Abstract
The hypothalamic–pituitary–adrenal (HPA) axis displays a characteristic circadian pattern of corticosterone release, with higher levels at the onset of the active phase and lower levels at the onset of the inactive phase. As corticosterone levels modify the response to stress and influence the susceptibility to and/or severity of stress-related sequelae, we examined the effects of an acute psychological trauma applied at different zeitgeber times (ZTs) on behavioral stress responses. Rats were exposed to stress either at the onset of the inactive-(light) phase (ZT=0) or at the onset of the active-(dark) phase (ZT=12). Their behavior in the elevated plus-maze and acoustic startle response paradigms were assessed 7 days post exposure for retrospective classification into behavioral response groups. Serum corticosterone levels and the dexamethasone suppression test were used to assess the stress response and feedback inhibition of the HPA axis. Immunoreactivity for neuropeptide Y (NPY) and NPY-Y1 receptor (Y1R) in the paraventricular (PVN) and arcuate (ARC) hypothalamic nuclei, hippocampus, and basolateral amygdala were measured. The behavioral effects of NPY/Y1R antagonist microinfused into the PVN 30 min before stress exposure during the inactive or active phase, respectively, were evaluated. PVN immunoreactivity for NPY and Y1R was measured 1 day after the behavioral tests. The time of day of the traumatic exposure markedly affected the pattern of the behavioral stress response and the prevalence of rats showing an extreme behavioral response. Rats exposed to the stressor at the onset of their inactive phase displayed a more traumatic behavioral response, faster post-exposure corticosterone decay, and a more pronounced stress-induced decline in NPY and Y1R expression in the PVN and arcuate hypothalamic nuclei. Blocking PVN Y1R before stress applied in the active phase, or administering NPY to the PVN before stress applied in the inactive phase, had a resounding behavioral effect. The time at which stress occurred significantly affected the behavioral stress response. Diurnal variations in HPA and NPY/Y1R significantly affect the behavioral response, conferring more resilience at the onset of the active phase and more vulnerability at the onset of the inactive phase, implying that NPY has a significant role in conferring resilience to stress-related psychopathology.
Similar content being viewed by others
INTRODUCTION
Glucocorticoid (GC) hormones have a major role in orchestrating the complex physiological and behavioral reactions essential for homeostasis (McEwen, 2002). As such, they enable the organism to prepare for, respond to, and cope with the acute demands of physical and emotional stressors. The release of GCs commensurate with stressor severity enables the body to properly contain stress responses and promote recovery by rapidly restoring homeostasis (Yehuda et al, 1998). Inadequate GC release following stress not only delays recovery by acutely disrupting biological homeostasis, but can also interfere with the processing or interpretation of stressful information, resulting in long-term disruptions of memory integration processes (McEwen, 2002). A salient example of such an impaired post-traumatic process in the clinic is post-traumatic stress disorder (PTSD; American Psychiatric Association, 1994). Conventional wisdom holds that, in individual who develop PTSD, GC levels are elevated following the traumatic event; however, several studies have reported that lower cortisol levels in the acute aftermath of trauma can also predict subsequent PTSD symptoms (Delahanty et al, 2000; McFarlane et al, 1997; McFarlane, 2000; Witteveen et al, 2010).
Our group has initiated a series of studies that examine the role of GCs in the susceptibility to extreme behavioral responses (EBRs) to stress (‘PTSD-like behaviors’) in a well-validated animal model for PTSD (Cohen et al, 2003a, 2005a). These studies have demonstrated a greater susceptibility to experimentally induced PTSD-like behavioral changes in Lewis rats, which have a hypoactive hypothalamic–pituitary–adrenal (HPA) axis, as compared with Fischer rats, which have a hyper-responsive HPA axis. Administration of corticosterone to Lewis rats before their exposure to the stressor effectively decreased the prevalence of subsequent extreme behavioral disruption (Cohen et al, 2006). Another study examined the effect of a single high-dose corticosterone intervention immediately following exposure to the stressor; this controlled prospective study showed a significant reduction in the incidence of PTSD-like behaviors and improved resilience to subsequent trauma (Cohen et al, 2008). In contrast, administration of corticosterone 14 days following stress exposure and immediately after memory reactivation had no effect on rat behavior. These findings highlight the pivotal role of the initial response of the HPA axis in producing normative stress responses and in determining the long-term neuro-hormonal imbalance underlying the behavioral symptoms of PTSD.
The secretion of GCs is a highly regulated process (Atkinson et al, 2010). The HPA axis displays a characteristic circadian pattern of corticosterone release, with lower levels at the onset of the inactive phase and higher levels at the onset of the active phase (Dallman and Yates, 1969; Lightman et al, 2000). It has been demonstrated that the time of onset of the exposure to stress, relative to the phase of the circadian cycle, can determine the physiological response to stress (Windle et al, 1998). This finding indicates a dynamic interaction between the basal GC levels and the ability of an animal to mount a response to stress. Therefore, it is reasonable to expect that endogenous variations in the basal activity of the HPA axis will influence the responsiveness to stress and, thereby, the susceptibility to or the severity of stress-related disorders, such as PTSD. Hence, the present study sought to assess the relationship between the time of stress exposure—either during the early inactive phase or the early active phase—and the magnitude of the resulting behavioral change. To this end, we analyzed the behavioral response of rats to predator scent stress (PSS) in an animal model that distinguishes between individuals according to the degree to which the stressor affects behavior (Cohen and Zohar, 2004a; Cohen et al, 2003b, 2005c, 2004b).
Another important goal of this study was to suggest a mechanism that may mediate the possible relationship between the circadian rhythm, the HPA axis activity, and the behavioral response to stress. Previous evidence have suggested a possible relationship between GCs and neuropeptide Y (NPY) in the brain (Akabayashi et al, 1994), which may mediate this relationship. Although NPY is widely distributed in the CNS (de Quidt and Emson, 1986; Kask et al, 2002), the hypothalamic PVN is particularly rich in innervation of NPY-immunoreactive (NPY-ir) terminals. Importantly, the PVN has a major role in controlling the release of corticosterone (Rivier and Plotsky, 1986) and shows a high concentration of GC receptors (Ahima and Harlan, 1990; Akabayashi et al, 1994; Fuxe et al, 1985). Moreover, most NPY-ir terminals in the PVN originate from non-catecholaminergic neurons of the hypothalamic ARC nucleus (Bai et al, 1985; Sawchenko et al, 1985), where GC receptors are also found in high concentrations (Ahima and Harlan, 1990; Fuxe et al, 1985). In a previous study, we reported that NPY has an active role in the stress-response cascade, interacting with other systems (including the HPA axis) to mediate stress-related processes, including behavioral responses, memory consolidation, recovery, and resilience (Cohen et al, 2012). Moreover, we found that an intra-hippocampal microinfusion of NPY elevated endogenous NPY and attenuated the behavioral effects to PSS exposure. These experiments indicate a direct link between NPY-ir cells/fibers and HPA axis activity.
In light of such accumulating evidence, we tested the hypothesis that the time of day at which an animal is exposed to a stressor significantly impacts on the behavioral response patterns of the animal (ie, vulnerability versus resilience to stress), and that these patterns are influenced by the interplay between the HPA axis and the NPY-ergic system. To this end, we first performed a controlled, prospective study to examine the behavioral, physiological, and biomolecular effects of acute psychological stress applied to rats (which are essentially nocturnal animals) at different zeitgeber (‘time-giver’; ZT) times. We exposed the rats to the stressor at the onset of either their active phase (ZT=12) or their inactive phase (ZT=0) and measured (a) their serum corticosterone levels and (b) their NPY and NPY-Y1 receptor (Y1R)-ir cells/fibers in different brain regions, including in the hypothalamic PVN and ARC, in different hippocampal subregions (CA1, CA3, and DG), and in the basolateral amygdala (BLA). Then, in a second set of experiments, we administered either NPY or the Y1R antagonist (Y1RA) BIBO3304 to rats during their active or inactive phase. Administration of these agents commenced 30 min before exposing the rats to the stressor, and their behavioral and biomolecular parameters were measured. We hypothesized that the time of exposure to the stressor would significantly affect behavioral stress response patterns, and that the interplay between the HPA axis and NPY-ergic system would influence the behavioral response to the stressor.
MATERIALS AND METHODS
Animals
A sample of 259 adult male Sprague–Dawley rats, weighing 150–200 g, was used. The rats were housed, four per cage, in a vivarium with a stable temperature and unlimited access to food and water. A 12 : 12 light–dark cycle (light phase: 200G50 lux) was maintained for all rats.
Experimental Design
Rats were randomly assigned to colony rooms maintained on either a ‘light regime’ (lights on at 0900 hours, ZT=0; Inactive Phase group) or a ‘dark (reversed) regime’ (lights off at 0900 hours, ZT=12; Active Phase group). All rats were maintained under this regime for a 1-w habituation period before behavioral testing commenced.
