at best and, in general, required higher doses of the drugs (Figure 5a). Consistent with the results seen with IMiDs, silencing of IKZF1 by two different shRNAs were selectively toxic to PEL cells (Figure 5b and Supplementary Figure S7A) and was accompanied by partially reduced expressions of IRF4 and MYC (Figure 5c). Additional studies revealed that IMiDs downregulate IKZF1 expression at the posttranslational level (Supplementary Figures S7B and C). Furthermore, time-course experiments revealed rapid and near-complete downregulation of IKZF1 expression as early as 12 h post treatment even at the lowest concentrations of both IMiDs (Figure 5d). In contrast, the levels of IRF4 and MYC were less sensitive to downregulation by IMiDs (Figure 5d). Thus, near-complete downregulation of these proteins was either not observed or required treatment with longer duration (that is, 48 h) and higher concentrations of the drugs (Figure 5d). Collectively, these results support the hypothesis that IKZF1 is an upstream target of IMiDs in PEL.
IMiDs rapidly downregulate IKZF1 and silencing of IKZF1 is toxic to PEL. (a) Immunoblot analysis showing the effect of treatment with lenalidomide (Len) and pomalidomide (Pom) at the indicated doses for 48 h on the expression of IKZF1, IKZF3 and GAPDH (glyceraldehyde 3-phosphate dehydrogenase) (loading control) in BC-3, BCBL-1, JSC-1 and DG-75 cells. Blots are representative of two individual experiments. (b) Change in % red fluorescent protein (RFP) positivity over time in BC-3 and BCBL-1 cells infected with viruses encoding RFP and the indicated shRNAs. The day 2% RFP for each virus was normalized to 1 and subsequent values were expressed relative to cells infected with a virus encoding RFP and a control shRNA. Data are representative of two individual experiments. (c) Immunoblot analysis of BC-3 and BCBL-1 cells transiently infected with lentiviruses expressing the indicated shRNAs for 72 h. Immunoblots were quantified (normalized to the expression of GAPDH) using image studio version 5.0 from LI-COR Biosciences (Lincoln, NE, USA). Blots are representative of two individual experiments. (d) Immunoblot analysis showing the expression IKZF1, IRF4, MYC, TUBA and HSP90 (loading controls) in BC-3 and BCBL-1 cells treated with indicated concentrations of IMiDs for 12, 24, 48 and 72 h. Blots are representative of two individual experiments.
We also checked the hypothesis that IKZF1 may be responsible for the high-level expression of IRF4 observed in PEL cells. We found that IRF4 and IKZF1 are both consistently expressed in PEL, myeloma, Waldenstrom macroglobulinemia, ABC-DLBCL and Hodgkin’s lymphoma cell lines. However, there was little correlation between IRF4 and IKZF1 expression in cell lines derived from other hematologic malignancies (Supplementary Figure S8). Therefore, although it is possible that IKZF1 may contribute to the overexpression of IRF4 (and MYC) in PEL, it is unlikely to be the sole regulator of their expression.
CRBN is dispensable for the survival of PEL
IMiDs exert their antiproliferative effect by binding to their cellular protein target CRBN.7, 8, 9 However, we failed to observe a significant and consistent effect of IMiDs on the expression of CRBN in BC-3 and BCBL-1 cells (Figure 6a). It has been shown that silencing of CRBN by shRNA significantly decreases the proliferation of MM8 and ABC-DLBCL cells.25 We generated polyclonal populations of BC-3 and BCBL-1 cells stably expressing a TO/U6 promoter-driven shRNA targeting CRBN (shCRBN).10 Treatment of shCRBN-expressing cells with Dox for 4 days significantly downregulated the expression of CRBN (Figure 6b, upper panel), whereas Dox treatment was without effect in control shRNA-expressing cells (Figure 6b, upper panel). Interestingly, silencing of CRBN expression did not have any significant effect on the proliferation of BC-3 and BCBL-1 cells (Figure 6b, lower panel). Thus, in contrast to myeloma and ABC-DLBCL cells, CRBN is dispensable for the survival of PEL.
