Abstract
Plant roots have greatly diversified in form and function since the emergence of the first land plants1,2, but the global organization of functional traits in roots remains poorly understood3,4. Here we analyse a global dataset of 10 functionally important root traits in metabolically active first-order roots, collected from 369 species distributed across the natural plant communities of 7 biomes. Our results identify a high degree of organization of root traits across species and biomes, and reveal a pattern that differs from expectations based on previous studies5,6 of leaf traits. Root diameter exerts the strongest influence on root trait variation across plant species, growth forms and biomes. Our analysis suggests that plants have evolved thinner roots since they first emerged in land ecosystems, which has enabled them to markedly improve their efficiency of soil exploration per unit of carbon invested and to reduce their dependence on symbiotic mycorrhizal fungi. We also found that diversity in root morphological traits is greatest in the tropics, where plant diversity is highest and many ancestral phylogenetic groups are preserved. Diversity in root morphology declines sharply across the sequence of tropical, temperate and desert biomes, presumably owing to changes in resource supply caused by seasonally inhospitable abiotic conditions. Our results suggest that root traits have evolved along a spectrum bounded by two contrasting strategies of root life: an ancestral ‘conservative’ strategy in which plants with thick roots depend on symbiosis with mycorrhizal fungi for soil resources and a more-derived ‘opportunistic’ strategy in which thin roots enable plants to more efficiently leverage photosynthetic carbon for soil exploration. These findings imply that innovations of belowground traits have had an important role in preparing plants to colonize new habitats, and in generating biodiversity within and across biomes.
Similar content being viewed by others
Main
Recent efforts to understand how functional traits are organized across land plants have revealed notable patterns across the leaf economic spectrum5,6, but whether such a high degree of organization is also seen in root traits remains controversial4,7. A key factor that has limited progress has been the paucity of data on root traits across plant species and biomes, as roots are difficult to sample and characterize8,9. Yet, roots are vital for the ability of plants to acquire nutrients and water—two functions of fundamental importance to whole-plant performance and for predicting how plants respond to elevated CO2 levels and to climate change10,11,12.
Roots face ecological and physiological challenges that differ fundamentally from those encountered by leaves. Roots must compete for and acquire nutrients and water in environments that greatly vary across global biomes, with biophysical conditions ranging from relatively stable (for example, tropical rainforests) to highly seasonal (for example, deserts or boreal forests). The high diversity that exists in root form and function, and in the degree of association with symbiotic mycorrhizal fungi, raises a fundamental question: how are root traits organized across the diverse taxa that inhabit different ecological conditions worldwide?
Here we propose a model of root trait organization that is functionally decoupled from the leaf economic spectrum and that derives from the phylogenetic history of root diameter and its evolutionary consequences for plant resource acquisition.
We evaluated a species- and biome-specific dataset of 10 root traits in 3 major categories3,13 (morphology, physiology and mycorrhizal association; Supplementary Information, note 1), collected from over 1,200 individual plants of 369 species (from 210 genera and 79 families), distributed across 7 major biomes and 3 continents (Extended Data Table 1). The observations in our dataset: (i) derive solely from native plant communities with natural soil and nutrient conditions; (ii) focus on first-order roots (the most distal and absorptive roots of the branching system) that are subject to strong selection by the local environment8,9,14; (iii) accurately identify species and root order (that is, measure of branching hierarchy8) in mixed-species ecosystems, by tracing roots to parent trees15; and (iv) apply consistent analytical methods to trait measures across all species and biomes. We collected 94% of the total observations used in our dataset (see Methods).
We first investigated whether first-order root traits are globally organized in a manner analogous to the leaf economic spectrum5,6, a composite axis of trait variation that ranges from nitrogen-rich leaves with high specific leaf area and short leaf lifespan to nitrogen-poor leaves with low specific leaf area and long leaf lifespan. In roots, nitrogen supports metabolic activity, including nutrient and water transport, enzyme functioning and mycorrhizal symbiosis16. As a result, nitrogen has previously been proposed to serve a similarly central role in the trait organization of roots, with high levels of nitrogen in roots occurring in species with high levels of nitrogen in their leaves, rapid growth and short root lifespans4,17.
Our results do not support the idea of an analogous organizing role for nitrogen in a global root economic spectrum, expanding on similar previous conclusions drawn from taxonomically and geographically smaller datasets4,18,19. First, a principal component analysis failed to identify root nitrogen, which is analogous to leaf nitrogen, as a significant contributor to the primary axis of trait variation (Extended Data Fig. 1 and Extended Data Table 2). Instead, root traits were most strongly explained by root diameter and by a group of traits associated with root construction and mycorrhizal association (principal component 1, 46%; Extended Data Fig. 1).
Second, root nitrogen was not correlated to specific root length (SRL, the length of root per unit of biomass invested) in a manner analogous to the relationship between specific leaf area and leaf nitrogen (Extended Data Figs 2a, b, 3). Third, in situ (n = 75) and hydroponic-based (n = 111) measures showed no systematic relationship between root nitrogen uptake—which is analogous to leaf photosynthetic capacity—and root diameter, SRL or plant growth form (Extended Data Figs 2c, d, 4b, d). Taken together, these results suggest that nitrogen is less important in belowground nutrient foraging than in aboveground light and CO2 capture (Supplementary Information, note 3). Furthermore, root lifespan, which is analogous to leaf lifespan, was correlated with root diameter and SRL but explained only 14% and 17% of the respective variance (P < 0.01 for both, linear model; Extended Data Fig. 4a, c).
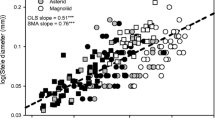
We next analysed the organizing role of root diameter in determining trait variation across plants. We found that the length of root per biomass invested (that is, the SRL) increases nonlinearly with decreasing root diameter (D) according to the allometric relationship,  (Fig. 1a, solid red line; Supplementary Information, note 1). This relationship indicates that as roots get thinner, plants can explore markedly greater volumes of soil per unit of carbon they invest. We also found that woody and herbaceous plants occupy different parts of the SRL versus root diameter relationship, although there is some overlap: woody plants (Fig. 1a, brown points) tend to occupy a region in which differences in root diameter have a limited effect on SRL, whereas herbaceous plants (Fig 1a, green points) reside in a region in which even small variations in diameter cause large changes in SRL.