To assess the behavioral and physiological responses to a stressor applied at different times of the day, three consecutive experiments were conducted, each stemming from the results of the preceding one. The experimental design used for each of these experiments is schematically depicted in the respective figures. In the first experiment (N=106), all rats were exposed to PSS or to Sham-PSS (see below) at 0900 hours (ZT=0) on day 1, namely, at the onset of their ZT active or inactive, depending on the group). Behavioral assessments were conducted on day 7, first in the elevated plus-maze (EPM) paradigm and then, 1 h later, in the acoustic startle reaction (ASR) paradigm. These data subsequently served for classification into behavioral response groups (Figure 1). The rats were killed at 0900 hours (ZT=0) on day 8 and their brains were collected for measurement of NPY- and Y1R-ir in different brain regions (see below). In the second experiment (N=70), circulating corticosterone levels were evaluated before and at different time points (15–240 min) after exposing rats to PSS. In light of the results, the dexamethasone (DEX) suppression test was used to assess negative feedback inhibition of the HPA axis and association with NPY levels (N=42 overall). The third experiment (N=41) was designed to evaluate the behavioral effects of pharmacologically manipulating NPY levels before the PSS exposure. To this end, rats in the Active Phase group were intra-PVN microinfused with a Y1RA (BIBO3304) 30 min before PSS or Sham-PSS exposure, whereas rats in the Inactive Phase group were intra-PVN microinfused with NPY 30 min before PSS or Sham-PSS exposure. Behavioral parameters were assessed on day 7 and, 1 day later, rats were killed and their brains were collected for measurement of NPY- and Y1R-ir in the PVN and ARC.
The cutoff behavioral criteria (CBC) algorithm. To approximate the behavioral model to contemporary clinical conceptions of post-traumatic stress disorder (PTSD), we classified animals into groups according to degree of response to the stressor (PSS, predator scent stressor), ie, the degree to which the behavior of an individual is altered or disrupted. To this end, behavioral criteria were defined and then complemented by the definition of cutoff criteria, which reflect the severity of response; this parallels inclusion and exclusion criteria applied in clinical research. The procedure requires the following steps: (a) verification of global effect: the data must demonstrate that the stressor has a significant effect on the overall behavior of PSS- vs Sham-PSS-exposed populations at the time of assessment; (b) application of the CBCs to the data: to maximize the resolution and minimize false positives, extreme responses to the stress in both the elevated plus-maze (EPM), and acoustic startle response (ASR) paradigms (performed sequentially) are required for ‘inclusion’ into the extreme behavioral response (EBR) group. A negligible response in both paradigms is required for inclusion into the minimal behavioral response (MBR) group. Individuals who are not classified as having an EBR or an MBR are, by default, classified as having a partial behavioral response (PBR).
Predator Scent Stress
Rats were individually placed on well-soiled cat litter, which was used by a cat for 2 days and sifted for stools. They were exposed to the litter for 10 min in a plastic cage (inescapable exposure) placed on a yard paving stone in a closed environment. Sham-PSS was administered under similar conditions, but the rats were exposed to a fresh, unused cat litter (Cohen et al, 2013, 2003c, 2005b).
Behavioral Measurements
The behavior of rats was assessed in the EPM and ASR paradigms, as described previously (Cohen et al, 2003a, 2005a, 2004c) and as briefly detailed below.
Elevated plus-maze
The maze was a plus-shaped platform with two opposing open arms and two opposing closed arms (closed arms surrounded by 14-cm high opaque walls on three sides; File et al, 1993). Rats were placed on the central platform, facing an open arm, and were allowed to freely explore the maze for 5 min. Each test was videotaped and the behavior of the rat was subsequently scored by an independent observer. Arm entry was defined as entering an arm with all four paws. At the end of the 5 min test period, the rat was removed from the maze, the floor was wiped with a damp cloth, and any fecal boluses removed. The percentage of time spent in the open arms [time spent in open arms/(time spent in open arms+time spent in closed arms) × 100] and the percentage of the number of open-arm entries [open arms entries/(open arms entries+closed arms entries) × 100] was used as a measure of anxiety.
Acoustic startle response
Startle responses were measured by using two ventilated startle chambers (SR-LAB system, San Diego Instruments, San Diego, CA). The SR-LAB calibration unit was used routinely to ensure consistent stabilimeter sensitivity between test chambers and over time. Each Plexiglas cylinder rested on a platform inside a soundproof, ventilated chamber. Movement inside the cylinder was detected by a piezoelectric accelerometer below the frame. Sound levels within each test chamber were measured routinely with a sound level meter (Radio Shack) to ensure consistent presentation. Each test session began with a 5-min acclimatization period to background white noise of 68 dB, followed by 30 acoustic startle trial stimuli presented in six blocks (110 dB white noise of 40 ms duration and 30 or 45 s inter-trial interval). Two behavioral parameters were assessed: (a) the mean startle amplitude (averaged over all 30 trials) and (b) the percent of startle habituation to repeated presentation of the acoustic pulse. For the latter, the percent change was calculated between the response to the first and last (6th) blocks of sound stimuli, as follows:

According to the EPM and ASR measurements, the response of each rat to the PSS or Sham-PSS exposure was classified as an EBR, minimal (MBR), or partial behavioral response (PBR; see below).
Immunohistochemistry
Twenty-four hours after the behavioral tests (ie, on day 8), animals were deeply anesthetized and perfused transcardially with cold 0.9% physiological saline followed by 4% paraformaldehyde (Sigma-Aldrich, Israel) in 0.1 M phosphate buffer. Brains were quickly removed, post-fixed in the same fixative for 12 h at 4 °C, cryoprotected overnight (30% sucrose in 0.1 M phosphate buffer at 4 °C), and then frozen and stored at −80 °C. Serial coronal sections (10 μm) were performed with a cryostat (Leica CM 1850) and mounted on coated slides.
Staining
Sections were air-dried and washed three times in PBS containing Tween 20 (PBS/T; Sigma-Aldrich). They were then incubated for 60 min in a blocking solution (normal goat serum, in PBS) and then overnight at 4 °C with the primary antibodies against NPY (mouse monoclonal anti-NPY antiserum (1 : 500), product code: sc-133080, Santa Cruz Biotechnology, Inc., Heidelberg Germany) and Y1R (rabbit polyclonal anti-Y1R antiserum (1 : 500), product code: sc-28949, Santa Cruz Biotechnology, Inc.). After three washes in PBS/T, sections were incubated for 2 h in DyLight-488-labeled goat-anti-rabbit IgG or in Dylight-594 goat anti-mouse IgG (1 : 250; KPL, MD, USA) in PBS containing 2% normal goat or horse serum. The sections were then washed and mounted with mounting medium (Vectastain; Vector Laboratories, CA, USA). Sections from the brains of different groups of rats were processed at the same time and under identical conditions to ensure reliable comparisons and to maintain stringency in tissue preparation and staining conditions. Control staining was performed in the absence of the primary antibodies. In addition, secondary fluorescent labels were swapped to test for cross-reactivity, and sections were incubated without primary antibodies to test for nonspecific binding of the secondary antibodies.
Relative quantitative analysis of NPY immunoreactivity
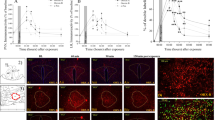
Brain sections showing NPY- and Y1R-ir in the PVN (–1.4 mm to –2.4 mm), ARC (–2.4 mm to –3.4 mm), CA1, CA3, DG (–3.6 mm to –4.0 mm), or BLA (–1.8 mm to –3.8 mm; all coordinates relative to Bregma) were subjected to image analysis. Each brain region was defined under the microscope according to cytoarchitectural landmarks (Paxinos and Watson, 1988). NPY- and Y1R-ir were measured in a 50 000-μm2 area in each area of interest and were digitized using microscopic images (Leica microscope DM4500B; Leica Microsystems, Wetzlar, Germany) and a DFC340FX digital imaging camera (Leica Microsystems). Measurements were taken from predetermined fields in each sub-region from both brain hemispheres. The density of fibers and cells expressing NPY- and Y1R-ir in each area was determined with the Leica LAS software (version 3.8). To compensate for background staining levels and to control for variations in the overall illumination level between images, the average pixel density of two regions that presumably contained only nonspecific staining (ie, in areas that are near each area of interest that is not thought to contain NPY/Y1R) was determined within each captured image, and this value was subtracted from all density measurements performed on that image (Brown et al 1998; Xavier et al 2005). Results are expressed as the average staining intensity and are presented as the mean±standard error of the mean (SEM). The data from all animals in each group were pooled separately for each brain region, and the mean±SEM was calculated. Figure 3 (panels 2 and 3) shows the rectangular area in which the cells and/or fibers were evaluated in each section.
Animal Sacrifice and Blood Sampling
In the second experiment, animals were decapitated with a guillotine. Situational stress was minimized by thoroughly cleaning the area between killing and removing the bodies. Trunk blood was collected, left at room temperature for 1 h, and then centrifuged at 1000 g for 10 min at 4 °C. Serum was collected and stored at –80 °C.
Measurement of Serum Corticosterone
Corticosterone was measured with a DSL-10-81000 ELISA kit (Diagnostic Systems Laboratories, Webster, TX, USA), according to the instructions of the manufacturer, by a person blind to the experimental procedures. All samples were measured in duplicates.
Drugs
NPY (10 μg; Bachem, Bubendorf, Switzerland) and Y1RA (the Y1R-selective argininamide derivative BIBO3304, (R)-N-[[4-(aminocarbonylaminomethyl)-phenyl]methyl]-N2-(diphenylacetyl)-argininamide trifluoroacetate (Wieland et al, 1998), 20 μg; Tocris Bioscience, Sweden) were dissolved in ACSF. Doses were chosen based on a previous study (Cohen et al, 2012).