CRBN is dispensable for the survival of PEL cells but is essential for the antiproliferative activity of IMiDs in PEL cells. (a) Immunoblot analysis showing the effect of lenalidomide (Len) and pomalidomide (Pom) at the indicated doses for 48 h on the expression of CRBN and GAPDH (glyceraldehyde 3-phosphate dehydrogenase) in BC-3 and BCBL-1 cells. The band corresponding to CRBN is marked with an asterisk. Blots are representative of two individual experiments. (b) Upper panel: BC-3 and BCBL-1 cells stably expressing tetracycline-inducible shRNA targeting CRBN (shCRBN) and shRNA targeting scrambled sequence (shCON) were treated with Dox (500 ng/ml) for 4 days and immunoblotted for the expression of CRBN, GAPDH and TUBA. Blots are representative of two individual experiments. Lower panel: BC-3 and BCBL-1 cells stably expressing shCON and shCRBN were treated with Dox for indicated time points and cell viability was measured by MTS (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. The values shown are mean±s.e. of a representative experiment performed in triplicate for two times. (c) BC-3 and BCBL-1 cells stably expressing shCON and shCRBN were pre-treated with Dox for 3 days followed by treatment with vehicle and IMiDs at indicated concentrations for 6 days in the presence of Dox and cell viability was measured by MTS assay. The values shown are mean±s.e. of a representative experiment performed in triplicate for three times. (d) BC-3 and BCBL-1 cells stably expressing shCON and shCRBN were pre-treated with Dox for 3 days followed by treatment with vehicle and IMiDs at indicated concentrations for 48 h in the presence of Dox, and cell lysates were collected and immunoblotted for indicated proteins. Blots are representative of two individual experiments. (e) BC-3 and BCBL-1 cells stably expressing shCON and shCRBN were pre-treated with Dox for 3 days followed by treatment with vehicle or IMiDs along with Dox in the presence of 100 μg/ml of cycloheximide (CHX) for 0, 1, 2 and 3 h, respectively. Whole-cell lysates were immunoblotted for IKZF1, CRBN and GAPDH. Blots are representative of two individual experiments. Note: The CRBN antibody gives a nonspecific band when CRBN is probed as first antigen but when the blot is probed for some other antigen, then stripped and probed for CRBN and then the intensity of the nonspecific band is decreased or gone.
CRBN is essential for the antiproliferative effect of IMiDs in PEL
We next asked the question whether CRBN is essential for the activity of IMiDs in PEL. Although IMiDs significantly inhibited the proliferation of shCON-expressing PEL cells, the antiproliferative activity of IMiDs was almost completely blocked in shCRBN-expressing PEL cells (Figure 6c). Furthermore, treatment with IMiDs failed to induce G1 cell cycle arrest in shCRBN-expressing PEL cells, but successfully did so in shCON-expressing PEL cells (Supplementary Figure S9). Further, IMiDs treatment resulted in a near-complete abrogation of IKZF1 expression in shCON-expressing BC-3 and BCBL-1, which was accompanied by a significant decrease in the expressions of IRF4 and MYC but was without any effect on the expression of CRBN (Figure 6d). Remarkably, IMiDs had no significant effect on the expression levels of IKZF1, IRF4 and MYC in the shCRBN-expressing BC-3 and BCBL-1 cells (Figure 6d). In addition, CRBN is essential for the posttranslational degradation of IKZF1 by IMiDs, as observed by a complete block in the degradation of IKZF1 by IMiDs in shCRBN-expressing cells (Figure 6e), whereas IKZF1 was degraded within 1 h by IMiDs in shCON-expressing cells (Figure 6e). These results clearly suggest that CRBN is essential for the antiproliferative potential of IMiDs in PEL. However, we did not observe a significant difference in the level of expression of CRBN between IMiD-sensitive and -resistant cell lines (Supplementary Figure S10), suggesting that the resistance to IMiDs in these cells is not linked to CRBN expression.
Knocking down MYC by shRNA enhances the sensitivity of IMiDs to PEL
To test whether the loss of MYC could synergize with IMiDs, we generated polyclonal population of BC-3 cells stably expressing TO/H1-driven shRNAs targeting MYC (shMYC) and a scrambled sequence (shSCR). Consistent with our published results,18 treatment of BC3-shMYC cells with Dox resulted in a significant downregulation of MYC (Figure 7a), which was accompanied by a decrease in cell proliferation (Figure 7b), without any effect on BC-3-shSCR cells (Figures 7a and b). Interestingly, knockdown of MYC significantly enhanced the antiproliferative effect of IMiDs (Figure 7c), which was accompanied by cell cycle arrest and apoptosis (Figures 7d and e). In contrast, no significant difference in cell proliferation, cell cycle progression and apoptosis was observed in BC-3 shSCR cells treated with IMiDs in the presence or the absence of Dox (Figures 7c–e). These results pointed to the existence of a potential synergism between IMiDs and inhibition of MYC.