(Fig. 1a, solid red line; Supplementary Information, note 1). This relationship indicates that as roots get thinner, plants can explore markedly greater volumes of soil per unit of carbon they invest. We also found that woody and herbaceous plants occupy different parts of the SRL versus root diameter relationship, although there is some overlap: woody plants (Fig. 1a, brown points) tend to occupy a region in which differences in root diameter have a limited effect on SRL, whereas herbaceous plants (Fig 1a, green points) reside in a region in which even small variations in diameter cause large changes in SRL.
a−c, At the species level, the diameter of first-order roots is inversely correlated with SRL (a, n = 323 species), positively related to the evolutionary time of divergence of major taxonomic groups (b; Ma, million years ago) and positively related to the length of root (in per cent) colonized by mycorrhizal fungi (c). ***P < 0.001, linear regression. d, Root tissue density differs across plant growth form, with herbaceous plants (green) displaying more constrained variation than woody plants (brown) (F-test, ***P < 0.001; note logarithmic scale (log10) on the y axis). The solid red line in a identifies the relationship  in which y denotes SRL and x denotes root diameter, and root tissue density is 0.25 g cm−3 (Supplementary Information, note 1); upper and lower dashed red lines identify tissue densities of 0.1 and 1.0 g cm−3, respectively. We used a linear regression weighted by number of species in b, and a linear regression with woody and non-woody growth forms as categorical variables in c. Root cross-section images in a are from the low root-tissue-density grass Agropyron cristatum (right) and the high root-tissue-density woody shrub Rhaphiolepis indica (left).
in which y denotes SRL and x denotes root diameter, and root tissue density is 0.25 g cm−3 (Supplementary Information, note 1); upper and lower dashed red lines identify tissue densities of 0.1 and 1.0 g cm−3, respectively. We used a linear regression weighted by number of species in b, and a linear regression with woody and non-woody growth forms as categorical variables in c. Root cross-section images in a are from the low root-tissue-density grass Agropyron cristatum (right) and the high root-tissue-density woody shrub Rhaphiolepis indica (left).
We further found that in thin-rooted species even modest evolutionary changes in the tissue density of first-order roots can greatly alter the soil length explored per unit of carbon invested. The dashed red lines in Fig. 1a indicate the sensitivity of the relationship between SRL and diameter to changes in root tissue density across a physiologically relevant range (0.1–1 g cm−3). For example, the low root density of the grass Agropyron cristatum enables it to explore approximately 350 m more soil per gram of biomass than can the shrub Rhaphiolepis indica, despite the fact that both have root diameters of approximately 0.2 mm (Fig. 1a, red arrows and cross-section images). We infer that over evolutionary time plants have used both root diameter (Fig. 1a, x axis) and tissue density (Fig. 1a, dashed lines) to influence SRL: thin and soft first-order roots have the advantage of efficient soil exploration, but incur the cost trade-off of sacrificing water conduction, tissue permanence and the ability to penetrate the soil matrix.
We next investigate the role of evolutionary history in structuring the differences in root diameter across all major vascular plant families in our dataset (Fig. 1b). We found that, on average, thick roots are associated with evolutionarily ancient taxa (for example, Magnoliaceae) and that thin roots are increasingly common in taxa that have recently diverged from their ancestral lineage (for example, Betulaceae) (weighted linear regression: r2 = 0.54; P < 0.001). Herbaceous plants evolved more recently (Fig. 1b, green circles) and—with the exception of Amaryllidaceae and Boraginaceae—are characterized by thin roots and SRLs that exceed those of woody plants (Fig. 1a). Together, these patterns broadly characterize an evolutionary transition from ancient tree taxa defined by thick first-order roots, to more recently radiated20,21 woody and herbaceous plants with thin roots that can explore markedly greater lengths of soil per unit of carbon invested.
The trend towards thinner roots has had major consequences for the symbiosis between plant roots and mycorrhizal fungi. We found that mycorrhizal colonization (that is, the percentage of root length colonized) declines as roots get thinner (Fig. 1c) and that herbaceous roots have approximately 30% less colonization than woody plants at the same root diameter (linear model; r2 = 0.63 with the difference between herbaceous and woody plants at P < 0.001). In addition, herbaceous plants have on average 33% lower root tissue density than woody plants (Fig. 1d; unequal variance t-test; P < 0.001), though considerable unexplained variation exists across taxa. These differences suggest that first-order roots have become less dependent on mycorrhizae as they have evolved thinner diameters, and that the innovation of the short-lived herbaceous growth form has fundamentally changed the relationship between root diameter and mycorrhizal colonization.
A phylogenetic independence contrasts analysis17 confirmed that variation in root diameter, SRL and mycorrhizal colonization is strongly influenced by evolutionary history (Blomberg’s K value in Extended Data Table1). By contrast, root chemical traits did not display a clear phylogenetic signal, which indicates that for these traits ecological variation overshadows evolutionary constraints22.
Taken together, our results identify a general evolutionary trend in vascular plants from thick roots that rely on mycorrhizal fungi for resource acquisition to thin roots that can explore the soil at high carbon-use efficiency, but with less reliance on mycorrhizae. The observed root trait combinations imply selection for two contrasting plant strategies: (i) a conservative strategy in which carbon allocation to mycorrhizae enhances the ability of plants to compete in environments with stable resources and intense plant–plant competition; and (ii) an opportunistic strategy in which thin roots benefit plants in less predictable environments (for example, seasonal drought or cold), where rapid root growth response to a fluctuating resource supply is rewarded.