DEX Suppression Test
Rats were subcutaneously injected with 50 μg/kg of DEX (Sigma Aldrich; dose based on a previous study (Cole et al, 2000)) or with saline. Then, 2.5 h later, the rats were intranasally (IN) administered with NPY, BIBO3304, or vehicle (ACSF). Blood samples were collected 30 and 60 min after the IN administration.
IN Administration
NPY, BIBO3304 (20 μg), or vehicle (ACSF) were infused into each nare by using a pipetteman with a disposable plastic tip under light isoflurane anesthesia. Extreme care was taken to avoid contact with the IN mucosa. Following the IN administration, the head of the animal was held in a tilted back position for approximately 15 s to prevent loss of solution from the nares (Serova et al, 2013).
Surgery
Rats were anesthetized with isoflurane and placed on a computer-guided motorized stereotaxic instrument (StereoDrive, Neurostar) that is fully integrated, via a control panel, with the coordinates of Paxinos and Franklin’s rat brain atlas (Paxinos and Watson, 1988). A bilateral stainless steel guide cannula (26 gauge, 1 mm cannula-to-cannula spacing, cut to extend 7 mm from the pedestal; Plastics One, Roanoke, VA, USA) was implanted with the tip just dorsal to the PVN (coordinates: 1.8 mm posterior to bregma, 6.5 mm below the skull surface). The guide cannula was affixed to the skull with three small machine screws surrounded by dental acrylic. A bilateral dummy cannula extending 1 mm beyond the end of the guide cannula was then inserted, and a dust cap was placed over the external end of the cannula. Animals were post-operatively administered buprenorphine (0.05 mg/kg, subcutaneously) and allowed to recover, singly housed, for 1 week. They were allowed 10 days to recover before experimental procedures were initiated.
Microinfusions
One microliter of NPY (1 nmol) was delivered bilaterally to the PVN by using a Hamilton syringe connected to a motorized nanoinjector, at a rate of 0.3 μl/min. To allow the solution to diffuse into the brain tissue, the needle was left in the brain for 2 min after injection.
Histology
At the end of the behavioral tests, 1 μl of India ink was microinjected to identify cannula placement. After decapitation, the brains were quickly removed, frozen on dry ice and kept at –20 °C. Coronal slices (30 μm) were cut in a cryostat, stained with a Nissl stain and analyzed to verify the microinfusion sites by using diagrams from the atlas by Paxinos and Watson (1988).
Statistical Analyses
For the behavioral and molecular results, statistical analyses were performed with a two-way ANOVA with PSS exposure (PSS vs Sham) and phase (Active (ZT=12) vs Inactive (ZT=0)) as the independent factors. For serum corticosterone levels, statistical analyses were performed with a one-way ANOVA. Post-hoc Bonferroni tests were used to examine differences between individual groups. In addition, the behavioral data were transformed to percentage using the Cutoff Behavioral Criteria (CBC) model: the prevalence of affected rats as a function of rat group was tested by using cross-tabulation and nonparametric χ2 tests. All nonparametric analyses were performed on raw data (and not on percentage).
The CBC model
Human responses to traumatic experiences clearly vary greatly in extent and in character. More importantly, PTSD occurs in a percentage (roughly 25–35%) of those exposed, underscoring the importance of contemporary definitions of stress-related disorders (DSM and ICD) and of inclusion and exclusion criteria applied in controlled clinical trials. In contrast, most animal studies have tended to relate to unclassified ‘global’ groups, ie, the entire exposed population vs control populations without distinction, whereas researchers who work with animals have long been aware that individual study subjects tend to display a variable range of responses to stimuli, certainly where stress paradigms are concerned. The heterogeneity in animal responses might be regarded as confirming the validity of animal studies, rather than as a problem. It stands to reason that a model of diagnostic criteria for psychiatric disorders can be applied to animal responses to augment the validity of study data, as long as the criteria for classification are clearly defined, reliably reproducible, and yield results that conform to findings in human subjects. The criteria used in this model were thus based on the EMP and ASR paradigms combined, and clearly defined two opposing extremes of the possible responses of each individual to stress. The one extreme of this model, termed here an ‘EBR’, indicates animals whose exploration of the open arms of the EPM was zero throughout the test, and whose startle response was maximal and did not undergo any habituation throughout the ASR test. An EBR thus parallels extreme PTSD-like responses and unabating maximal stress. The other extreme, termed here a ‘MBR’, indicates animals whose behavior in both the EMP and ASR paradigms was virtually unaffected by the stressor. A MBR thus parallels no PTSD-like response to the stressor. Rats that did not meet the criteria of either an EBR or a MBR were considered, by default, to have a PBR (Cohen et al, 2013, 2003c, 2005b). The CBC procedure and its predefined classification criteria are detailed in Figure 1.
RESULTS
Behavioral Analyses
EPM
A two-way ANOVA revealed a significant main effect for PSS exposure on the percentage of time spent in the open arms (F(1,102)=5.8, p<0.02, Figure 2a) and on the percentage of open-arm entries (F(1,102)=16.6, p<0.0001, Figure 2b). A PSS exposure × phase interaction was also found for these two EPM parameters (F(1,102)=31.0, p<0.00001; and F(1,102)=9.1, p<0.0035, respectively). Bonferroni post-hoc tests indicated that, in both the Active and the Inactive Phase groups, the overall time spent in the open arms was lower in the groups exposed to PSS than in the groups exposed to a Sham-PSS (p<0.035 and p<0.0001, respectively). In addition, Inactive/PSS rats spent significantly less time (p<0.05) in the open arms of the EPM, and entered significantly less (p<0.05) to these arms, than Active/PSS rats. This difference was not observed in rats exposed to a Sham-PSS. Thus, in the EPM, the behavioral outcomes of PSS exposure were greater when the stressor was applied at the onset of the inactive phase than at the onset of the active phase of the rats.
The effects of predator scent stress (PSS) applied at different zeitgeber times (ZTs) on behavior: Behavioral procedure (1). Behavior was compared between rats exposed to Sham-PSS at the onset of the active (dark; N=20) or inactive (light; N=23) phase, and between rats exposed to PSS at the onset of the active (N=32) and inactive (N=31) phase. (a) Percentage of time spent in the open arms of the EPM. (b) Percentage of entries to the open arms of the EPM. (c) Startle amplitude in the ASR paradigm. (d) Percentage of startle habituation in the ASR paradigm. (e) Prevalence of extreme behavioral response (EBR). (f) Prevalence of minimal behavioral response (MBR). (g) prevalence of partial behavioral response (PBR). Rats exposed to PSS during the inactive phase showed a significant decrease in the percentage of time spent in the open arms of the EPM, as compared with rats exposed to PSS during the active phase. Rats exposed to PSS during the inactive phase exhibited a significantly higher mean startle amplitude, as compared with rats exposed to PSS during the active phase. The prevalence of EBR was markedly higher in rats exposed to PSS at the onset of the inactive phase, as compared with rats exposed to PSS at the onset of the active phase. All data represent group mean±SEM.
ASR
A two-way ANOVA revealed a significant main effect for PSS exposure (F(1,102)=9.3, p<0.003) and a significant PSS exposure × phase interaction (F(1,102)=9.06, p<0.0035) for the startle amplitude (Figure 2c). Bonferroni post-hoc tests indicated that Inactive/PSS rats showed a significantly higher mean startle amplitude than Active/PSS rats (p<0.008). No differences in the mean startle amplitude were observed between Active/PSS and Active/Sham rats, or between the two Sham groups. Thus, in the ASR, the behavioral outcomes of PSS exposure were greater when the stressor was applied at the onset of the inactive phase than at the onset of the active phase of the rats. No differences in startle habituation were observed between any of the groups (Figure 2d).
CBC model classification
Significant differences were found between groups in the prevalence rates of individuals displaying an EBR, MBR, and PBR (Pearson χ2=30.55, d.f.=6, p<0.0025). Significant differences were found between groups in the prevalence rates of individuals displaying an EBR (Pearson χ2=14.4, d.f.=3, p<0.0025; Figure 2e). Specifically, the prevalence of an EBR was significantly higher in Inactive/PSS than in Active/PSS rats (22.5% (7/31) and 3.1% (1/32), respectively; χ2=4.18, p<0.05), and in Inactive/PSS than Inactive/Sham (χ2=5.76, p<0.02). No significant differences were found between the Active/PSS and the Active/Sham groups. Significant differences were also found between groups in the prevalence rates of individuals displaying a MBR (Pearson χ2=21.5, d.f.=3, p<0.0001; Figure 2f). Specifically, the prevalence of a MBR was markedly lower in Inactive/PSS rats than in Active/PSS rats (6.5% (2/31) and 31.3% (10/32), respectively; χ2=4.3, p<0.04). No significant differences were found between groups in the prevalence of a PBR (Figure 2g).