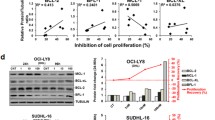
Knocking down MYC enhances the antiproliferative effect of IMiDs in PEL, BRD4 inhibitors and IMiDs display synergistic antiproliferative activity against PEL. (a) BC-3 cells stably expressing tetracycline-inducible H1 promoter (H1/TO)-driven shRNA targeting MYC (shMYC) and shRNA targeting scrambled sequence (shSCR) were treated with Dox (500 ng/ml) for 4 days and immunoblotted for the expression of MYC and GAPDH (glyceraldehyde 3-phosphate dehydrogenase). Blots are representative of two individual experiments. (b) BC-3 cells stably expressing shSCR and shMYC were treated with Dox for indicated time points and cell viability was measured by MTS (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. The values shown are mean±s.e. of a representative experiment performed in triplicate for three times. (c) BC-3 cells stably expressing shSCR and shMYC were treated in the presence/absence of Dox with indicated concentrations of IMiDs or vehicle for 72 h and cell viability was measured by MTS assay. ***Significance at the level of P⩽0.001. The values shown are mean±s.e. of a representative experiment performed in triplicate for three times. (d) Cell cycle analysis of BC-3 cells stably expressing shSCR and shMYC that were treated in the presence/absence of Dox with indicated concentrations of IMiDs or vehicle for 72 h. Data are representative of two individual experiments. (e) Apoptosis analysis of BC-3 cells stably expressing shSCR and shMYC that were treated in the presence/absence of Dox with indicated concentrations of IMiDs or vehicle for 48 h. Data are representative of two individual experiments. (f) BC-3 and BCBL-1 cells were treated with low doses of lenalidomide (Len) in combination with low doses of three structurally different BRD4 inhibitors (JQ-1, IBET151 and PFI-1) for 5 days and then assessed for viability using MTS assay. Combination index (CI) was calculated using the calcusyn software, which is based on the method of Chou and Talalay.33 Each BRD4 inhibitor was tested in combination with lenalidomide at 12 different combinations (please see supplementary Tables 2–8 for details). CI values of <1 denotes synergism and CI values >1 denotes antagonism. Data presented are representative of three individual experiments performed in triplicate.
BRD4 inhibitors JQ-1, IBET151 and PFI-1 are synergistic with IMiDs in PEL
Recently, it has been shown that MYC transcription can be targeted using BRD4 inhibitors.16, 17, 18 JQ-1, IBET151 and PFI-1 are three structurally distinct BRD4 inhibitors.13, 14, 15 To test whether IMiDs show synergistic antiproliferative activity when combined with BRD4 inhibitors, BC-3 and BCBL-1 cells were treated with low doses of lenalidomide in combination with low doses of JQ-1, IBET151 and PFI-1, respectively. The combination index was calculated using the calcusyn software, which is based on the method of Chou and Talalay.33 Lenalidomide was highly synergistic with all BRD4 inhibitors at all the combination doses tested in both BC-3 and BCBL-1 cells (Figure 7f and Supplementary Tables 2). In contrast, lenalidomide is not synergistic with the inactive isomer of JQ-1 in either cell line (Supplementary Tables 9 and 10). Furthermore, combined treatment with lenalidomide and JQ-1 in BC-3 and BCBL-1 cells significantly decreased the expression of MYC and IRF4 at both protein (Figure 8a) and mRNA (Supplementary Figure S11A) levels as compared with treatment with either drug alone. The combination of lenalidomide with JQ-1 also resulted in G1 cell cycle arrest, cleavage of poly (ADP-ribose) polymerase and the appearance of apoptotic cells as compared with treatment with either drug alone (Supplementary Figure S11B and Figures 8a and b, respectively).
(a) BC-3 and BCBL-1 cells were treated with vehicle, lenalidomide 1 μM (L1), pomalidomide 100 nM (P100), JQ-1 50 nM (J50), JQ-1 100 nM (J100) and the combinations for 48 h. Whole-cell lysates were immunoblotted for MYC, IRF4, poly (ADP-ribose) polymerase (PARP) and GAPDH (glyceraldehyde 3-phosphate dehydrogenase). Blots are representative of three independent experiments. (b) Apoptosis analysis of BC-3 and BCBL-1 cells treated with vehicle or indicated concentrations of IMiDs and JQ-1, or the combination for 72 h. Data presented are representative of two individual experiments. (c) BC-3 cells stably expressing tetracycline-inducible H1 promoter (H1/TO)-driven shRNA targeting BRD4 (shBRD4) and shRNA targeting scrambled sequence (shSCR) were treated with Dox (500 ng/ml) for 4 days and immunoblotted for the expression of BRD4 and GAPDH. The band corresponding to BRD4 is marked with an asterisk. Blots are representative of two individual experiments. (d) BC-3 cells stably expressing shSCR and shBRD4 were treated in the presence/absence of Dox with indicated concentrations of IMiDs or vehicle for 4 days, and cell viability was measured by MTS (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. The values shown are mean±s.e. of a representative experiment performed in triplicate for two times. (e) Body weight gain of mice injected with BC-3 cells followed by indicated treatments (n=7 in each group) over the period of experiment. Statistically significant differences (on day 27 of the treatment) are shown by asterisks (*) and (**) at the levels of P≤0.05 and 0.01, respectively. The black arrows in the x axis mark the start (day1) and end (day28) of the treatment. (f) Survival curves (Kaplan–Meier) of mice injected with BC-3 cells followed by indicated treatments (n=7 in each group). The survival curve was generated in GraphPad Prism 5 software and statistical values for the curves are calculated by log rank (Mantel–Cox) test. Asterisks (*) and (**) indicate significance at the level of P⩽0.05 and 0.01, respectively.