It is less clear, however, why herbaceous plants have lower mycorrhizal colonization than woody plants at similar root diameters (Fig. 1c), although the softer tissues of herbaceous plants may cause their roots to be less permanent than those of woody plants (Extended Data Fig. 4c) and therefore less able to maintain stable mycorrhizal relationships.
We next evaluated whether the distribution of root diameter changed across biomes that may differ in the pattern and stability of their resource supplies. First, we found an overall trend of decreasing variance in root diameter of woody plants from the more stable conditions of tropical and sub-tropical forests to the highly seasonal conditions of boreal and desert biomes (Fig. 2, Levene’s test, Extended Data Fig. 5c). Second, woody plants were limited to thin-rooted species in the most seasonal biomes, but the diameter of herbaceous plant roots did not differ systematically across biomes (Extended Data Fig. 5a, b).
The variance in root diameter declines from biomes with equable conditions (for example, tropical forests) to biomes with pronounced seasonality in soil resource supplies (for example, deserts). Numbers in brackets identify species-specific observations. Letters denote groups; pairwise differences between members of different groups are significant, Levene’s variance test (for P values, see Extended Data Fig. 5c). Woody biomes are identified as shades of tan-to-yellow and non-woody biomes as shades of green.
These patterns are consistent with biome-specific differences in both evolutionary history and the stability of resource supply and abiotic conditions. The tropical forest biome is evolutionarily ancient23, characterized by seasonally stable supplies of soil resources and holds species that range from ancestral thick-rooted to more-derived thin-rooted taxa. By contrast, boreal and desert biomes are evolutionarily young24 and have been colonized mainly by thin-rooted species that, in theory, can rapidly respond25 to fluctuating soil resources and seasonally inhospitable conditions. The coexistence in the tropical biome of thin-rooted plants and plants pursuing more-ancient thick-rooted strategies suggests that the heterogeneity of this biome is sufficient to maintain a range of niche conditions for diverse belowground strategies.
Our findings suggest that at the timescale of plant evolution innovations of belowground traits have been important for preparing plants to colonize new habitats, and for the rich generation of biodiversity within and across biomes. The dominant dimension of trait evolution for first-order roots has been a decrease in diameter, which has reduced dependence on mycorrhizal fungi, increased the efficiency of root growth and thus enhanced the ability of plants to leverage photosynthetic carbon for soil exploration. An improved functional understanding of root traits is critical for comprehending the history and distribution of plant life, and may help to predict the risk of species extinction and to conserve biodiversity in the face of environmental change.
Methods
Sampling approach
We collected roots from natural plant communities across seven major biomes and three continents (Asia, Europe and North America) between 2004 and 2016. Our sampling sites range from −1.4 °C to 22.4 °C in mean annual temperature, and from 35 mm to 2,651 mm in mean annual precipitation. At each site, we selected common indigenous species that are representative of the local plant community. For each species, we sampled multiple root branches or segments from at least three individual plants to derive the mean species trait value. For species that occupied more than one sample location, we merged the local means into one species trait value. Eleven species occurred in more than a single biome; for these we calculated a mean value for each biome.
In mixed-species ecosystems we identified roots to the species level by tracing a root to its parent tree. During the growing season, we selected mature individuals and excavated the surface soil (0–20 cm) around the plant stem to expose lateral roots. We then sampled multiple intact root branches and gently cleared the attached soil. Sampled roots were bagged and immediately placed in a cooler, and then either transferred to a refrigerator for processing within the next few days or kept frozen until later laboratory analyses.
Laboratory analyses of root functional traits
We adopted a previously established (for details, see Supplementary Information, note 2, and ref. 8) approach based on root branching-order, in which absorptive fine roots are sorted on the basis of their position in the branching architecture. We dissected root branches according to standard methodology15 and analysed four morphological traits (diameter, SRL, root tissue density and root length), three physiological–chemical traits (root nitrogen content, root carbon content and root carbon-to-nitrogen ratio) and the extent of mycorrhizal colonization (Supplementary Information, note 1).
Root diameter and length were measured using a stereomicroscope with an ocular micrometer. SRL was determined by dividing root length by the dry biomass weight. We calculated the volume of root segments from root diameter and length, assuming segments are cylinders. Root tissue density was then calculated using dry mass and volume. Sampled roots were dried in an oven at 60 °C for 48 h, and then ground to fine powder with a ball-mill for subsequent measurements of carbon and nitrogen on an elemental analyser (Vario EL Cube; Elementar).
We measured the length of root colonized by mycorrhizal fungi in 137 species from sub-tropical19 forests, temperate forests15 and temperate grasslands26. We calculated the percentage of mycorrhizal colonization by sampling first-order roots, and determining by microscope the presence of either arbuscular mycorrhizal or ectomycorrhizal fungal structures within an individual root segment. For arbuscular mycorrhizal fungi we used a standard staining technique to identify coils and arbuscules19,26; no stain was needed to identify ectomycorrhizal fungal sheaths19. For each individual plant, we selected at least ten root branches (containing multiple orders of roots). For each species, the percentage of colonization was calculated across 20–150 randomly selected first-order root segments19,26 to ensure that each segment length was consistent across all roots sampled. The percentage of length colonization was calculated as the ratio of the sum of infected root segments over all root segments examined. We used two different techniques: one based on cross-section analysis (MC1, denoting mycorrhizal colonization technique 1; n = 110 species) and one based on scanning the root surface (MC2, denoting mycorrhizal colonization technique 2; n = 27 species); both enabled us to quantify fungal association within a standardized root area. We kept the effective area examined per root segment approximately the same for both methods (173 versus 169 mm2), such that the results are equivalent (MC2 = 1.02 × MC1 – 0.02; r2 = 0.988).
