Abstract
Empirical and modeling studies of the N cycle in temperate forests of eastern North America have focused on the mechanisms regulating the production of inorganic N, and assumed that only inorganic forms of N are available for plant growth. Recent isotope studies in field conditions suggest that amino acid capture is a widespread ecological phenomenon, although northern temperate forests have yet to be studied. We quantified fine root biomass and applied tracer-level quantities of U–13C2–15N-glycine, 15NH4 + and 15NO3 − in two stands, one dominated by sugar maple and white ash, the other dominated by red oak, beech, and hemlock, to assess the importance of amino acids to the N nutrition of northeastern US forests. Significant enrichment of 13C in fine roots 2 and 5 h following tracer application indicated intact glycine uptake in both stands. Glycine accounted for up to 77% of total N uptake in the oak–beech–hemlock stand, a stand that produces recalcitrant litter, cycles N slowly and has a thick, amino acid-rich organic horizon. By contrast, glycine accounted for only 20% of total N uptake in the sugar maple and white ash stand, a stand characterized by labile litter and rapid rates of amino acid production and turnover resulting in high rates of mineralization and nitrification. This study shows that amino acid uptake is an important process occurring in two widespread, northeastern US temperate forest types with widely differing rates of N cycling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Research on the nitrogen (N) cycle of temperate forests has traditionally focused on the mechanisms regulating the production and turnover of inorganic N (Binkley et al. 1986; Marschner 1995; Matson and Vitousek 1981; Nadelhoffer et al. 1985; Parton and Rasmussen 1994). This long-standing focus reflects the importance of inorganic N to the N nutrition of temperate forest species (Lajtha 1994; Nadelhoffer et al. 1984; Templer and Dawson 2004; Zerihun and Bassirirad 2001), an observation substantiated by strong correlations between the net rate of inorganic N production and forest productivity (Pastor et al. 1984; Reich et al. 1997).
With the development of isotopic methods, studies in arctic, alpine, and boreal ecosystems have applied double-labeled (13C/15N) amino acids in situ and found intact amino acid uptake in mycorrhizal and non-mycorrhizal plants in direct competition with soil microbes (McFarland et al. 2002; Näsholm et al. 1998; Nordin et al. 2001; Persson et al. 2003). This research suggests that in cold regions where slow rates of decomposition result in low supply rates of inorganic N, plants take up a significant fraction of their annual N requirement in the form of organic N (Kielland et al. 2006). By extension it has been argued that organic N uptake should be substantially reduced in warmer climates where decomposition is faster and microbial populations mineralize organic N more rapidly (Schimel and Bennett 2004). However, recent field studies conducted under varying climate and land use (Harrison et al. 2007; Hofmockel et al. 2007; Näsholm et al. 2000; Rains and Bledsoe 2007; Schmidt and Stewart 1999; Turnbull et al. 1996; Warren and Adams 2007) suggest that amino acid capture is a widespread ecological phenomenon. Although organic N is increasingly recognized as a major contributor to plant nutrition in many ecosystems, data for northeastern temperate forests are still lacking.
In previous research, we studied two widely distributed forest types in northeastern North America—a sugar maple and white ash forest type, and a red oak, beech and hemlock forest type. We found that N cycling was strongly influenced by the species composition of these stands because of individualistic variations in the litter chemistry (Finzi and Canham 1998; Finzi et al. 1998a, b; Talbot and Finzi 2008), and that amino acids serve as important substrates for mineralization and nitrification (Finzi and Berthrong 2005). Moreover, we found that surface organic horizons had among the highest concentrations of extractable amino acids ever measured (Berthrong and Finzi 2006), suggesting that soil horizon development may affect amino acid uptake. Finally, an excised-root study showed that the dominant tree species in these northern hardwood and mixed-hardwood conifer forests took up NH4 + significantly more rapidly than glycine, and in turn, that glycine was taken up more rapidly than NO3 − (Finzi and Berthrong 2005). While this pattern was consistent between forest types, sugar maple- and white ash-dominated stands had significantly greater rates of inorganic N uptake than glycine uptake compared to the oak–beech–hemlock-dominated forests.
Although excised root studies may provide insights into the form of N taken up by plants growing in different ecosystems (Chapin et al. 2002), field studies are required to validate lab results, because of the many-fold factors affecting organic N cycling and availability in the soil. These factors include the composition and activity of the microbial community (Chalot and Brun 1998; Hodge et al. 2000), the chemistry of soil organic matter (Talbot and Finzi 2008), the mobility and concentration of different N forms (Owen and Jones 2001) and the mass and distribution of fine roots (Chapin et al. 2002). In situ experiments that include the full suite of plant–microbe–soil interactions are therefore required to assess amino acid uptake by trees in different forest types.