Knocking down BRD4 by shRNA enhances the sensitivity of IMiDs to PEL
To confirm whether the observed synergism between lenalidomide and the BRD4 inhibitors is due to inhibition of BRD4, we generated stable clones of BC-3 and BCBL-1 cells expressing TO/H1 promoter-driven shRNA targeting BRD4 (shBRD4). Treatment of BC-3 and BCBL-1 cells expressing shBRD4 with Dox resulted in a significant downregulation of BRD4 (Figure 8c and Supplementary Figure S11C) and decrease in cellular proliferation (Figure 8d and Supplementary Figure S11D), whereas Dox treatment had no effect on BRD4 expression or cellular proliferation in BC-3 and BCBL-1 cells expressing a scrambled shRNA sequence (shSCR) as control (Figures 8c and d and Supplementary Figures S11C and D). More importantly, Dox enhanced the antiproliferative activity of IMiDs in the shBRD4-expressing BC-3 and BCBL-1 cells but was without effect in shSCR-expressing cells (Figure 8d and Supplementary Figure S11D).
Lenalidomide and JQ-1 are synergistic against PEL in vivo
To check the in vivo efficacy of lenalidomide, alone and in combination with JQ-1, BC-3 cells were injected into the intraperitoneal cavity of NOD–SCID mice. Five days after the injection, animals were randomly assigned to vehicle control, lenalidomide (50 mg/kg once daily for 28 days), JQ-1 (50 mg/kg once daily for 28 days) and the combination. Intraperitoneal inoculation of BC-3 cells resulted in rapid tumor growth and massive ascites, which resulted in weight gain (Figure 8e). There was a significant reduction (P⩽0.01) in body weight gain (a measure of ascites)34 of animals treated with lenalidomide and JQ-1 when compared with vehicle control (Figure 8e). In addition, the combination of lenalidomide and JQ-1 showed a further reduction in body weight gain over time when compared with mice treated with either agent alone (Figure 8e). Furthermore, the median survival of mice that received combination treatment (51 days) was significantly (P⩽0.01) increased as compared with the median survival of mice treated with lenalidomide (35 days) or JQ-1(42 days) alone (Figure 8f).
Discussion
In this study, we demonstrate that a majority of the PEL cells are highly sensitive to lenalidomide and pomalidomide, two Food and Drug Administration-approved drugs for the treatment of MM. Both drugs have predictable and manageable safety profiles and limited cumulative long-term toxicity,35, 36 making them attractive treatment options for PEL.
The antiproliferative action of lenalidomide in PEL cell lines was associated with the activation of the IFN signaling pathway. However, we did not detect IFN in the supernatant of IMiD-treated cells and neutralizing antibodies against IFNs failed to block the activity of IMiDs against PEL. As IRF4 has been recently shown to modulate IFN signaling,25 these results prompted us to explore the role of IRF4 in the antiproliferative effects of IMiDs in PEL. We observed that IRF4 is not only uniformly expressed in PEL cell lines but is significantly downregulated following treatment with IMiDs. Furthermore, shRNA-mediated silencing of IRF4 was toxic to PEL cells, thereby supporting the argument that downregulation of IRF4 contributes to the antiproliferative effect of IMiDs in PEL. Expression of IRF4 in myeloma cells has been attributed to their plasmacytic differentiation.24 As PEL cells resemble plasma cells in the gene expression profile,37 the uniform expression of IRF4 in these cells may also reflect their plasma cell lineage. IRF4 expression is also a feature of ABC-DLBCL.25, 38 In these cells, oncogenic mutations affecting the B-cell receptor and MYD88 signaling pathways induce NF-κB,39, 40 which is a strong inducer of cytotoxic IFNβ.41 IRF4, however, places a brake on IFNβ production by repressing IRF7, thereby allowing ABC-DLBCL to survive and proliferate.25 Furthermore, IRF4 is believed to promote ABC-DLBCL survival by transactivating CARD11 and potentiating NF-κB signaling.25 Although oncogenic mutations affecting the B-cell receptor and MYD88 signaling pathways have not been reported in PEL, they do possess constitutively active NF-κB signaling pathway due to the activity of KSHV-encoded viral FLICE inhibitory protein K13.27, 28, 42 Therefore, similar to ABC-DLBCL, IRF4 may be upregulated in PEL cells to augment the prosurvival aspect of NF-κB signaling while simultaneously protecting against the deleterious effects (for example, IFNβ production) of uncontrolled NF-κB activation. IRF4 is also known to bind to MYC promoter and stimulate MYC gene expression.24 Even though PEL cells lack structural alterations in the MYC gene,43 they nevertheless demonstrate elevated MYC expression, which has been shown to be essential for their survival and proliferation.44 The overexpression of IRF4 in PEL might contribute to the elevated MYC expression observed in these cells.