Because distal fine roots (that is, first-order roots) are primarily responsible for plant nutrient acquisition8,9, we focused our analyses on first-order root traits. We accumulated 480 species-specific observations, of which 187 are unpublished and 256 have previously been published14,15,19,25,26. To enhance the coverage of some biomes (for example, boreal and Mediterranean), we added 37 previously published8,27,28,29 observations into our dataset (comprising ~5% of the final dataset), taking care to include only studies of first-order roots and consistent methods. In total, we gathered traits from 369 species (281 woody, and 88 herbaceous, species; Extended Data Fig. 6 and Extended Data Table 1) covering 210 genera and 79 families.
Root lifespan
We collected data on plant root lifespan for 40 species using in situ minirhizotrons in boreal and temperate forests of Europe30, Asia31,32 and North America33,34, with individual measures spanning at least one year. We acquired additional previously published lifespan data, selecting only studies of distal roots using in situ minirhizotrons or root windows35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56. When corresponding root traits (for example, diameter or SRL) were not available, we used species-specific observations from our own dataset to match the lifespan data. In total, we obtained 70 species-specific observations and 13 community observations across 5 biomes.
Root nitrogen uptake rates
We measured per-biomass root nitrogen uptake rates in 34 plant species using 2 standard approaches: (i) by isolating an intact living root branch and exposing it to a hydroponic solution with isotopically labelled ammonium nitrate (intrusive approach, elevated nitrogen concentration; see ref. 57); and (ii) by applying nutrient solution to soil and allowing plant roots to take up nutrients in situ (non-intrusive, low nitrogen concentration; see ref. 58). The first approach enables an estimation of the maximum uptake rate of absorptive roots and the second approach more accurately reflects the uptake rate of roots in natural conditions; we analysed the resulting two datasets separately. We acquired previously published data for an additional 107 species59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101. The resulting dataset is summarized in Extended Data Fig. 2d.
Species and phylogeny
We conducted phylogenetic analyses of our species from 210 genera and 79 families, confirming species names using The Plant List (http://www.theplantlist.org). We followed the APG III phylogenetic system102 in all analyses and used PHYLOCOM103 to construct phylogenetic trees (Extended Data Fig. 7). Following a previous analysis104, we defined the divergence time of a plant family using the earliest diverging genus within that family. We calculated Blomberg’s K statistic105 using the ‘Picante’ package in R and evaluated the strength of the phylogenetic signal for each trait; a large Blomberg’s K value is thought to indicate phylogenetic conservatism. We performed phylogenetic independent contrasts analyses to correct for shared evolutionary histories among traits and to look for the effect of environmental influences (Extended Data Table 3).
Quantitative and statistical analyses
Shapiro–Wilk tests revealed that all of our traits were significantly non-normal (P < 0.05), which we corrected by log10-transforming our data. We derived the nonlinear relationship between SRL and root diameter based on inherent biophysical constraints, as discussed in Supplementary Information, note 1. We fit the equation using the average root tissue density across all species and evaluated the sensitivity to variation in root tissue density across a tenfold range.
All statistical analyses were performed using the R software (version 2.15.0.), using the ‘Factor R’ package for principal component analyses and the ‘lme4’ package for mixed linear effect models. The linear regression between root diameter and divergence time was weighted by the number of species within each family. We used linear regression to test the effect of root diameter and growth form (woody versus herbaceous) on root mycorrhizal colonization. Across biomes, we examined equality of variance in root diameter using Levene’s test and differences in mean root diameter using a linear mixed effects model.
Code availability
The R scripts used in Figs 1 and 2 are available from the corresponding author upon reasonable request.
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Change history
04 April 2018
Please see accompanying Erratum (https://doi.org/10.1038/nature26163). Both authors D.G. and L.O.H. should have been listed as corresponding authors. This has been corrected online.
References
Kenrick, P. & Crane, P. R. The origin and early evolution of plants on land. Nature 389, 33–39 (1997)
Field, K. J., Pressel, S., Duckett, J. G., Rimington, W. R. & Bidartondo, M. I. Symbiotic options for the conquest of land. Trends Ecol. Evol. 30, 477–486 (2015)
Bardgett, R. D., Mommer, L. & De Vries, F. T. Going underground: root traits as drivers of ecosystem processes. Trends Ecol. Evol. 29, 692–699 (2014)
Weemstra, M. et al. Towards a multidimensional root trait framework: a tree root review. New Phytol. 211, 1159–1169 (2016)
Díaz, S. et al. The global spectrum of plant form and function. Nature 529, 167–171 (2016)
Wright, I. J. et al. The worldwide leaf economics spectrum. Nature 428, 821–827 (2004)
Roumet, C. et al. Root structure–function relationships in 74 species: evidence of a root economics spectrum related to carbon economy. New Phytol. 210, 815–826 (2016)