Immunoreactivity in Hypothalamic Nuclei
NPY immunostaining
In both the PVN and ARC regions, a two-way ANOVA revealed a significant main effect of PSS exposure (F(1,20)=230.3, p<0.0001, and F(1,20)=164.7, p<0.0001 for the two regions, respectively), a significant effect of phase (F(1,20)=65.7, p<0.0001, and F(1,20)=59.6, p<0.0001, respectively), and a significant PSS exposure × phase interaction (F(1,20)=5.3, p<0.035, and F(1,20)=4.4, p<0.05, respectively) on the immunocytochemical profile of NPY-ir fibers and cells (Figure 3a and d). Bonferroni post-hoc tests indicated that the density of NPY-ir cells and fibers in the PVN (Figure 3a) and ARC (Figure 3d) was significantly higher in Active/Sham rats than in Inactive/Sham rats (p<0.0035 and p<0.0007, respectively), indicating higher baseline levels at the onset of the active phase. In addition, the density of NPY-ir cells and fibers in the PVN and ARC significantly decreased in both the Active/PSS and the Inactive/PSS groups, as compared with the Active/Sham and Inactive/Sham groups, respectively (PVN: p<0.0005 and p<0.0001; ARC: p<0.0001 and p<0.0001, for the Active and Inactive Phase groups, respectively; Figure 3a and d). The decrease from baseline NPY-ir levels (indicated by the Sham-PSS groups) was greater in Inactive/PSS rats—which exhibited relatively low baseline NPY-ir levels—than in the Active/PSS rats.
The effects of predator scent stress (PSS) applied at different zeitgeber times (ZTs) on neuropeptide Y (NPY) and neuropeptide Y1 receptor (Y1R) levels: Behavioral procedure (1) and a schematic drawing representing the regions of the paraventricular nucleus (PVN; 2) and the arcuate nucleus (ARC; 3; coordinates relative to bregma: −1.80 mm and −2.56 mm, respectively; (Paxinos and Watson, 1988)), from which the measurements were collated. The quantitative morphometric analysis of NPY- (a and d) and of Y1R- (b and e) immunoreactivity in fibers and/or cells of the PVN and ARC of rats exposed to PSS or Sham-PSS at the onset of the dark (active) and the light (inactive) phases (n=6 for each group). Also shown are representative images of NPY- and Y1R-immunoreactivity in the PVN (c) and ARC (f). Images were acquired at × 10 magnification. Scale bar: 200 μm. The cells in green are NPY-positive and the cells in red are Y1R-positive. NPY- and Y1R-immunoreactivity in the PVN and ARC varied significantly in relation to the ZTs, with a steep rise at the onset of the active phase and a steep decline at the onset of the inactive phase. The decrease in the density of NPY- and Y1R- immunoreactivity in response to PSS exposure was smaller in rats exposed at the onset of the active phase than at the onset of the inactive phase. Active, dark (active) phase; Inactive, light (inactive) phase; AHC, central part of the anterior hypothalamus; DMH, dorsomedial nucleus of the hypothalamus; Rch, retrochiasmatic nucleus; VMH, ventromedial nucleus of the hypothalamus; 3V, third ventricle. All data represent group mean±SEM.
Y1R immunostaining
In both the PVN and ARC regions, a two-way ANOVA revealed a significant main effect of PSS exposure (F(1,20)=171.9, p<0.0001 and F(1,20)=112.6, p<0.0001 for the two regions, respectively) and of phase (F(1,20)=6.45, p<0.02 and F(1,20)=5.7, p<0.03, respectively) on the immunocytochemical profile of Y1R-ir (Figure 3b and e). Bonferroni post-hoc tests indicated that the density of Y1R-ir in the PVN (Figure 3b) and ARC (Figure 3e) was significantly higher in Active/Sham rats than in Inactive/Sham rats (p<0.05 and p<0.02, respectively), indicating higher baseline levels at the onset of the active phase. The density of Y1R-ir in the PVN and ARC was lower in the Active/PSS and in Inactive/PSS rats than in the Active/Sham and the Inactive/Sham groups, respectively (p<0.0001 for both comparisons).
Immunoreactivity in Hippocampal Subregions
NPY immunostaining
In the CA1 and DG subregions of the hippocampus, a two-way ANOVA revealed a significant main effect for PSS exposure (F(1,20)=40.7, p<0.0001 and F(1,20)=17.8, p<0.0001 for the two regions, respectively; Figure 4a and c) but not for phase. In addition, no PSS exposure × phase interaction was found. Bonferroni post-hoc tests indicated that, in both subregions, PSS exposure decreased the density of NPY-ir in both the Active and Inactive Phase groups, as compared with their Sham counterparts (CA1: p<0.0002 and p<0.001, and DG: p<0.0001 and p<0.0001, respectively). No significant differences were found between groups in the CA3 subregion (Figure 4b).
The effects of predator scent stress (PSS) applied at different zeitgeber times (ZTs) on hippocampal neuropeptide Y (NPY)- and neuropeptide Y1 receptor (Y1R)-immunoreactivity. A schematic drawing representing the regions—cornu ammonis 1/3 (CA1, CA3), dentate gyrus (DG; 1), and basolateral amygdala (BLA; 2) —from which measurements were collated. The quantitative morphometric analysis of NPY- (a–c and g) and Y1R (d–f and h) -immunoreactivity in cells of the hippocampal subregions and BLA of rats exposed to PSS or to Sham-PSS at the onset of the dark (active) or the light (inactive) phases. In the CA1, DG, and BLA, PSS exposure at the onset of either the active or inactive phase decreased the levels of NPY and Y1R. In the CA3 sub-region, no significant differences were found between groups. Active, dark (active) phase; Inactive, light (inactive) phase. All data represent group mean±SEM.
Y1R immunostaining
In the CA1 and DG subregions of the hippocampus, a two-way ANOVA revealed a significant main effect for PSS exposure (F(1,20)=31.9, p<0.0002 and F(1,20)=110.2, p<0.0001 in the two regions, respectively; Figure 4d and f) but not for phase. No significant PSS exposure × phase interaction was found. Bonferroni post-hoc tests indicated that, in both subregions, PSS exposure decreased the density of Y1R-ir in both the Active and Inactive Phase groups, as compared with their Sham counterparts (CA1: p<0.015 and p<0.002 and DG: p<0.0001 and p<0.0001, respectively). No significant differences were found between groups in the CA3 subregion (Figure 4e).
Immunoreactivity in the Basolateral Amygdala
NPY immunostaining
A two two-way ANOVA revealed a significant main effect for PSS exposure (F(1,19)=14.7, p<0.0015; Figure 4g) but not for phase. No significant PSS exposure × phase interaction was found. Bonferroni post-hoc tests indicated that PSS exposure decreased the density of NPY-ir in both the Active and Inactive Phase groups, as compared with their Sham counterparts (p<0.02 and p<0.05, respectively).
Y1R immunostaining
A two-way ANOVA revealed a significant main effect for PSS exposure (F(1,20)=31.9, p<0.0002; Figure 4h) but not for phase. No significant PSS exposure × phase interaction was found. Bonferroni post-hoc tests indicated that PSS exposure decreased the density of Y1R-ir in both the Active and Inactive Phase groups, as compared with their Sham counterparts (p<0.025 and p<0.05, respectively).
Plasma Corticosteroid Levels
Circulating corticosterone levels were measured at 5–15 min intervals over a period of 2.5 h, commencing immediately following PSS exposure at the onset of the active or inactive phase (Figure 5a).
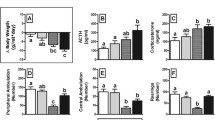
The effects of predator scent stress (PSS) applied at different zeitgeber times on plasma corticosteroid levels. (a) Serum corticosterone levels before and after exposure to PSS at the onset of the dark (active) and the light (inactive) phases, at 15-min intervals. (b) The exponential rate (R0) was calculated from baseline to corticosterone peak value. (c) The decay constant (t1) was calculated by using an exponential decay order 1 model. Dark—active phase; Light—inactive phase. All data represent group mean±SEM.
PSS applied at the onset of the inactive phase
Corticosterone concentrations were low (∼0.5 ng/ml) immediately after the onset of the inactive phase (lights on) and before the PSS exposure. They increased rapidly in response to PSS exposure, peaking approximately 15 min after the exposure, and then declined exponentially.
PSS applied at the onset of the active phase
In response to PSS exposure, corticosterone concentrations increased, peaked approximately 30 min after the exposure, and then declined steeply.
Comparison between phases
In rats exposed to a Sham-PSS, serum corticosterone concentrations were significantly higher in the Active Phase group than in the Inactive Phase group (p<0.0006). In rats exposed to PSS exposure, corticosterone concentrations rose similarly in the Active and Inactive Phase groups, reaching similar peak levels of 77–85 ng/ml. The corticosterone concentrations appeared to peak earlier in the Inactive Phase group than in the Active Phase group (15 vs 30 min, respectively, after PSS exposure), although this could not be verified with a sampling frequency of 10–15 min from different animals. Active/PSS rats displayed significantly higher and more prolonged serum corticosterone levels than Inactive/PSS rats. A nonlinear (exponential) fitting carried out between 3 and 4 time points before PSS exposure and the peak corticosterone concentrations indicated that the ‘exponential rate’ (R0) of the corticosterone response to PSS did not differ significantly between groups (Figure 5b). A nonlinear (exponential decay order 1 model) fitting carried out on the 6–7 time points following corticosterone peak concentrations indicated significantly different decay constants (t1) between groups, such that Active/PSS rats exhibited a slower decay in corticosterone levels than Inactive/PSS rats (t1=80.1±7.4 min and t1=39.5±11.3 min, respectively; p<0.05; Figure 5c).