IMiDs were shown to degrade both IKZF1 and IKZF3 in MM.10, 11 In contrast, we observed that IKZF1 was the primary target of IMiDs in PEL cells. The expression of IKZF1 was downregulated earlier than IRF4 and MYC. In addition, IKZF1-specific shRNAs was not only toxic to PEL cells but also partially downregulated the expression of IRF4 and MYC. IKZF1 has been previously shown to bind to IRF4 promoter and regulate its expression at the transcriptional level.10 Furthermore, MYC is a known transcriptional target of IRF4.24 Taken together with prior studies, our results suggest that degradation of IKZF1 by IMiDs downregulates IRF4 expression at the transcriptional level, which in turn downregulates MYC expression.
CRBN is the direct cellular-binding target of IMiDs7 and is essential for their immunomodulatory and antiproliferative activities.8, 9 We observed that CRBN is dispensable for the survival of PEL, which is in contrast to the situtation in MM and ABC-DLBCL cells where shRNA-mediated knockdown of CRBN has been reported to be toxic.8, 25 However, although CRBN is not essential for the survival of PEL, it is essential for the antiproliferative activity of IMiDs in PEL, as all the IMiD-induced anti-PEL effects are blunted in cells expressing an shRNA targeting CRBN.
Our study along with work of others26 suggest that MYC is one of the downstream target of IMiDs in PEL. We found that shRNA-mediated knockdown of MYC enhanced the antiproliferative effect of IMiDs on PEL, thus suggesting a potential synergism between IMiDs and inhibition of MYC. BRD4 inhibitors have been shown to block MYC expression.16, 17, 18 In support of this premise, we observed striking synergy between low doses of lenalidomide and BRD4 inhibitors (JQ-1, IBET151 and PFI-1) against PEL. Furthermore, shRNA-mediated BRD4 knockdown also enhanced the cytotoxicity of IMiDs toward PEL, suggesting that the synergism observed between IMiDs and BRD4 inhibition may not be limited only to the BRD4 inhibitors used in our study. There are several potential explanations for the observed synergism between IMiDs and BRD4 inhibitors. First, as inhibition of MYC is not complete on treatment with lower doses of IMiDs (Figure 5d), addition of low doses of BRD4 inhibitors may eliminate any residual MYC expression seen following IMiD treatment. Second, apart from MYC, BRD4 inhibitors are known to modulate the expression of other genes45, which may have synergistic cytotoxicity when combined with IMiDs. Finally, in addition to degrading IKZF1/IKZF3 via CRBN, IMiDs may also degrade other proteins, which may result in synergistic cytotoxicity when combined with BRD4 inhibitors.
In summary, we provide strong in vitro and in vivo data showing that IMiDs are effective against PEL, and combined treatment of IMiDs with BRD4 inhibitors have synergistic activity against this deadly incurable cancer. BRD4 inhibitors have shown promising activity against multiple cancers in pre-clinical studies, and at present there are five BRD4 inhibitors in phase 1–2 clinical trials.12 Our results suggest that IMiDs, alone and in combination with BRD4 inhibitors, deserve further testing for the treatment of PEL. While this study was in its final stage of completion, a case report was published describing the successful treatment of a PEL patient with lenalidomide,46 which supports our pre-clinical data.
Materials and methods
Cell lines
BC-3, BCBL-1, JSC-1, BC-1, BCP-1, VG-1 and APK-1 were obtained from Dr Jae Jung (University of Southern California, Los Angeles, CA, USA). UMPEL-1 and UMPEL-3 were provided by Drs Izidore Lossos and Juan Ramos, respectively (both from University of Miami, Miami, FL, USA). DG-75 was obtained from Dr Alan Epstein (University of Southern California). All the cells were grown in conditions as described previously.47 The cell lines were expanded, stored in liquid nitrogen and used within 3 months after resuscitation. The identities of the PEL cell lines were routinely authenticated by western blotting detection of KSHV LANA. No further authentication of cell line characteristics was done. The authentication information for the remaining cell lines is not available.
Cell viability, cell cycle, apoptosis, luciferase assays and western blotting
Cell viability, cell cycle, apoptosis, luciferase assays and western blotting were performed as described earlier.47, 48, 49, 50, 51
Lentiviral shRNA constructs
shRNA oligonucleotides (Supplementary Table 11) directed against human IRF4, MYC and BRD4 mRNAs were annealed and cloned into a modified pENT entry vector containing a TO/H1 promoter as described previously.18 Lentiviral shRNAs for CRBN, IKZF1-1 and IKZF1-2 along with their respective controls were obtained from Dr. Willian Kaelin Jr (Harvard University, Cambridge, MA, USA).10
Real-time reverse transcriptase–PCR
Real-time quantitative reverse transcriptase–PCR analysis was performed as described earlier47 using gene-specific PCR primers listed in Supplementary Table 12.
PEL orthotopic tumor model
A total of 2 × 107 BC-3 cells were injected intraperitoneally into female NOD–SCID mice (NCI Frederick, 6 weeks old). Five days later, the mice were randomly divided in to four groups (n=7 each). Investigators are not blinded. Vehicle control (10% hydroxypropyl-β-cyclodextrin), lenalidomide 50 mg/kg b.w. (once daily), JQ-1 50 mg/kg b.w. (once daily) and the combination were administered intraperitoneally for 28 days. Then, the animals were monitored for survival. Body weight gain was measured once in 3 days as a surrogate measure of tumor progression.34 The experiments were performed following the approval of the Institutional Animal Ethics Committee of the University of Southern California.