Pregitzer, K. S. et al. Fine root architecture of nine North American trees. Ecol. Monogr. 72, 293–309 (2002)
McCormack, M. L. et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 207, 505–518 (2015)
Chen, W. et al. Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proc. Natl Acad. Sci. USA 113, 8741–8746 (2016)
Norby, R. J., Ledford, J., Reilly, C. D., Miller, N. E. & O’Neill, E. G. Fine-root production dominates response of a deciduous forest to atmospheric CO2 enrichment. Proc. Natl Acad. Sci. USA 101, 9689–9693 (2004)
Jackson, R. B., Mooney, H. A. & Schulze, E. D. A global budget for fine root biomass, surface area, and nutrient contents. Proc. Natl Acad. Sci. USA 94, 7362–7366 (1997)
McCormack, M. L. et al. Building a better foundation: improving root-trait measurements to understand and model plant and ecosystem processes. New Phytol. 215, 27–37 (2017)
Chen, W. L., Zeng, H., Eissenstat, D. M. & Guo, D. Variation of first-order root traits across climatic gradients and evolutionary trends in geological time. Glob. Ecol. Biogeogr. 22, 846–856 (2013)
Guo, D. et al. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol. 180, 673–683 (2008)
Reich, P. B. et al. Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol. Lett. 11, 793–801 (2008)
Valverde-Barrantes, O. J., Freschet, G. T., Roumet, C. & Blackwood, C. B. A worldview of root traits: the influence of ancestry, growth form, climate and mycorrhizal association on the functional trait variation of fine-root tissues in seed plants. New Phytol. 215, 1562–1573 (2017)
Iversen, C. M. et al. A global fine-root ecology database to address below-ground challenges in plant ecology. New Phytol. 215, 15–26 (2017)
Kong, D. et al. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytol. 203, 863–872 (2014)
Feild, T. S. & Arens, N. C. Form, function and environments of the early angiosperms: merging extant phylogeny and ecophysiology with fossils. New Phytol. 166, 383–408 (2005)
Comas, L. H. et al. Evolutionary patterns and biogeochemical significance of angiosperm root traits. Int. J. Plant Sci. 173, 584–595 (2012)
Ackerly, D. D. & Reich, P. B. Convergence and correlations among leaf size and function in seed plants: a comparative test using independent contrasts. Am. J. Bot. 86, 1272–1281 (1999)
Wing, S. L. et al. Late Paleocene fossils from the Cerrejon Formation, Colombia, are the earliest record of Neotropical rainforest. Proc. Natl Acad. Sci. USA 106, 18627–18632 (2009)
Woodward, F. I., Lomas, M. R. & Kelly, C. K. Global climate and the distribution of plant biomes. Philos. Trans. Roy. Soc. Lond. B. 359, 1465–1476 (2004)
Eissenstat, D. M., Kucharski, J. M., Zadworny, M., Adams, T. S. & Koide, R. T. Linking root traits to nutrient foraging in arbuscular mycorrhizal trees in a temperate forest. New Phytol. 208, 114–124 (2015)
Li, H., Liu, B., McCormack, M. L., Ma, Z. & Guo, D. Diverse belowground resource strategies underlie plant species coexistence and spatial distribution in three grasslands along a precipitation gradient. New Phytol. 216, 1140–1150 (2017)
Hummel, I. et al. Relating root structure and anatomy to whole-plant functioning in 14 herbaceous Mediterranean species. New Phytol. 173, 313–321 (2007)
Ostonen, I. et al. Fine root foraging strategies in Norway spruce forests across a European climate gradient. Glob. Chang. Biol. 17, 3620–3632 (2011)
Leppälammi-Kujansuu, J. et al. Fine root longevity and carbon input into soil from below- and aboveground litter in climatically contrasting forests. For. Ecol. Manage. 326, 79–90 (2014)
Withington, J. M., Reich, P. B., Oleksyn, J. & Eissenstat, D. M. Comparisons of structure and life span in roots and leaves among temperate trees. Ecol. Monogr. 76, 381–397 (2006)
Sun, K., McCormack, M. L., Li, L., Ma, Z. & Guo, D. Fast-cycling unit of root turnover in perennial herbaceous plants in a cold temperate ecosystem. Sci. Rep. 6, 19698 (2016)
Xia, M., Guo, D. & Pregitzer, K. S. Ephemeral root modules in Fraxinus mandshurica. New Phytol. 188, 1065–1074 (2010)
McCormack, M. L., Adams, T. S., Smithwick, E. A. H. & Eissenstat, D. M. Predicting fine root lifespan from plant functional traits in temperate trees. New Phytol. 195, 823–831 (2012)
Valenzuela-Estrada, L. R., Vera-Caraballo, V., Ruth, L. E. & Eissenstat, D. M. Root anatomy, morphology, and longevity among root orders in Vaccinium corymbosum (Ericaceae). Am. J. Bot. 95, 1506–1514 (2008)
Liu, B., He, J., Zeng, F., Lei, J. & Arndt, S. K. Life span and structure of ephemeral root modules of different functional groups from a desert system. New Phytol. 211, 103–112 (2016)
Hansson, K., Helmisaari, H.-S., Sah, S. P. & Lange, H. Fine root production and turnover of tree and understorey vegetation in Scots pine, silver birch and Norway spruce stands in SW Sweden. For. Ecol. Manage. 309, 58–65 (2013)
Huang, J. X. et al. Fine root longevity and controlling factors in subtropical Altingia grlilipes and Castanopsis carlesii forests. Acta Ecol. Sin. 32, 1932–1942 (2012)
Yavitt, J. B., Harms, K. E., Garcia, M. N., Mirabello, M. J. & Wright, S. J. Soil fertility and fine root dynamics in response to 4 years of nutrient (N, P, K) fertilization in a lowland tropical moist forest, Panama. Austral. Ecol. 36, 433–445 (2011)
Ling, H. et al. Influencing factors of fine root lifespans in two Chinese fir plantations in subtropical China. Acta Ecol. Sin. 31, 1130–1138 (2011)
Stover, D. B., Day, F. P., Drake, B. G. & Hinkle, C. R. The long-term effects of CO2 enrichment on fine root productivity, mortality, and survivorship in a scrub-oak ecosystem at Kennedy Space Center, Florida, USA. Environ. Exp. Bot. 69, 214–222 (2010)