DEX Suppression Test
DEX or saline (Sal) were administered before PSS exposure, and corticosterone levels were measured 30 and 60 min after the IN administration of NPY, Y1RA, or ACSF (Figure 6). The serum corticosterone levels were significantly higher in Sal/NPY rats than in Sal/ACSF or Sal/Y1RA rats, both 30 and 60 min after the IN administration (Bonferroni post-hoc test: p<0.0075 and p<0.05, respectively, after 30 min; and p<0.0004 and p<0.00015, respectively, after 60 min). The corticosterone levels were significantly lower in DEX/ACSF and in DEX/Y1RA rats than in Sal/ACSF rats (p<0.015 and p<0.0015, respectively) 30 min after IN administration. In contrast, a prior administration of DEX did not suppress plasma corticosterone after the IN administration of NPY, such that no difference was observed between Sal/ACSF and DEX/NPY rats. In addition, corticosterone concentrations were significantly higher in DEX/NPY rats than in DEX/Y1RA rats (p<0.015).
The effects of dexamethasone on corticosterone levels after external manipulation of the neuropeptide Y (NPY)-ergic system. Behavioral procedure (1). The thick empty arrow on the left represents a subcutaneous injection of dexamethasone (DEX; 50 μg/kg) or saline. The thick empty arrow on the right represents intranasal administration of NPY (10 μg), neuropeptide Y1 receptor antagonist (Y1RA (BIBO3304); 20 μg), or artificial cerebrospinal fluid (ACSF). Rats administered with NPY displayed significantly higher circulating corticosterone levels than rats treated with Y1RA or with the vehicle. Rats administered with DEX+NPY displayed significantly higher levels of corticosterone than rats administered with DEX+Y1RA. All data represent group mean±SEM.
The Behavioral Consequences of Intra-PVN Microinfusion of Y1RA or NPY Applied 30 min Before PSS Exposure
EPM
The percentage of time spent in the open arms of the EPM was significantly different between the groups (F(3,37)=7.9, p<0.00035; Figure 7a). Bonferroni post-hoc tests indicated that, in the Active Phase group, the overall time spent in the open arms was significantly lower in Active/Y1RA rats than in Active/ACSF rats (p<0.005). In addition, in the Inactive Phase group, the overall time spent in the open arms was significantly higher in Inactive/NPY rats than in Inactive/ACSF rats (p<0.0045). The percentage of open-arm entries was similar in all Active Phase rats (Figure 7b), whereas the percentage of open-arm entries was significantly higher in Inactive/NPY rats than in Inactive/ACSF rats (p<0.035).
The behavioral effects of an intra-PVN microinfusion of neuropeptide Y (NPY) or neuropeptide Y1 receptor agonist (Y1RA) applied 30 min before predator scent stress (PSS) exposure at the onset of the inactive or active phase. Experimental procedure (1). Thick arrow on top line represents the time of exposure to PSS in rats at the onset of the inactive phase. Thin arrow on top line represents the time of intra-PVN infusion of NPY (10 μg) in these rats. Thick arrow on bottom line represents the time of exposure to PSS in rats at the onset of the active phase (zeitgeber time (ZT)=12). Thin arrow on the bottom line represents the intra-PVN infusion of the Y1RA BIBO3304 (20 μg). Panel (2) shows a schematic diagram of a coronal section of the brain, indicating the localization of a correctly implanted cannula. (a) Percentage of time spent in the open arms of the elevated plus-maze (EPM). (b) Percentage of entries to the open arms of the EPM. (c) Startle amplitude and (d) percentage of startle habituation in the ASR paradigm. (e) Prevalence of rats exhibiting an extreme behavioral response (EBR). (f) Prevalence of rats exhibiting a minimal behavioral response (MBR) and (g) prevalence of rats exhibiting a partial behavioral response (PBR). Comparisons were between rats microinfused with NPY/artificial cerebrospinal fluid (ACSF) and exposed to PSS at the onset of the light (inactive) phase, and rats microinfused with Y1RA/ACSF and exposed to PSS at the onset of the dark (active) phase. An intra-PVN microinfusion of the Y1RA (20 mg) increased the prevalence of PTSD-like behavioral responses (EBR) as compared with ACSF treatment. An intra-PVN microinfusion of NPY before PSS exposure at the onset of the inactive phase decreased the prevalence of PTSD-like behavioral responses (EBR). Active, dark (active) phase; Inactive, light (inactive) phase. All data represent group mean±SEM.
ASR
Significant differences in the startle amplitude were found between the groups (F(3,37)=5.4, p<0.0035; Figure 7c). Bonferroni post-hoc tests indicated that the mean startle amplitude was significantly higher in Active/Y1RA rats than in Active/ACSF rats (p<0.015), and significantly lower in Inactive/NPY rats than in Inactive/ACSF rats (p<0.009). No differences in the startle habituation were observed between groups (Figure 7d).
CBC model classification
Significant differences were found between groups in the prevalence rates of individuals displaying an EBR, MBR, and PBR (Pearson χ2=14.3, d.f.=6, p<0.025). Significant differences were found between groups in the prevalence of individuals displaying an EBR (Pearson χ2=8.83, d.f.=3, p<0.035; Figure 7e). Specifically, the prevalence of an EBR was higher in Active/Y1RA rats than in Active/ACSF rats (36.36% (4//11) and 0% (0/10), respectively; p=0.055 Fisher Exact Test). In contrast, the prevalence of an EBR was lower in Inactive/NPY rats (0%) than in Inactive/ACSF rats (10% (1/10)). Significant differences were also found between groups in the prevalence of individuals displaying a MBR (Pearson χ2=9.0, d.f.=3, p<0.03; Figure 7f). Specifically, the prevalence of a MBR was lower in Active/Y1RA rats than in Active/ACSF rats (9.1% (1/11) and 50% (5/10), respectively; p=0.055 Fisher Exact Test), and was higher in Inactive/NPY rats than in Inactive/ACSF rats (70% (7/10) and 30% (3/10), respectively). No significant differences were found in the prevalence of PBR between groups (Figure 7g).
The Effects of a Microinfusion of NPY to Inactive Phase rats, and of Y1RA to Active Phase rats, on NPY and Y1R Immunoreactivity in the PVN
PVN NPY immunostaining
Significant differences were found between the groups (F(3,16)=8.9, p<0.0015; Figure 8a). Bonferroni post-hoc tests indicated that PVN NPY-ir was significantly lower in Active/Y1RA rats than in Active/ACSF rats (p<0.015), and was significantly higher in Inactive/NPY rats than in Inactive/ACSF rats (p<0.015).
The effects of intra-paraventricular nucleus (PVN) microinfusion of neuropeptide Y (NPY) or neuropeptide Y1 receptor agonist (Y1RA) applied 30 min before exposure to predator scent stress (PSS) at the onset of the inactive or active phase on NPY and Y1R levels. Experimental procedure (1). The thick arrow on top line represents the time of exposure to PSS (inactive phase, zeitgeber time (ZT)=0) and thin arrow represents the intra-PVN infusion of NPY (10 μg) to these rats. The thick arrow on the bottom line represents the time of exposure to PSS (active phase, ZT=12) and the thin arrow represents the intra-PVN infusion of Y1RA (20 μg) to these rats. Panel (2) shows a schematic drawing of the regions of the PVN (coordinates relative to bregams: −1.80 mm) from which the measurements were collected. The quantitative morphometric analysis of NPY- (a) and Y1R- (b) immunoreactivity in fibers and/or cells of the PVN of rats microinfused with NPY/artificial cerebrospinal fluid (ACSF) and exposed to PSS at the onset of the inactive phase and rats microinfused with Y1RA/ACSF and exposed to PSS at the onset of the dark (active) phase. (c) Representative images of NPY- and Y1R-immunoreactivity in the PVN. Images were acquired at × 10 magnification. Scale bar: 200 μm. The cells in green are NPY-positive and the cells in red are Y1R-positive. Thirty minutes after intra-PVN microinfusion of the Y1RA, a significant decrease was observed in the density of NPY- and Y1R-immunoreactivity in the PVN. Intra-PVN microinfusion of NPY before PSS exposure at the onset of the inactive phase was accompanied by a significant upregulation of the density of NPY- and Y1R-immunoreactivity in the PVN. Active, dark (active) phase; Inactive, light (inactive) phase; Rch, retrochiasmatic nucleus; 3V, third ventricle. All data represent group mean±SEM.
PVN Y1R immunostaining
Significant differences were found between the groups (F(3,16)=5.7, p<0.0075; Figure 8b). Bonferroni post-hoc tests indicated that the Y1R-ir was significantly lower in Active/Y1RA rats than in Active/ACSF rats (p<0.02), and was significantly higher in Inactive/NPY rats than in Inactive/ACSF rats (p<0.045).
DISCUSSION
The most significant findings of this study are: (a) the ZT at which rats were exposed to psychological stress influenced on the behavioral response of the rats. Rats appeared to be more vulnerable to stress applied at the onset of the inactive phase and, conversely, more resilient to stress applied at the onset of the active phase. (b) The behavioral response to stress displayed diurnal variations, suggesting a circadian-dependent modulation of the behavioral response by the NPY-ergic system in the PVN and ARC, in association with HPA axis activity. This effect is in line with our previously reported hypothesis that the NPY-ergic system, together with the HPA axis, has a significant role in the resilience to stress (Cohen et al, 2012).