Statistical analysis
Two-tailed unpaired Student’s t-test was used to test for differences between two groups. Differences with a P-value⩽0.05 were considered statistically significant. Data were given as mean±s.e. All the experiments were reproduced atleast twice. No inclusion/exclusion criteria are applied and none of the samples or animals was excluded from the analysis. The vehicle and drug treatments were performed at the same time in the same condition. The investigators are not blinded for any of the experiments.
Detailed information about Materials and Methods is provided in the Supplementary Information.
Accession codes
References
Patel S, Xiao P . Primary effusion lymphoma. Arch Pathol Lab Med 2013; 137: 1152–1154.
Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM . Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 1995; 332: 1186–1191.
Kobayashi Y, Kamitsuji Y, Kuroda J, Tsunoda S, Uoshima N, Kimura S et al. Comparison of human herpes virus 8 related primary effusion lymphoma with human herpes virus 8 unrelated primary effusion lymphoma-like lymphoma on the basis of HIV: report of 2 cases and review of 212 cases in the literature. Acta Haematol 2007; 117: 132–144.
Shortt J, Hsu AK, Johnstone RW . Thalidomide-analogue biology: immunological, molecular and epigenetic targets in cancer therapy. Oncogene 2013; 32: 4191–4202.
Zhu YX, Kortuem KM, Stewart AK . Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma. Leuk Lymphoma 2013; 54: 683–687.
Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med 1999; 341: 1565–1571.
Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y et al. Identification of a primary target of thalidomide teratogenicity. Science 2010; 327: 1345–1350.
Zhu YX, Braggio E, Shi CX, Bruins LA, Schmidt JE, Van Wier S et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood 2011; 118: 4771–4779.
Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi AK, Kang J et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 2012; 26: 2326–2335.
Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 2014; 343: 305–309.
Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014; 343: 301–305.
Filippakopoulos P, Knapp S . Targeting bromodomains: epigenetic readers of lysine acetylation. Nat Rev Drug Discov 2014; 13: 337–356.
Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O et al. Selective inhibition of BET bromodomains. Nature 2010; 468: 1067–1073.
Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature 2011; 478: 529–533.
Picaud S, Da Costa D, Thanasopoulou A, Filippakopoulos P, Fish PV, Philpott M et al. PFI-1, a highly selective protein interaction inhibitor, targeting BET Bromodomains. Cancer Res 2013; 73: 3336–3346.
Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 2011; 146: 904–917.
Mertz JA, Conery AR, Bryant BM, Sandy P, Balasubramanian S, Mele DA et al. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl Acad Sci USA 2011; 108: 16669–16674.
Tolani B, Gopalakrishnan R, Punj V, Matta H, Chaudhary PM . Targeting Myc in KSHV-associated primary effusion lymphoma with BET bromodomain inhibitors. Oncogene 2014; 33: 2928–2937.
Chen N, Lau H, Kong L, Kumar G, Zeldis JB, Knight R et al. Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. J Clin Pharmacol 2007; 47: 1466–1475.
Kasserra C, Assaf M, Hoffmann M, Li Y, Liu L, Wang X et al. Pomalidomide: evaluation of cytochrome P450 and transporter-mediated drug-drug interaction potential in vitro and in healthy subjects. J Clin Pharmacol 2015; 55: 168–178.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102: 15545–15550.
Sun R, Lin SF, Gradoville L, Yuan Y, Zhu F, Miller G . A viral gene that activates lytic cycle expression of Kaposi’s sarcoma-associated herpesvirus. Proc Natl Acad Sci USA 1998; 95: 10866–10871.
Zhao J, Punj V, Matta H, Mazzacurati L, Schamus S, Yang Y et al. K13 blocks KSHV lytic replication and deregulates vIL6 and hIL6 expression: a model of lytic replication induced clonal selection in viral oncogenesis. PLoS One 2007; 2: e1067.
Shaffer AL, Emre NC, Lamy L, Ngo VN, Wright G, Xiao W et al. IRF4 addiction in multiple myeloma. Nature 2008; 454: 226–231.
Yang Y, Shaffer 3rd AL, Emre NC, Ceribelli M, Zhang M, Wright G et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell 2012; 21: 723–737.
Lopez-Girona A, Heintel D, Zhang LH, Mendy D, Gaidarova S, Brady H et al. Lenalidomide downregulates the cell survival factor, interferon regulatory factor-4, providing a potential mechanistic link for predicting response. Br J Haematol 2011; 154: 325–336.
Liu L, Eby MT, Rathore N, Sinha SK, Kumar A, Chaudhary PM . The human herpes virus 8-encoded viral FLICE inhibitory protein physically associates with and persistently activates the Ikappa B kinase complex. J Biol Chem 2002; 277: 13745–13751.