Krasowski, M. J., Lavigne, M. B., Olesinski, J. & Bernier, P. Y. Advantages of long-term measurement of fine root demographics with a minirhizotron at two balsam fir sites. Can. J. For. Res. 40, 1128–1135 (2010)
Huang, G., Zhao, X., Zhao, H., Huang, Y. & Zuo, X. Linking root morphology, longevity and function to root branch order: a case study in three shrubs. Plant Soil 336, 197–208 (2010)
Girardin, C. A. J. et al. Net primary productivity allocation and cycling of carbon along a tropical forest elevational transect in the Peruvian Andes. Glob. Chang. Biol. 16, 3176–3192 (2010)
Espeleta, J. F., West, J. B. & Donovan, L. A. Tree species fine-root demography parallels habitat specialization across a sandhill soil resource gradient. Ecology 90, 1773–1787 (2009)
Vargas, R. & Allen, M. F. Dynamics of fine root, fungal rhizomorphs, and soil respiration in a mixed temperate forest: integrating sensors and observations. Vadose Zone J. 7, 1055–1064 (2008)
Graefe, S., Hertel, D. & Leuschner, C. Fine root dynamics along a 2,000-m elevation transect in South Ecuadorian mountain rainforests. Plant Soil 313, 155–166 (2008)
Green, J. J., Dawson, L. A., Proctor, J., Duff, E. I. & Elston, D. A. Fine root dynamics in a tropical rain forest is influenced by rainfall. Plant Soil 276, 23–32 (2005)
Baddeley, J. A. & Watson, C. A. Influences of root diameter, tree age, soil depth and season on fine root survivorship in Prunus avium. Plant Soil 276, 15–22 (2005)
Kern, C. C., Friend, A. L., Johnson, J. M. F. & Coleman, M. D. Fine root dynamics in a developing Populus deltoides plantation. Tree Physiol. 24, 651–660 (2004)
Wells, C. E., Glenn, D. M. & Eissenstat, D. M. Changes in the risk of fine-root mortality with age: a case study in peach, Prunus persica (Rosaceae). Am. J. Bot. 89, 79–87 (2002)
King, J. S. et al. Below-ground carbon input to soil is controlled by nutrient availability and fine root dynamics in loblolly pine. New Phytol. 154, 389–398 (2002)
Gill, R. A., Burke, I. C., Lauenroth, W. K. & Milchunas, D. G. Longevity and turnover of roots in the shortgrass steppe: influence of diameter and depth. Plant Ecol. 159, 241–251 (2002)
Wells, C. E. & Eissenstat, D. M. Marked differences in survivorship among apple roots of different diameters. Ecology 82, 882–892 (2001)
Tierney, G. L. & Fahey, T. J. Evaluating minirhizotron estimates of fine root longevity and production in the forest floor of a temperate broadleaf forest. Plant Soil 229, 167–176 (2001)
Majdi, H., Damm, E. & Nylund, J.-E. Longevity of mycorrhizal roots depends on branching order and nutrient availability. New Phytol. 150, 195–202 (2001)
Coleman, M. D., Dickson, R. E. & Isebrands, J. G. Contrasting fine-root production, survival and soil CO2 efflux in pine and poplar plantations. Plant Soil 225, 129–139 (2000)
Chapin, F. S. III, Moilanen, L. & Kielland, K. Preferential use of organic nitrogen for growth by a nonmycorrhizal Arctic sedge. Nature 361, 150–153 (1993)
McKane, R. B. et al. Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 415, 68–71 (2002)
Aanderud, Z. T. & Bledsoe, C. S. Preferences for 15N-ammonium, 15N-nitrate, and 15N-glycine differ among dominant exotic and subordinate native grasses from a California oak woodland. Environ. Exp. Bot. 65, 205–209 (2009)
Andersen, K. M. & Turner, B. L. Preferences or plasticity in nitrogen acquisition by understorey palms in a tropical montane forest. J. Ecol. 101, 819–825 (2013)
Andresen, L. C., Michelsen, A., Jonasson, S. & Ström, L. Seasonal changes in nitrogen availability, and root and microbial uptake of 15N13C9-phenylalanine and 15N-ammonium in situ at a temperate heath. Appl. Soil Ecol. 51, 94–101 (2011)
Averill, C. & Finzi, A. Increasing plant use of organic nitrogen with elevation is reflected in nitrogen uptake rates and ecosystem δ15N. Ecology 92, 883–891 (2011)
Bardgett, R. D., Streeter, T. C. & Bol, R. Soil microbes compete effectively with plants for organic-nitrogen inputs to temperate grasslands. Ecology 84, 1277–1287 (2003)
Boczulak, S. A., Hawkins, B. J. & Roy, R. Temperature effects on nitrogen form uptake by seedling roots of three contrasting conifers. Tree Physiol. 34, 513–523 (2014)
Cheng, X. & Bledsoe, C. S. Competition for inorganic and organic N by blue oak (Quercus douglasii) seedlings, an annual grass, and soil microorganisms in a pot study. Soil Biol. Biochem. 36, 135–144 (2004)
Dunn, R. M., Mikola, J., Bol, R. & Bardgett, R. D. Influence of microbial activity on plant–microbial competition for organic and inorganic nitrogen. Plant Soil 289, 321–334 (2006)
Finzi, A. C. & Berthrong, S. T. The uptake of amino acids by microbes and trees in three cold-temperate forests. Ecology 86, 3345–3353 (2005)
Gallet-Budynek, A . et al. Intact amino acid uptake by northern hardwood and conifer trees. Oecologia 160, 129–138 (2009)
Harrison, K. A., Bol, R. & Bardgett, R. D. Preferences for different nitrogen forms by coexisting plant species and soil microbes. Ecology 88, 989–999 (2007)
Henry, H. A. L. & Jefferies, R. L. Interactions in the uptake of amino acids, ammonium and nitrate ions in the Arctic salt-marsh grass,Puccinellia phryganodes. Plant Cell Environ. 26, 419–428 (2003)
Jin, V. L. & Evans, R. D. Microbial 13C utilization patterns via stable isotope probing of phospholipid biomarkers in Mojave Desert soils exposed to ambient and elevated atmospheric CO2 . Glob. Chang. Biol. 16, 2334–2344 (2010)
Jin, V. L., Romanek, C. S., Donovan, L. A. & Sharitz, R. R. Soil nitrogen availability and in situ nitrogen uptake by Acer rubrum L. and Pinus palustris Mill. in the southeastern U. S. Coastal Plain. J. Torrey Bot. Soc. 137, 339–347 (2010)