The time of day at which rats were exposed to the stressor altered the sequelae of the resulting trauma. Phenotypically, marked behavioral differences were observed in the EPM and ASR paradigms in response to stress applied at different ZTs. In the EPM, rats exposed to PSS at the onset of the active phase spent a higher proportion of the test duration in the open arms, as compared with rats exposed to PSS at the onset of the inactive phase. The startle amplitude similarly exhibited a diurnal modulation, as was also reported previously (Chabot and Taylor, 1992a, 1992b; Davis and Sollberger, 1971): at baseline, rats exposed to PSS at the onset of the active phase exhibited a higher (albeit not significant) startle amplitude but a low-range response to the PSS. On the other hand, rats exposed to PSS at the onset of the inactive phase exhibited low baseline startle responses but a more marked post-exposure behavioral disruption. Data analysis in which the more robustly affected rats were distinguished from other rats (based on the CBC procedure) provided a more precise assessment of the magnitude of these behavioral effects: the prevalence of severely affected rats (ie, an EBR) among rats exposed to PSS at the onset of the inactive phase was 22.6%, as compared with 3.3% in rats exposed at the onset of the active phase. We therefore suggest that PSS exposure at the onset of the active phase, as compared with the inactive phase, confers a greater resistance to the negative consequences of the experimental stress paradigm (ie, decreased anxiety-like, fearful- and PTSD-like responses).
To test whether the behavioral response to PSS exposure was commensurate with HPA axis profiles, we assessed the influence of the exposure applied at different ZTs on plasma corticosterone levels. The basal levels of circulating corticosterone at the onset of the active phase were significantly different than those at the onset of the inactive phase, reflecting the expected circadian variation (Torrellas et al, 1981). This notwithstanding, the magnitude of response of the HPA axis to stress was similar in the two circadian phases, although, in rats at the active phase, a slower return to baseline corticosterone levels after PSS exposure was observed. A nonlinear regressive fitting model (exponential decay order 1) indicated significantly different ‘half-life’ (t1/2) for corticosterone between the groups, with rats in the active phase showing a prolonged corticosterone stress response as compared with rats in the inactive phase. At the systemic level, this result indicates a less efficient negative feedback during the active phase. Taken together, these findings imply a possible association between the circadian phase, HPA regulation, and the behavioral response to stress. The specific mechanisms underlying these effects are, however, yet unclear.
Stress responses related to diurnal responsiveness of the HPA axis have been extensively reported in the past. A considerable amount of data suggests that the responsiveness of the HPA is increased in the early inactive phase (Choi et al, 1996; Retana-Marquez et al, 2003; Torrellas et al, 1981), although some data suggest increased responsiveness at the onset of the active phase (Kalsbeek et al, 2003; Lilly et al, 2000), or equal responsiveness at the two phases (Mathias et al, 2000; Retana-Marquez et al, 2003). One interpretation of these data is that the diurnal variations in the magnitude of the corticosteroid response to stress is stressor-specific (Atkinson et al, 2006). For example, hemorrhage (Lilly et al, 2000) and hypoglycemia (Kalsbeek et al, 2003) have been reported to produce a greater corticosterone response in the evening, whereas restraint, immobilization, and foot-shocks (Retana-Marquez et al, 2003; Torrellas et al, 1981) produce a greater corticosterone response in the morning. Other stressors, such as an immune challenge or immersion in cold water, produce equivalent HPA responses throughout the day (Mathias et al, 2000; Retana-Marquez et al, 2003). However, findings are not uniform across studies and are difficult to compare accurately, mostly due to methodological differences in the stress paradigms used, in the duration and number of exposures to stress, and in the time of assessment.
Densities of NPY- and Y1R-ir in the PVN and ARC hypothalamic nuclei, in hippocampal sub-regions, and in the BLA were evaluated in rats exposed to real or sham-PSS at the onset of the two phases for a number of reasons. First, the NPY-ergic system has a significant role in resilience to stress (Cohen et al, 2012), as hypothalamic NPY is critically implicated in the homeostatic regulation of the HPA axis (Hanson and Dallman, 1995; Wahlestedt et al, 1987). Second, NPY has been shown to be involved in behavioral functions that depend upon the integrity of the hippocampus and BLA (Redrobe et al, 1999). In the hippocampus, in vitro studies have shown that NPY exerts a neuro-proliferative effect on neuronal precursors (Decressac et al, 2010; Howell et al, 2007). In the current study, we found that, under basal conditions, NPY and Y1R levels in the PVN and ARC vary significantly in relation to the light/dark cycle, showing a high immunostaining density at the onset of the active phase and a low immunostaining density at the onset of the inactive phase. In contrast to the hypothalamic nuclei, no significant cycle was apparent in the hippocampal sub-regions and in the BLA, where the density of NPY-ir was considerably lower and relatively stable at both ZTs tested. These data are consistent with previous reports (Akabayashi et al, 1994; Jhanwar-Uniyal et al, 1990; McKibbin et al, 1991; van Dijk and Strubbe, 2003). In response to stress, a striking association was found between the degree of behavioral disruption and the pattern of NPY- and Y1R-ir, in relation to the active/inactive phases. More specifically, the density of NPY- and Y1R-ir in the PVN and ARC decreased in response to stress at both ZTs. However, rats exposed to PSS at the onset of the inactive phase—and who exhibited a low density of NPY-ir at baseline—displayed a greater change, ie, a greater reduction in the density of NPY-ir, as compared with rats exposed at the onset of the active phase. Consistent with the hypothesis that the NPY-ergic system promotes neuronal function and/or structural changes, the protective qualities attributed to NPY are supported by its increased levels at the onset of the active phase. Thus, NPY appears to promote resilience to and/or recovery from stress. These results are in line with our hypothesis that vicissitudes of NPY have an important role in the regulation of anxiety and depression, as was also suggested previously (Angelucci et al, 2002, 2003, 2000; Domschke et al, 2010; Heilig et al, 2004; Mathe’, 1999; Mathe’ et al, 1996; Neumann et al, 2011; Redrobe et al, 1999; Sajdyk et al, 2008).
We found that corticosterone levels were significantly increased at around the same time in which the rise in NPY levels was observed in the PVN and ARC. This finding suggests a possible association between central NPY levels and circulating corticosterone levels. As NPY systems in both the ARC and PVN contribute to the modulation of the HPA axis, we hypothesized that the prolonged expression of the HPA axis at the onset of the active phase could be due to the synergistic action of NPY and the HPA axis. The sensitivity to steroid feedback inhibition was tested by comparing the circulating corticosterone responses to DEX with and without treating the rats by an intranasal administration of NPY or Y1RA. The NPY-treated rats displayed significantly higher circulating corticosterone levels than vehicle-treated controls and Y1RA-treated rats. Moreover, elevated serum corticosterone levels were found in rats pre-treated with DEX and then treated with intranasal NPY. Such an increase was prevented in rats pre-treated with DEX and then with intranasal Y1RA, suggesting a strong influence of NPY/Y1R activation on the regulation of the HPA axis.
Studies in which NPY was administered to animals support the suggestion that this peptide may integrate within and regulate HPA systems (Cohen et al, 2012; Serova et al, 2013). Our data are consistent with the hypothesis that the corticosterone peak at the active phase is mediated by activation of neuronal inputs to the hypothalamus, which render the HPA axis less sensitive to negative feedback (Akana et al, 1986; Holmes et al, 1995). In light of the strong association between the circadian rhythm of circulating corticosterone levels and central NPY levels, an interaction between the HPA axis and central NPY neurons may have a particular significance in determining daily rhythms of several physiological processes, including behavioral responses to stress (Akabayashi et al, 1994).
The contribution of NPY to the diurnal variation in the behavioral response to stress was further assessed by experimentally manipulating the NPY levels, namely, by administrating NPY or its receptor antagonist. To manipulate NPY levels, NPY was microinfused into the PVN 30 min before exposure to PSS at the onset of the inactive phase, whereas Y1RA was microinfused into the PVN 30 min before exposure to PSS at the onset of the active phase. This manipulation was indeed effective; the behavioral effects were most striking in the NPY experiment, wherein a significant increase was observed in the overall time spent in the open arms and in the open arm entries, and a significant decrease was observed in the startle amplitude, as compared with the Inactive/ACSF group. However, the Y1RA treatment significantly decreased the overall time spent in the open arms and significantly increased the startle amplitude, as compared with the Active/ACSF group. Although not statistically significant (due to a small sample size), there was a clear trend showing that the Y1RA treatment increased the prevalence rates of EBRs (from 0 to 36.36%), with a concomitant decrease in the prevalence rates of MBRs (50 to 9.1%). Thus, microinfusion of the antagonist to rats in the active phase resulted in an overall shift toward an extreme, PTSD-like behavioral disruption ensuing from the traumatic stress exposure. In addition, administration of NPY before PSS exposure at the onset of the inactive phase reduced the prevalence rates of EBRs (from 10 to 0%), with a concomitant increase in the prevalence rates of MBRs (from 30 to 70%). This change was relatively small (as compared with the change observed in the Y1RA experiment), probably because the circadian cycle involves numerous factors other than NPY, which dampened the effect of the NPY manipulation.
In rats exposed to PSS at the onset of the inactive phase, the anxiolytic-like effect of microinjected NPY was accompanied by a significant upregulation of NPY- and NPY-Y1-ir in the PVN. In contrast, a microinjection of Y1RA at the onset of the active phase was accompanied by a significant downregulation of NPY- and Y1R-ir in the PVN. The use of the NPY manipulation under both diurnal and nocturnal conditions elicited interesting results. The fact that an NPY microinjection 30 min before PSS exposure at the onset of the inactive phase not only improved the behavioral response pattern but also normalized the PVN NPY levels 8 days later, combined with the fact that a Y1RA infusion 30 min before PSS exposure at the onset of the active phase not only impaired behavior but also exerted an opposite effect on the PVN NPY levels, are pivotal. This is because such results (a) point to a significant protective role of endogenous NPY; (b) reinforce the role of the dysregulated NPY transmission in anxiety and depression (Bjørnebekk et al, 2010; Husum and Mathé, 2002; Jiménez-Vasquez et al, 2007; Mathé et al, 2007); and (c) are consistent with our hypothesis that Y1RAs are good candidates for developing new treatments for affective disorders and anxiety (for review, see Wu et al, 2011).