The primary objective of this study was to use in situ methods to quantify the importance of amino acid uptake relative to NH4 + and NO3 − uptake in two dominant forest types throughout northeastern North America. The secondary objective was to identify the factors responsible for variability in the form of N taken up from the soil. The northern hardwood and hardwood-conifer forests we studied here are well characterized in terms of species effects on soil processes (Finzi and Canham 1998; Finzi et al. 1998a, b; Talbot and Finzi 2008), and rates of N cycling including amino acid cycling (Berthrong and Finzi 2006; Finzi and Berthrong 2005). By working in these well-characterized systems, we were able to link variability in amino acid and inorganic N uptake to variability in the production and turnover of N in the soil in two contrasting forest–soil systems. To meet our research objective, we quantified the depth distribution of fine root biomass, the concentration of inorganic and amino acid N at each site, and applied double-labeled (U–13C2/15N) glycine, and 15N-enriched NH4Cl or KNO3 into the organic horizon and top 15 cm of mineral soil. We then measured the concentration of 13C and/or 15N in fine roots 2 and 5 h after tracer application.
Materials and methods
Study sites and soil sampling
This research was conducted in two forest types common to the northeastern US. Trees in the oak–beech–hemlock stand grow on glacial till overlying mica-schist bedrock. Because of the production of recalcitrant litter, this forest type is characterized by the development of a thick organic horizon above the mineral soil horizon (Berthrong and Finzi 2006). The concentration of amino acids in the organic horizon is nearly an order of magnitude higher than that in the top 15 cm of mineral soil (Berthrong and Finzi 2006). Gross and net rates of mineralization and nitrification are low (Finzi et al. 1998b; Finzi and Berthrong 2005). Trees in the sugar maple–white ash forest grow on glacial till overlying dolomitic bedrock. Rapid rates of litter decomposition and the occurrence of earthworms result in no surface organic horizon in these stands. By contrast to the oak–beech–hemlock stand, rates of amino acid production and turnover are significantly higher in the sugar maple–white ash stand, as are rates of gross and net mineralization and nitrification (Finzi and Berthrong 2005). Additional site and soil properties are presented in Table 1.
At each research site, we located four replicate 20 × 20-m plots. We quantified fine root biomass (<2 mm) in each plot in July 2005. The organic horizon was sampled as a 10 × 20-cm monolith. The mineral soil was sampled with a 5-cm diameter soil bulk density sampler to a depth of 60 cm in 15-cm increments.
Immediately after coring, the samples were weighed and then sieved through 2-mm mesh in the field. The concentration of amino acid N, NH4 +, and NO3 − in each sample was extracted in 150 ml of 2 M KCl from root-free subsamples of the mineral soil horizon (50 g) and O horizon (25 g; Shepherd et al. 2001). The remainder of the sieved soil sample was transported back to the laboratory on ice, and quantitatively root picked. All the roots were then carefully washed and dried at 60°C to determine the total fine root biomass.
The concentration of NH4 +–N in each sample was measured by the phenolate method on an autoanalyzer (Lachat Quickchem 8000; Zellweger Analytics, Milwaukee, Wis.). The concentration of NO3 −–N was measured by the cadmium reduction method (Lachat Quickchem 8000). The concentration of amino acid N in each sample was measured by reaction with ninhydrin (Rosen 1957), and comparing the optical absorbance of the samples at 570 nm relative to a leucine standard curve, correcting for interference by NH4 + (Berthrong and Finzi 2006).
In August 2005 we conducted an isotope-labeling experiment in the field. Within each plot, four randomly selected sampling points received an injection of either double-labeled (U–13C2/15N) 98% atom-enriched glycine, 98% atom-enriched 15NH4Cl, 98% atom-enriched K15NO3, or water (control). The O-horizon was injected from its boundary with the mineral soil horizon upwards at 50 points within a 10 × 20-cm sampling grid. Each point received an 0.8-ml aliquot of labeling solution. The total volume of labeling solution applied to the O-horizon was 40 ml. The mineral soil horizon was injected to a depth of 15 cm using a coring-injection grid, made of a 20 × 20-cm piece of Lexan (modified from McFarland et al. 2002). The coring grid consisted of a single, centralized 5.5-cm, outside-diameter hole through which a soil corer could fit. The coring grid was fastened to the soil by four no. 16 galvanized nails. The injection template was the 5.5-cm-diameter piece of Lexan cut from the coring grids with 13 separate injection points. Two milliliters of labeling solution was delivered at each injection point using a syringe inserted at 15-cm depth. As the needle was removed from the soil, the syringe was progressively emptied.