Kahmen, A., Livesley, S. J. & Arndt, S. K. High potential, but low actual, glycine uptake of dominant plant species in three Australian land-use types with intermediate N availability. Plant Soil 325, 109–121 (2009)
Kaštovská, E. & Šantrůčková, H. Comparison of uptake of different N forms by soil microorganisms and two wet-grassland plants: a pot study. Soil Biol. Biochem. 43, 1285–1291 (2011)
Li, C. et al. Inorganic and organic nitrogen uptake by nine dominant subtropical tree species. iForest (Viterbo) 9, 253–258 (2015)
McFarland, J. W. et al. Cross-ecosystem comparisons of in situ plant uptake of amino acid-N and NH4+. Ecosystems 13, 177–193 (2010)
Metcalfe, R. J., Nault, J. & Hawkins, B. J. Adaptations to nitrogen form: comparing inorganic nitrogen and amino acid availability and uptake by four temperate forest plants. Can. J. For. Res. 41, 1626–1637 (2011)
Mozdzer, T. J., Zieman, J. C. & McGlathery, K. J. Nitrogen uptake by native and invasive temperate coastal macrophytes: importance of dissolved organic nitrogen. Estuaries Coast. 33, 784–797 (2010)
Nie, M. et al. Plants’ use of different nitrogen forms in response to crude oil contamination. Environ. Pollut. 159, 157–163 (2011)
Nordin, A., Högberg, P. & Näsholm, T. Soil nitrogen form and plant nitrogen uptake along a boreal forest productivity gradient. Oecologia 129, 125–132 (2001)
Öhlund, J. & Näsholm, T. Growth of conifer seedlings on organic and inorganic nitrogen sources. Tree Physiol. 21, 1319–1326 (2001)
Ouyang, S. et al. Nitrogen competition between three dominant plant species and microbes in a temperate grassland. Plant Soil 408, 121–132 (2016)
Paulding, E. M., Baker, A. J. M. & Warren, C. R. Competition for nitrogen by three sympatric species of Eucalyptus. Ann. For. Sci. 67, 406 (2010)
Persson, J. et al. Nitrogen acquisition from inorganic and organic sources by boreal forest plants in the field. Oecologia 137, 252–257 (2003)
Persson, J. & Näsholm, T. Regulation of amino acid uptake in conifers by exogenous and endogenous nitrogen. Planta 215, 639–644 (2002)
Pfautsch, S., Rennenberg, H., Bell, T. L. & Adams, M. A. Nitrogen uptake by Eucalyptus regnans and Acacia spp. – preferences, resource overlap and energetic costs. Tree Physiol. 29, 389–399 (2009)
Rains, K. C. & Bledsoe, C. S. Rapid uptake of 15N-ammonium and glycine-13C, 15N by arbuscular and ericoid mycorrhizal plants native to a Northern California coastal pygmy forest. Soil Biol. Biochem. 39, 1078–1086 (2007)
Schmidt, S. & Stewart, G. R. Waterlogging and fire impacts on nitrogen availability and utilization in a subtropical wet heathland (wallum). Plant Cell Environ. 20, 1231–1241 (1997)
Schmidt, S. & Stewart, G. R. Glycine metabolism by plant roots and its occurrence in Australian plant communities. Aust. J. Plant Physiol. 26, 253–264 (1999)
Scott, E. E. & Rothstein, D. E. Amino acid uptake by temperate tree species characteristic of low- and high-fertility habitats. Oecologia 167, 547–557 (2011)
Simon, J. et al. Competition for nitrogen between adult European beech and its offspring is reduced by avoidance strategy. For. Ecol. Manage. 262, 105–114 (2011)
Simon, J., Waldhecker, P., Brüggemann, N. & Rennenberg, H. Competition for nitrogen sources between European beech (Fagus sylvatica) and sycamore maple (Acer pseudoplatanus) seedlings. Plant Biol. 12, 453–458 (2010)
Stoelken, G., Simon, J., Ehlting, B. & Rennenberg, H. The presence of amino acids affects inorganic N uptake in non-mycorrhizal seedlings of European beech (Fagus sylvatica). Tree Physiol. 30, 1118–1128 (2010)
Wallander, H., Arnebrant, K., Östrand, F. & Kårén, O. Uptake of N15-labelled alanine, ammonium and nitrate in Pinus sylvestris L. ectomycorrhiza growing in forest soil treated with nitrogen, sulphur or lime. Plant Soil 195, 329–338 (1997)
Wanek, W., Arndt, S. K., Huber, W. & Popp, M. Nitrogen nutrition during ontogeny of hemiepiphytic Clusia species. Funct. Plant Biol. 29, 733–740 (2002)
Warren, C. R. Potential organic and inorganic N uptake by six Eucalyptus species. Funct. Plant Biol. 33, 653–660 (2006)
Warren, C. R. Does nitrogen concentration affect relative uptake rates of nitrate, ammonium, and glycine? J. Plant Nutr. Soil Sci. 172, 224–229 (2009)
Warren, C. R. & Adams, P. R. Uptake of nitrate, ammonium and glycine by plants of Tasmanian wet eucalypt forests. Tree Physiol. 27, 413–419 (2007)
Wei, L., Chen, C. & Yu, S. Uptake of organic nitrogen and preference for inorganic nitrogen by two Australian native Araucariaceae species. Plant Ecol. Divers. 8, 259–264 (2015)
Weigelt, A., Bol, R. & Bardgett, R. D. Preferential uptake of soil nitrogen forms by grassland plant species. Oecologia 142, 627–635 (2005)
Wu, J. et al. Mycorrhizas alter nitrogen acquisition by the terrestrial orchid Cymbidium goeringii. Ann. Bot. 111, 1181–1187 (2013)
The Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 161, 105–121 (2009)
Webb, C. O., Ackerly, D. D. & Kembel, S. W. Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 24, 2098–2100 (2008)
Wikström, N ., Savolainen, V. & Chase, M. W. Evolution of the angiosperms: calibrating the family tree. Proc. R. Soc. B 268, 2211–2220 (2001)
Blomberg, S. P., Garland, T., Jr & Ives, A. R. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745 (2003)
Acknowledgements
We thank C. Ma, L. Li, Y. Yue, M. Liu, F. Ma, H. Li, D. Kong, B. Liu and K. Sun for collecting data; X. Liu and X. Deng for their assistance in field sampling; and all members of field research stations of the Chinese Academy of Sciences for their support. This study was funded by the Natural Science Foundation of China (NSFC Grants 31325006, 31530011, and 41571130041).