Matta H, Chaudhary PM . Activation of alternative NF-kappa B pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1 beta-converting enzyme inhibitory protein (vFLIP). Proc Natl Acad Sci USA 2004; 101: 9399–9404.
Keller SA, Schattner EJ, Cesarman E . Inhibition of NF-kappaB induces apoptosis of KSHV-infected primary effusion lymphoma cells. Blood 2000; 96: 2537–2542.
Shaffer AL, Emre NC, Romesser PB, Staudt LM . IRF4: Immunity. Malignancy! Therapy? Clin Cancer Res 2009; 15: 2954–2961.
Libermann TA, Baltimore D . Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol 1990; 10: 2327–2334.
John LB, Ward AC . The Ikaros gene family: transcriptional regulators of hematopoiesis and immunity. Mol Immunol 2011; 48: 1272–1278.
Chou TC, Talalay P . Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 1984; 22: 27–55.
Qin Z, Dai L, Trillo-Tinoco J, Senkal C, Wang W, Reske T et al. Targeting sphingosine kinase induces apoptosis and tumor regression for KSHV-associated primary effusion lymphoma. Mol Cancer Ther 2014; 13: 154–164.
Dimopoulos MA, Terpos E, Niesvizky R . How lenalidomide is changing the treatment of patients with multiple myeloma. Crit Rev Oncol Hematol 2013; 88 (Suppl 1): S23–S35.
Richardson PG, Mark TM, Lacy MQ . Pomalidomide: new immunomodulatory agent with potent antiproliferative effects. Crit Rev Oncol Hematol 2013; 88 (Suppl 1): S36–S44.
Klein U, Gloghini A, Gaidano G, Chadburn A, Cesarman E, Dalla-Favera R et al. Gene expression profile analysis of AIDS-related primary effusion lymphoma (PEL) suggests a plasmablastic derivation and identifies PEL-specific transcripts. Blood 2003; 101: 4115–4121.
Zhang LH, Kosek J, Wang M, Heise C, Schafer PH, Chopra R . Lenalidomide efficacy in activated B-cell-like subtype diffuse large B-cell lymphoma is dependent upon IRF4 and cereblon expression. Br J Haematol 2013; 160: 487–502.
Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 2010; 463: 88–92.
Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 2011; 470: 115–119.
Lenardo MJ, Fan CM, Maniatis T, Baltimore D . The involvement of NF-kappa B in beta-interferon gene regulation reveals its role as widely inducible mediator of signal transduction. Cell 1989; 57: 287–294.
Guasparri I, Keller SA, Cesarman E . KSHV vFLIP is essential for the survival of infected lymphoma cells. J Exp Med 2004; 199: 993–1003.
Nador RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, Sald J et al. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood 1996; 88: 645–656.
Li X, Chen S, Feng J, Deng H, Sun R . Myc is required for the maintenance of Kaposi’s sarcoma-associated herpesvirus latency. J Virol 2010; 84: 8945–8948.
Zhang H, Neely L, Lundgren K, Yang YC, Lough R, Timple N et al. BIIB021, a synthetic Hsp90 inhibitor, has broad application against tumors with acquired multidrug resistance. Int J Cancer 2010; 126: 1226–1234.
Antar A, El Hajj H, Jabbour M, Khalifeh I, El-Merhi F, Mahfouz R et al. Primary effusion lymphoma in an elderly patient effectively treated by lenalidomide: case report and review of literature. Blood Cancer J 2014; 4: e190.
Gopalakrishnan R, Matta H, Chaudhary PM . A purine scaffold HSP90 inhibitor BIIB021 has selective activity against KSHV-associated primary effusion lymphoma and blocks vFLIP K13-induced NF-kappaB. Clin Cancer Res 2013; 19: 5016–5026.
Tolani B, Matta H, Gopalakrishnan R, Punj V, Chaudhary PM . NEMO is essential for Kaposi's sarcoma-associated herpesvirus-encoded vFLIP K13-induced gene expression and protection against death receptor-induced cell death, and its N-terminal 251 residues are sufficient for this process. J Virol 2014; 88: 6345–6354.
Yang Y, Groshong JS, Matta H, Gopalakrishnan R, Yi H, Chaudhary PM . Constitutive NF-kappaB activation confers interleukin 6 (IL6) independence and resistance to dexamethasone and janus kinase inhibitor INCB018424 in murine plasmacytoma cells. J Biol Chem 2011; 286: 27988–27997.
Matta H, Gopalakrishnan R, Graham C, Tolani B, Khanna A, Yi H et al. Kaposi’s sarcoma associated herpesvirus encoded viral FLICE inhibitory protein K13 activates NF-kappaB pathway independent of TRAF6, TAK1 and LUBAC. PLoS One 2012; 7: e36601.