To avoid any fertilization effect, we added the 13C and 15N at tracer levels, increasing the concentration of the background pool of N no more than 10% and enriching to ~2.0 atom%-15N. In the O horizon, the total quantity of 15N applied was 0.54, 0.21, and 0.03 μg 15N g soil−1 for the glycine, NH4 + and NO3 − treatments, respectively. In the mineral soil horizon, the total quantity of 15N applied was 0.11, 0.07, and 0.05 μg 15N g soil−1 for the glycine, NH4 + and NO3 − treatments, respectively. The quantity of 13C applied in the glycine treatment was 0.94 and 0.19 μg 13C g soil−1 in the organic and the mineral soil horizons, respectively. Given the rapid turnover time of amino acids in soil (Finzi and Berthrong 2005; Jones and Hodge 1999), we sampled 2 and 5 h following isotope addition with the expectation that these time intervals would allow us to observe intact uptake if it indeed occurred.
Immediately after coring, the samples were weighed, and then sieved through 2-mm mesh in the field. The fine roots were removed from the sieve, rinsed in water for 3 min, rinsed in 1 mmol l−1 KCl for 3 min to remove adsorbed 15N, and frozen in liquid N to prevent further metabolism of the amino acids. These frozen samples were returned to the laboratory, oven dried at 60°C, weighed and ground under liquid nitrogen with a mortar and pestle. The remainder of each soil sample was refrigerated, brought back to Boston University, and thereafter all the roots and rocks were removed by hand, oven dried and weighed. Total fine root biomass was calculated as the sum of the root biomass frozen in liquid nitrogen and the remaining fine root biomass of the soil core. The bulk density of each horizon was calculated after determining the water content and the mass of roots and rocks in each sample.
Given that the glycine treatment contained very little 13C relative to background C pools in the root (Bennett and Prescott 2004; Nordin et al. 2001), we extracted the soluble C fraction within the root (i.e., amino acids, peptides, proteins) and measured the recovery of 13C in this fraction to determine whether intact glycine uptake had occurred (Näsholm et al. 1998). In brief, the soluble C fraction was extracted as follows: 70 mg of dried ground-root samples was weighed in 15-ml centrifuge tubes, extracted with 1 ml of 10 mM phosphate buffer (pH 8), and centrifuged at 3,700 r.p.m. for 20 min. The supernatant was decanted and stored, and the extraction process was repeated with an additional 1 ml of buffer. The two supernatants were combined, filtered, and the total volume was reduced to 100 μl in a reduced pressure evaporator (Savant Speed Vac). The reduced extracts were pipetted into 9 × 10-mm tin capsules containing two Whatman GF/D filter punches that had been ashed previously at 510°C for 5 h. The sample then was placed in a desiccator overnight. All the samples were sent for analysis of δ15N, δ13C, %N, and %C on a mass spectrometer (Europa Integra, Cheshire, UK) at the University of California, Davis.
Under the assumption that the spacing of the injection points within the injection grid allowed for the diffusion of the labeling solutions throughout the entire 5-cm-diameter × 15-cm-depth volume of soil with no tracer loss, we used the isotope mixing model presented in (McKane et al. 2002) to calculate the uptake of N per unit of root mass (UN; μg N g root−1 h−1) from the soil. The mass-specific rate of N uptake depends upon knowing the rate at which each labeled form of N was taken up from the soil, and isotope dilution of each form due to variably sized pools of N in the soil. The uptake of 15N after 2 and 5 h (U15N; μg 15N g root−1 h−1) was calculated as the product of the N content of fine roots and the atom%-15N excess of the bulk fine root sample. For each soil horizon, the concentration of 15N applied (C15N label; μg 15N g soil−1) was calculated by dividing the total quantity of 15N applied (volume applied in ml × concentration of labeling solution in μg15N ml−1) by the weight of each soil core (g soil). The dilution of the isotope was accounted for by dividing the concentration of each N form in the soil (Cavailable N; μg N g soil−1), by the concentration of the applied label (C15N label; μg 15N g soil−1). When tracer quantities of label are applied, there is, in theory, no perturbation of the system, and the ratio 15N/14N of the N taken up by trees is assumed to be the same as the 15N/14N ratio of available N in the soil. Hence the mass-specific rate of N uptake for each form was calculated according to:
To calculate the total quantity of N taken up from the soil for each form separately, we multiplied the mass-specific rate of N uptake (i.e., UN) for each form by the biomass of the fine roots in each plot. The percent contribution of each N form to total N uptake was calculated as the total quantity of a particular form taken up from the soil divided by the uptake rate of all forms combined. This calculation is valid under the assumption that: (1) the uptake of different forms was unaffected by the addition of the label, which is likely in this case given the low levels of tracer application; (2) that there was no difference in the transfer of the different N forms out of the roots toward the shoot over the 2- and 5-h time frame; and (3) that glycine was representative of the total amino acid pool extracted by 2 M KCl, and that all amino acids were equally taken up by trees.