Our study is not devoid of limitations, which should be addressed in future studies. First, measuring corticosterone levels in response to an NPY/Y1RA microinfusion at several time points following the exposure to stress could shed light on the association between the NPY-ergic system and the HPA axis activity; the focus of an ongoing follow-up study. Second, in the last experiment presented in this article, the overall sample size was insufficient for a nonparametric statistical analysis. Future studies should thus employ larger sample sizes.
CONCLUSIONS
We show that the ZT at which the exposure to a traumatic stressful event occurs significantly affects the behavioral response to stress. Animals were more vulnerable to the traumatic stress exposure at the onset of their inactive phase and, conversely, more resilient at the onset of their active phase. The diurnal rhythms in the responsiveness to stress are modulated by the neural network that regulates NPY levels. This system has a significant role in the stress-response cascade and interacts with other systems, including the HPA axis, to mediate processes involved in stress-related behavioral responses, recovery, and resilience. Thus, the subjective time of day at which an animal is exposed to a stressful and potentially traumatic event impacts on the vulnerability or resilience of the individual to the stressor. This process may have an adaptive value in the ecological context, considering an environment especially relevant to a diurnal animal where time-of-day information carried by light has to be separated from a noisy background (Daan, 2000; Yannielli and Harrington, 2001).
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
References
Ahima RS, Harlan RE (1990). Charting of type II glucocorticold receptor-like immunoreactivity in the rat central nervous system. Neuroscience 39: 579–604.
Akabayashi A, Watanabe Y, Wahlestedt C, McEwen BS, Paez X, Leibowitz SF (1994). Hypothalamic neuropeptide Y, its gene expression and receptor activity: relation to circulating corticosterone in adrenalectomized rats. Brain Res 665: 201–212.
Akana SF, Cascio CS, Du JZ, Levin N, Dallman MF (1986). Reset of feedback in the adrenocortical system: an apparent shift in sensitivity of adrenocorticotropin to inhibition by corticosterone between morning and evening. Endocrinology 119: 2325–2332.
American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders 4 edn American Psychiatric Association: Washington, DC.
Angelucci F, Aloe L, Jimenez-Vasquez P, Mathé AA (2002). Electroconvulsive stimuli alter the regional concentrations of nerve growth factor, brain-derived neurotrophic factor, and glial cell line-derived neurotrophic factor in adult rat brain. J Ect 18: 138–143.
Angelucci F, Aloe L, Jimenez-Vasquez P, Mathé AA (2003). Lithium treatment alters brain concentrations of nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor in a rat model of depression. Int J Neuropsychopharmacol 6: 225–231.
Angelucci F, Aloe L, Vasquez PJ, Mathé AA (2000). Mapping the differences in the brain concentration of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in an animal model of depression. Neuroreport 11: 1369–1373.
Atkinson HC, Leggett JD, Wood SA, Castrique ES, Kershaw YM, Lightman SL (2010). Regulation of the hypothalamic-pituitary-adrenal axis circadian rhythm by endocannabinoids is sexually diergic. Endocrinology 151: 3720–3727.
Atkinson HC, Wood SA, Kershaw YM, Bate E, Lightman SL (2006). Diurnal variation in the responsiveness of the hypothalamic-pituitary-adrenal axis of the male rat to noise stress. J Neuroendocrinol 18: 526–533.
Bai FL, Yamano M, Shiotani Y, Emson PC, Smith AD, Powell JF et al (1985). An arcuato-paraventricular and -dorsomedial hypothalamic neuropeptide Y-containing system which lacks noradrenaline in the rat. Brain Res 331: 172–175.
Bjørnebekk A, Mathé A, Brené S (2010). The antidepressant effects of running and escitalopram are associated with levels of hippocampal NPY and Y1 receptor but not cell proliferation in a rat model of depression. Hippocampus 20: 820–828.
Brown HE, Garcia MM, Harlan RE (1998). A two focal plane method for digital quantification of nuclear immunoreactivity in large brain areas using NIH-image software. Brain Res Brain Res Protoc 2: 264–272.
Chabot CC, Taylor DH (1992a). Circadian modulation of the rat acoustic startle response. Behav Neurosci 106: 846–852.
Chabot CC, Taylor DH (1992b). Daily rhythmicity of the rat acoustic startle response. Physiol Behav 51: 885–889.
Choi S, Horsley C, Aguila S, Dallman MF (1996). The hypothalamic ventromedial nuclei couple activity in the hypothalamo-pituitary-adrenal axis to the morning fed or fasted state. J Neurosci 16: 8170–8180.
Cohen H, Joseph Z, Matar M (2003a). The relevance of differential response to trauma in an animal model of post-traumatic stress disorder. Biol Psychiatry 53: 463–473.
Cohen H, Liu T, Kozlovsky N, Kaplan Z, Zohar J, Mathe AA (2012). The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology 37: 350–363.
Cohen H, Matar MA, Buskila D, Kaplan Z, Zohar J (2008). Early post-stressor intervention with high dose corticosterone attenuates post traumatic stress response in an animal model of PTSD. Biol Psychiatry 64: 708–717.
Cohen H, Matar MA, Joseph Z (2013). Animal models of post-traumatic stress disorder. Curr Protoc Neurosci Chapter 9: Unit 9 45.
Cohen H, Zohar J (2004a). An animal model of posttraumatic stress disorder: the use of cut-off behavioral criteria. Ann NY Acad Sci 1032: 167–178.
Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U et al (2006). Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol Psychiatry 59: 1208–1218.
Cohen H, Zohar J, Matar M (2003b). The relevance of differential response to trauma in an animal model of post-traumatic stress disorder. Biol Psychiatry 53: 463–473.
Cohen H, Zohar J, Matar M (2003c). The relevance of differential response to trauma in an animal model of posttraumatic stress disorder. Biol Psychiatry 53: 463–473.
Cohen H, Zohar J, Matar MA, Kaplan Z, Geva AB (2005a). Unsupervised fuzzy clustering analysis supports behavioral cutoff criteria in an animal model of posttraumatic stress disorder. Biol Psychiatry 58: 640–650.
Cohen H, Zohar J, Matar MA, Kaplan Z, Geva AB (2005b). Unsupervised fuzzy clustering analysis supports behavioral cutoff criteria in an animal model of posttraumatic stress disorder. Biol Psychiatry 58: 640–650.
Cohen H, Zohar J, Matar MA, Kaplan Z, Geva AB (2005c). Unsupervised fuzzy clustering analysis supports behavioral cutoff criteria in an animal model of posttraumatic stress disorder. Biol Psychiatry 58: 640–650.
Cohen H, Zohar J, Matar MA, Zeev K, Loewenthal U, Richter-Levin G (2004b). Setting apart the affected: the use of behavioral criteria in animal models of post traumatic stress disorder. Neuropsychopharmacology 29: 1962–1970.
Cohen H, Zohar J, Matar MA, Zeev K, Loewenthal U, Richter-Levin G (2004c). Setting apart the affected: the use of behavioral criteria in animal models of post traumatic stress disorder. Neuropsychopharmacology 29: 1962–1970.
Cole MA, Kim PJ, Kalman BA, Spencer RL (2000). Dexamethasone suppression of corticosteroid secretion: evaluation of the site of action by receptor measures and functional studies. Psychoneuroendocrinology 25: 151–167.
Daan S (2000). The Colin S. Pittendrigh Lecture. Colin Pittendrigh, Jurgen Aschoff, and the natural entrainment of circadian systems. J Biol Rhythms 15: 195–207.
Dallman M, Yates F (1969). Dynamic asymmetries in the corticosteroid feedback path and distribution-metabolism-binding elements of the adrenocortical system. Ann NY Acad Sci 156: 696–721.
Davis M, Sollberger A (1971). Twenty-four-hour periodicity of the startle response in rats. Psychonom Sci 25: 37–39.
de Quidt ME, Emson PC (1986). Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system-II. Immunohistochemical analysis. Neuroscience 18: 545–618.
Decressac M, Wright B, David B, Tyers P, Jaber M, Barker RA et al (2010). Exogenous neuropeptide Y promotes in vivo hippocampal neurogenesis. Hippocampus 21: 233–238.
Delahanty DL, Raimonde AJ, Spoonster E (2000). Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biol Psychiatry 48: 940–947.
Domschke K, Dannlowski U, Hohoff C, Ohrmann P, Bauer J, Kugel H et al (2010). Neuropeptide Y (NPY) gene: Impact on emotional processing and treatment response in anxious depression. Eur Neuropsychopharmacol 20: 301–309.
File SE, Zangrossi H Jr, Sanders FL, Mabbutt PS (1993). Dissociation between behavioral and corticosterone responses on repeated exposures to cat odor. Physiol Behav 54: 1109–1111.