Matta H, Gopalakrishnan R, Punj V, Yi H, Suo Y, Chaudhary PM . A20 is induced by Kaposi sarcoma-associated herpesvirus-encoded viral FLICE inhibitory protein (vFLIP) K13 and blocks K13-induced nuclear factor-kappaB in a negative feedback manner. J Biol Chem 2011; 286: 21555–21564.
Arvanitakis L, Mesri EA, Nador RG, Said JW, Asch AS, Knowles DM et al. Establishment and characterization of a primary effusion (body cavity- based) lymphoma cell line (BC-3) harboring kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood 1996; 88: 2648–2654.
Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D et al. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med 1996; 2: 342–346.
Cannon JS, Ciufo D, Hawkins AL, Griffin CA, Borowitz MJ, Hayward GS et al. A new primary effusion lymphoma-derived cell line yields a highly infectious Kaposi’s sarcoma herpesvirus-containing supernatant. J Virol 2000; 74: 10187–10193.
Jones D, Ballestas ME, Kaye KM, Gulizia JM, Winters GL, Fletcher J et al. Primary-effusion lymphoma and Kaposi’s sarcoma in a cardiac-transplant recipient. N Engl J Med 1998; 339: 444–449.
Sarosiek KA, Cavallin LE, Bhatt S, Toomey NL, Natkunam Y, Blasini W et al. Efficacy of bortezomib in a direct xenograft model of primary effusion lymphoma. Proc Natl Acad Sci USA 2010; 107: 13069–13074.
Bhatt S, Ashlock BM, Natkunam Y, Sujoy V, Chapman JR, Ramos JC et al. CD30 targeting with brentuximab vedotin: a novel therapeutic approach to primary effusion lymphoma. Blood 2013; 122: 1233–1242.
Cesarman E, Moore PS, Rao PH, Inghirami G, Knowles DM, Chang Y . In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi’s sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood 1995; 86: 2708–2714.
Gao SJ, Kingsley L, Li M, Zheng W, Parravicini C, Ziegler J et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med 1996; 2: 925–928.
Lee BS, Connole M, Tang Z, Harris NL, Jung JU . Structural analysis of the Kaposi’s sarcoma-associated herpesvirus K1 protein. J Virol 2003; 77: 8072–8086.
Acknowledgements
We thank the following investigators for their generous gift of cell lines: Dr Jae Jung (BC-3, BCBL-1, JSC-1, BC-1, BCP-1, VG-1 and APK-1); Drs Izidore Lossos and Juan Ramos (UMPEL-1 and UMPEL-3); Dr Art Shaffer (TMD8, U-2932, HBL-1, OCI-Ly7, OCI-Ly8 and OCI-Ly19); Dr Alan Epstein (DG-75); Randall Rossi (SUDHL-4, SUDHL-6, Granta, Toledo, KG-1 and MV-4-11); Dr Markus Mapara (L428, L540, L1236 and KM-H2); Dr Irene Ghobrial (BCWM.1 and WMCL-1); and Dr Alan Lichenstein (MM.1S and RPMI8226). We are grateful to Dr William Kaelin Jr for providing the shRNA constructs (CRBN, IKZF1-1 and IKZF1-2), Dr James Bradner for his generous contribution of (+) and (−) JQ-1, Dr Peter Howley for BRD4 antibody and Dr Gary Hayward for KSHV replication and transcription activator antibody. This work was supported by grants from the National Institutes of Health (CA139119, DE019811, SC CTSI UL1TR000130 and P30CA014089) and Stop Cancer Foundation. Flow Cytometry was analyzed in the USC Flow Cytometry Core Facility that is supported in part by the National Cancer Institute Cancer Center Shared Grant award P30CA014089 and the USC Provost Office Dean’s Development Funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
RG and PMC are inventors on a patent application (No. 62/031,053) filed to US patent office pertaining to the compositions and methods for treating primary effusion lymphoma. All other authors declare no conflict of interest.
Additional information
Accession codes
Microarray gene expression data has been deposited under accession number GSE60618 at the website Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/).
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Gopalakrishnan, R., Matta, H., Tolani, B. et al. Immunomodulatory drugs target IKZF1-IRF4-MYC axis in primary effusion lymphoma in a cereblon-dependent manner and display synergistic cytotoxicity with BRD4 inhibitors. Oncogene 35, 1797–1810 (2016). https://doi.org/10.1038/onc.2015.245
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.245
- Springer Nature Limited
This article is cited by
-
A non-randomized risk-adjusted comparison of lenalidomide + R-CHOP versus R-CHOP for MYC-rearranged DLBCL patients
Blood Cancer Journal (2023)
-
IRF4 drives clonal evolution and lineage choice in a zebrafish model of T-cell lymphoma
Nature Communications (2022)
-
Key regulators of sensitivity to immunomodulatory drugs in cancer treatment
Biomarker Research (2021)
-
A phase 1b dose-escalation/expansion study of BET inhibitor RO6870810 in patients with advanced multiple myeloma
Blood Cancer Journal (2021)
-
A novel thermostable beetle luciferase based cytotoxicity assay
Scientific Reports (2021)