Statistical analyses
In order to account for any correlation between depths at given locations, a mixed model was used to test for the effect of forest type and soil depth on the biomass of fine roots and the concentration of amino acid N, NH4 +, and NO3 − in the soil, with site, depth and site × depth as fixed effects, and sampling plot as a random effect. To test for the effect of tracer form (glycine, NH4 +, NO3 −), forest type and sampling time on the recovery of 13C in the soluble C fraction and 15N in bulk fine roots, the uptake of different N forms from the soil, and the total quantity of N taken up from the soil, we used a mixed model with tracer form and time as fixed effects, and tracer nested in plot as a random effect. Because the sugar maple–white ash stand had no O horizon the forest type variable in the isotope-labeling experiment had three levels combining both site and soil horizon information (i.e., sugar maple–white ash mineral soil, oak–beech–hemlock organic horizon, and oak–beech–hemlock mineral soil). Both data sets were analyzed in SAS (version 9; SAS Institute, Cary, N.C.) with the MIXED procedure. The Tukey’s adjustment was used for comparisons of least square means at the P < 0.05 level. We log-transformed the data when they did not conform to assumptions of normality and homogeneity of variance.
Results
Fine root biomass was higher (P < 0.05) in the oak–beech–hemlock stand than in the sugar maple–white ash stand (Fig. 1a). Fine root biomass decreased with depth in the soil (P < 0.0001), but the decrease was significantly greater (P = 0.01) in the sugar maple–white ash forest. Amino acid and inorganic N concentrations were highest in the O horizon and decreased with depth (P < 0.0001; Fig. 1b, c). Amino acid concentrations were higher in the top 15 cm of mineral soil of the oak–beech–hemlock stand than the sugar maple–white ash stand (P < 0.05), whereas the opposite was true for inorganic N (P = 0.001, Table 1). The total quantity of amino acid N to 60-cm depth was slightly higher in the oak–beech–hemlock stand than in the sugar maple–white ash stand, but this result was not statistically significant (Fig. 1b). The total quantity of inorganic N was higher in the sugar maple–white ash stand (P < 0.05; Fig. 1c).
Depth distribution of (a) fine roots, (b) amino acid N concentrations, and (c) inorganic N concentrations in the different horizons of the oak–beech–hemlock (O–B-H) and white ash–sugar maple (WA-SM) stands. Different capital letters indicate significant (P < 0.05) differences among depths in the O–B-H stand, and different lower-case letters indicate significant (P < 0.05) differences in the WA-SM stand. Bar charts plot total fine root biomass (a), and the quantity of amino acid N (b) and inorganic N (c) in the profile
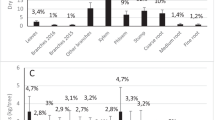
Excess 13C was not detected in bulk-root samples (data not shown); the label was masked by the large concentration of C in root tissues. By contrast, the soluble C pool in the fine roots was significantly enriched in 13C at both sites (P < 0.05) and all horizons 2 h following tracer application (Fig. 2). After 5 h, the soluble C pool was enriched in 13C in the top 15 cm of mineral soil but not in the O horizon (Fig. 2). 15N was readily detected in bulk, fine root samples. 15N enrichment was significantly higher 2 and 5 h following the application of glycine (P < 0.05) and NH4 + (P < 0.01) tracers at both sites in both horizons (Fig. 3a, b). The application of 15NO3 − only increased 15N enrichment of the fine roots in the sugar maple–white ash stand (P = 0.01; Fig. 3c).
Mean (± 1 SE) δ13C in the soluble C fraction of the fine roots in the glycine treatment 2 and 5 h following tracer application in the organic horizon at the O–B-H stand and in the mineral soil horizons at the O–B-H and WA-SM stands. Different capital letters indicate a significant (P < 0.05) difference in tracer enrichment within a particular site and horizon. For abbreviations, see Fig. 1
Mean (± 1 SE) δ15N in bulk fine roots of the (a) glycine, (b) NH4 +, and (c) NO3 − treatments 2 and 5 h following tracer application at both sites. Different capital letters indicate a significant (P < 0.05) difference in tracer enrichment within a particular site and horizon. For abbreviations, see Fig. 1
In the O horizon of the oak–beech–hemlock forest, mass-specific rates of glycine uptake (μg N g root−1 h−1) were significantly higher than NH4 + uptake rates which, in turn, were significantly greater than NO3 − uptake rates (P < 0.001; Fig. 4a). In the top 15 cm of mineral soil, mass-specific rates of glycine and NH4 + uptake were comparable but both were significantly greater than NO3 − uptake (P < 0.01; Fig. 4b). By contrast, in the sugar maple–white ash stand mass-specific rates of NO3 − uptake (P < 0.01) were significantly greater than glycine uptake rates, with mass-specific NH4 + uptake rates intermediate between these two forms (P < 0.05; Fig. 4c). In general, there was a decrease in the mass-specific rate of N uptake for all forms between the 2- and the 5-h sampling.