Fuxe K, Wikstrom A-C, Okret S, Agnati LF, Harfstrand A, Yu A-Y et al (1985). Mapping of glucocorticoid receptor immunoreactive neurons in the rat tel- and diencephalon using a monoclonal antibody against rat liver glucocorticoid receptor. Endocrinology 117: 1803–1812.
Hanson E, Dallman M (1995). Neuropeptide Y (NPY) may integrate responses of hypothalamic feeding systems and the hypothalamo-pituitary-adrenal axis. J Neuroendocrinol 7: 273–279.
Heilig M, Zachrisson O, Thorsell A, Ehnvall A, Mottagui-Tabar S, Sjogren M et al (2004). Decreased cerebrospinal fluid neuropeptide Y (NPY) in patients with treatment refractory unipolar major depression: preliminary evidence for association with preproNPY gene polymorphism. J Psychiatr Res 38: 113–121.
Holmes MC, French KL, Seckl JR (1995). Modulation of serotonin and corticosteroid receptor gene expression in the rat hippocampus with circadian rhythm and stress. Brain Res Mol Brain Res 28: 186–192.
Howell OW, Silva S, Scharfman HE, Sosunov AA, Zaben M, Shatya A et al (2007). Neuropeptide Y is important for basal and seizure-induced precursor cell proliferation in the hippocampus. Neurobiol Dis 26: 174–188.
Husum H, Mathé A (2002). Early life stress changes concentrations of neuropeptide Y and corticotropin-releasing hormone in adult rat brain. Lithium treatment modifies these changes. Neuropsychopharmacology 27: 756–764.
Jhanwar-Uniyal M, Beck B, Burlet C, Leibowitz SF (1990). Diurnal rhythm of neuropeptide Y-like immunoreactivity in the suprachiasmatic, arcuate and paraventricular nuclei and other hypothalamic sites. Brain Res 536: 331–334.
Jiménez-Vasquez P, Diaz-Cabiale Z, Caberlotto L, Bellido I, Overstreet D, Fuxe K et al (2007). Electroconvulsive stimuli selectively affect behavior and neuropeptide Y (NPY) and NPY Y(1) receptor gene expressions in hippocampus and hypothalamus of Flinders Sensitive Line rat model of depression. Eur Neuropsychopharmacol 17: 298–308.
Kalsbeek A, Ruiter M, La Fleur SE, Van Heijningen C, Buijs RM (2003). The diurnal modulation of hormonal responses in the rat varies with different stimuli. J Neuroendocrinol 15: 1144–1155.
Kask A, Harro J, von Horsten S, Redrobe JP, Dumont Y, Quirion R (2002). The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci Biobehav Rev 26: 259–283.
Lightman SL, Windle RJ, Julian MD, Harbuz MS, Shanks N, Wood SA et al (2000). Significance of pulsatility in the HPA axis. Novartis Found Symp 227: 244–257 discussion 257-260.
Lilly MP, Jones RO, Putney DJ, Carlson DE (2000). Post-surgical recovery and time-of-day mask potentiated responses of ACTH to repeated moderate hemorrhage in conscious rats. J Endocrinol 167: 205–217.
Mathe’ A (1999). Neuropeptides and electroconvulsive treatment. J Ect 15: 60–75.
Mathe’ A, Rudorfer M, Stenfors C, Manji H, Potter W, Theodorsson E (1996). Effects of electroconvulsive treatment on somatostatin, neuropeptide Y, endothelin and neurokinin A concentration in cerebrospinal fluid of depressed patients. Depression 3: 250–257.
Mathias S, Schiffelholz T, Linthorst AC, Pollmacher T, Lancel M (2000). Diurnal variations in lipopolysaccharide-induced sleep, sickness behavior and changes in corticosterone levels in the rat. Neuroendocrinology 71: 375–385.
Mathé A, Husum H, El Khoury A, Jiménez-Vasquez P, Gruber S, Wörtwein G et al (2007). Search for biological correlates of depression and mechanisms of action of antidepressant treatment modalities. Do neuropeptides play a role? Physiol Behav 92: 226–231.
McEwen BS (2002). The neurobiology and neuroendocrinology of stress. Implications for post-traumatic stress disorder from a basic science perspective. Psychiatr Clin North Am 25: 469–494.
McFarlane AC, Atchison M, Yehuda R (1997). The acute stress response following motor vehicle accidents and its relation to PTSD. Ann N Y Acad Sci 821: 437–441.
McFarlane S (2000). Dendritic morphogenesis: building an arbor. Mol Neurobiol 22: 1–9.
McKibbin PE, Rogers P, Williams G (1991). Increased neuropeptide Y concentrations in the lateral hypothalamic area of the rat after the onset of darkness: possible relevance to the circadian periodicity of feeding behavior. Life Sci 48: 2527–2533.
Neumann ID, Wegener G, Homberg JR, Cohen H, Slattery DA, Zohar J et al (2011). Animal models of depression and anxiety: What do they tell us about human condition? Prog Neuropsychopharmacol Biol Psychiatry 35: 1357–1375.
Paxinos G, Watson C (1988) The Rat Brain in Stereotaxic Coordinates. London.
Redrobe JP, Dumont Y, St-Pierre JA, Quirion R (1999). Multiple receptors for neuropeptide Y in the hippocampus: putative roles in seizures and cognition. Brain Res 848: 153–166.
Retana-Marquez S, Bonilla-Jaime H, Vazquez-Palacios G, Dominguez-Salazar E, Martinez-Garcia R, Velazquez-Moctezuma J (2003). Body weight gain and diurnal differences of corticosterone changes in response to acute and chronic stress in rats. Psychoneuroendocrinology 28: 207–227.
Rivier CL, Plotsky PM (1986). Mediation by corticotropin releasing factor (CRF) of adenohypophysial hormone secretion. Ann Rec Physiol 48: 475–494.
Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz SD, Dietrich A, Morin M et al (2008). Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. J Neurosci 28: 893–903.
Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, Polak JM (1985). Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol 241: 138–153.
Serova LI, Tillinger A, Alaluf LG, Laukova M, Keegan K, Sabban EL (2013). Single intranasal neuropeptide Y infusion attenuates development of PTSD-like symptoms to traumatic stress in rats. Neuroscience 236: 298–312.
Torrellas A, Guaza C, Borrell J, Borrell S (1981). Adrenal hormones and brain catecholamines responses to morning and afternoon immobilization stress in rats. Physiol Behav 26: 129–133.
van Dijk G, Strubbe JH (2003). Time-dependent effects of neuropeptide Y infusion in the paraventricular hypothalamus on ingestive and associated behaviors in rats. Physiol Behav 79: 575–580.
Wahlestedt C, Skagerberg G, Ekman R, Heilig M, Sundler F, Hakanson R (1987). Neuropeptide Y (NPY) in the area of the hypothalamic paraventricular nucleus activates the pituitary-adrenocortical axis in the rat. Brain Res 417: 33–38.
Wieland HA, Engel W, Eberlein W, Rudolf K, Doods HN (1998). Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO 3304 and its effect on feeding in rodents. Br J Pharmacol 125: 549–555.
Windle RJ, Wood SA, Shanks N, Lightman SL, Ingram CD (1998). Ultradian rhythm of basal corticosterone release in the female rat: dynamic interaction with the response to acute stress. Endocrinology 139: 443–450.
Witteveen AB, Huizink AC, Slottje P, Bramsen I, Smid T, van der Ploeg HM (2010). Associations of cortisol with posttraumatic stress symptoms and negative life events: a study of police officers and firefighters. Psychoneuroendocrinology 35: 1113–1118.
Wu G, Feder A, Wegener G, Bailey C, Saxena S, Charney D et al (2011). Central functions of neuropeptide Y in mood and anxiety disorders. Expert Opin Ther Targets 15: 1317–1331.
Xavier LL, Viola GG, Ferraz AC, Da Cunha C, Deonizio JM, Netto CA et al (2005). A simple and fast densitometric method for the analysis of tyrosine hydroxylase immunoreactivity in the substantia nigra pars compacta and in the ventral tegmental area. Brain Res Brain Res Protoc 16: 58–64.
Yannielli PC, Harrington ME (2001). Neuropeptide Y in the mammalian circadian system: effects on light-induced circadian responses. Peptides 22: 547–556.
Yehuda R, McFarlane AC, Shalev AY (1998). Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biol Psychiatry 44: 1305–1313.
Acknowledgements
This study was supported by a grant from the Israel Academy of Science and Humanities (grant #416/09) and by the Ministry of Health (grant #6086) to HC and, in part by the Swedish Medical Research Council (grant #10414) to AAM.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Cohen, S., Vainer, E., Matar, M. et al. Diurnal Fluctuations in HPA and Neuropeptide Y-ergic Systems Underlie Differences in Vulnerability to Traumatic Stress Responses at Different Zeitgeber Times. Neuropsychopharmacol 40, 774–790 (2015). https://doi.org/10.1038/npp.2014.257
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2014.257
- Springer Nature Switzerland AG
This article is cited by
-
Spontaneous recovery, time course, and circadian influence on habituation of the cardiovascular responses to repeated restraint stress in rats
Pflügers Archiv - European Journal of Physiology (2020)
-
The wake-promoting drug modafinil stimulates specific hypothalamic circuits to promote adaptive stress responses in an animal model of PTSD
Translational Psychiatry (2016)
-
A novel approach to PTSD modeling in rats reveals alternating patterns of limbic activity in different types of stress reaction
Molecular Psychiatry (2016)