Author information
Authors and Affiliations
Contributions
Z.M., D.G. and L.O.H. developed the overall conceptual approach and analysis. Z.M. compiled and analysed the data, and X.X. provided nitrogen-uptake-rate data. R.D.B., D.M.E. and M.L.M. contributed to the formulation of research questions and interpretations. Z.M., D.G., M.L. and L.O.H. wrote the paper and all authors contributed to revisions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Principal component analysis of 7 root functional traits across 104 species.
Trait loading on the plane defined by principal components 1 and 2 (PC1 and PC2). Brown arrows indicate four morphological traits; diameter, length, SRL and root tissue density (RTD). Green arrows indicate two physiological–chemical traits; root carbon (RootC) and root nitrogen (RootN). The yellow arrow shows mycorrhizal colonization. Three different analyses confirm the results shown here (detailed in Extended Data Table 2): (i) all data excluding the mycorrhizal colonization trait (n = 217 species); (ii) gaps in mycorrhizal colonization data interpolated using the regression from Fig. 1c (n = 217 species); and (iii) gaps in any trait value interpolated (n = 369) using the regressions in Fig. 1a, c or the multiple imputation method in the MICE R package).
Extended Data Figure 2 Root nitrogen concentration and root nitrogen uptake rate.
a, There is no correlation between root nitrogen and SRL (r2 = 0.002, P = 0.81, n = 269). b, There is no correlation between root nitrogen and diameter (r2 = 0.02, P = 0.01, n = 274). Each point represents one species: brown, woody plants; green, herbaceous plants (a, b). c, Across plant growth forms, nitrogen uptake rates per root biomass did not vary significantly (P > 0.05) based on hydroponic measurements (brown). For in situ experiments (green) we observed a growth-form effect (P < 0.01), which was caused solely by higher root uptake in graminoid species compared to trees. All other growth forms were statistically indistinguishable. The letters ‘a’ and ‘b’ indicate significant difference based on an ANOVA across growth forms. d, Summary table of nitrogen uptake rates across different biomes by two approaches. This dataset included previously unpublished data from 22 species. A detailed description of these two approaches can be found in the Methods. Additional data were collected from refs 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101.
Extended Data Figure 3 First-order-root nitrogen concentration across biomes and plant functional groups.
a, We did not detect a distinct pattern in first-order-root nitrogen concentration across biomes (a) (ANOVA; P > 0.14, n = 284 species). b, We detected a slight difference in first-order-root nitrogen concentration among plant functional groups (b) (ANOVA; P < 0.01, n = 284 species), which was mostly driven by the higher root nitrogen concentrations found in legumes. Each point represents one species; brown, woody plants; green, herbaceous plants. The letters ‘a’, ‘b’ and ‘ab’ indicate significant differences between categories.
Extended Data Figure 4 Relationship between root functional and morphological traits.
a, c, Root median lifespan is significantly correlated with root diameter (a, r2 = 0.14, P < 0.01, linear regression) and SRL (c, r2 = 0.17, P < 0.01, linear regression). b, d, Root nitrogen uptake rate is not correlated with root diameter (b, r2 = 0.07, P > 0.05, linear regression) or with SRL (d, r2 = 0.07, P = 0.29, linear regression) in woody plants. Data are presented on a logarithmic scale (log10), with each point representing one species.
Extended Data Figure 5 Distribution of first-order-root diameter for woody and herbaceous plants across biomes.
a, Woody plant root diameter deceases from tropical to desert biomes, with the most frequent occurrence of coarse-root ancestral woody species in tropical and subtropical biomes. b, Herbaceous plant root diameters do not display a clear trend across biomes. In both panels, the letters ‘a’, ‘b’ and ‘c’ denote significant differences (P < 0.05) between biomes based on a linear mixed effects model (generated using the lmer function in R) with species included as a random effect. Diameter was first log10-transformed to correct for non-normality. Each point represents a species-specific observation at one site. The background violin plot characterizes the distribution of points in each biome. c, Pairwise comparisons for equal variance in first-order-root diameter using Levene’s test. Levene’s test is used for testing the homogeneity of variance, and is used here to explain biome differences in variance of root diameter.
Extended Data Figure 6 Frequency distributions of nine root functional traits.
a–c, Cyan bars identify the distribution of herbaceous plants, yellow bars identify woody plants, and green colour is where two distributions overlap. n, total number of species; s, skewness of all data.
Extended Data Figure 7 Phylogenetic tree of 365 taxa in the study.
The oldest taxonomic groups are highlighted in orange (gymnosperms), yellow (monocotyledons) and green (for example, Magnoliales, Lauraceae). The youngest taxonomic groups are highlighted in purple (for example, Betulaceae, Fagaceae).
Supplementary information
Supplementary Information
This file contains Supplementary Notes 1-3 and Supplementary References. (PDF 151 kb)
PowerPoint slides
Rights and permissions
About this article
Cite this article
Ma, Z., Guo, D., Xu, X. et al. Evolutionary history resolves global organization of root functional traits. Nature 555, 94–97 (2018). https://doi.org/10.1038/nature25783
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature25783
- Springer Nature Limited
This article is cited by
-
Variations and trade-offs in leaf and culm functional traits among 77 woody bamboo species
BMC Plant Biology (2024)
-
Drought intensity and duration effects on morphological root traits vary across trait type and plant functional groups: a meta-analysis
BMC Ecology and Evolution (2024)
-
Molecular-level carbon traits underlie the multidimensional fine root economics space
Nature Plants (2024)
-
A trait-based root acquisition-defence-decomposition framework in angiosperm tree species
Nature Communications (2024)
-
IPD3, a master regulator of arbuscular mycorrhizal symbiosis, affects genes for immunity and metabolism of non-host Arabidopsis when restored long after its evolutionary loss
Plant Molecular Biology (2024)