The mass-specific rate of N uptake (μg N g−1 root h−1 ± 1 SE) as glycine, NH4 +, and NO3 − in the (a) organic horizon of the O–B-H stand, and in the top 15 cm of mineral soil in the (b) O–B-H and (c) WA-SM stands, in addition to the total quantity of N taken up from the soil (μg N m−2 h−1 ± 1 SE) as (d) glycine, (e) NH4 + and (f) NO3 − 2 and 5 h following tracer addition. a–c Bars with different letters indicate a significant (P < 0.05) difference in the rate of N uptake among forms. d–f Bars with different letters indicate differences in the total quantity of each N form taken up across sites and soil horizons. For abbreviations, see Fig. 1
Averaging across the 2- and 5-h sampling times, glycine accounted for 77% of total N uptake (μg N m−2 h−1) from the O horizon of the oak–beech–hemlock stand, with NH4 + and NO3 − accounting for 22 and 1%, respectively (Fig. 4d–f). In the mineral soil of the oak–beech–hemlock stand, glycine and NH4 + accounted for 48 and 45% of total N uptake, respectively, while NO3 − uptake only accounted for 7% of total N uptake. In the mineral soil of the sugar maple–white ash stand, NO3 − accounted for 47% of total N uptake whereas NH4 + and glycine accounted for 33 and 20% of total N uptake, respectively (Fig. 4d–f). Total N uptake was significantly higher (P < 0.05) 2 h after labeling than after 5 h for each form of N (Fig. 4d–f).
Discussion
The objective of this study was to quantify the importance of amino acid uptake relative to NH4 + and NO3 − uptake in two hardwood-conifer forests differing in rates of N cycling. Canopy trees at both research sites took up the amino acid glycine in direct competition with the microbial community (Figs. 2, 3, 4). The total quantity of glycine taken up in the slow N cycling oak–beech–hemlock stand was significantly greater than that taken up in the fast N cycling sugar maple–white ash stand, particularly in the surface organic horizon where glycine accounted for ~77% of total N uptake (Fig. 4). In the sugar maple–white ash stand NO3 − and NH4 + dominated uptake with glycine accounting for only 20% of total N uptake (Fig. 4).
The results presented here suggest that soil horizon development may affect organic N uptake in temperate forests, and if so, implies an important link between the chemistry of tree species litter and the form of N taken up from the soil (Northup et al. 1995). The oak–beech–hemlock stand is characterized by a relatively thick O horizon that is not present in the sugar maple–white ash stand. Oak, beech, and hemlock trees produce litter with significantly higher lignin content and higher C-to-N ratios than sugar maple and white ash trees (Aber and Melillo 1982; Finzi and Canham 1998). As a consequence, leaf litter decomposition is slow and litter accumulates above the mineral soil horizon. The O horizon in this stand also has very high concentrations of extractable amino acids (Berthrong and Finzi 2006; Fig. 1c), thereby maximizing the potential for amino acid uptake (Jones et al. 2005b). In the sugar maple–white ash stand by contrast, leaf litter and soil organic matter decomposition are significantly more rapid (Talbot and Finzi 2008), as are rates of mineralization and nitrification (Finzi and Berthrong 2005), both of which result in high concentrations of inorganic N in the mineral soil horizon and the apparent dominance of inorganic N uptake (Fig. 4f). Whether soil horizon development is important to organic N uptake in all temperate forest soils is currently unknown. Additional work on organic N uptake in other sites will be necessary to confirm the observations presented here.
To quantify in situ uptake from two functionally distinct soil horizons, we had to make three assumptions. First, we labeled soil cores assuming no loss of the tracer, a reasonable assumption given the spacing within the injection grids and the small volume of water associated with tracer application. Since we did not measure tracer recovery in the different soil solution N pools, however, we cannot exclude the possibility of some loss. If so, the tracer dilution associated with loss will result in an underestimation of actual uptake rates (i.e., C15N label would be smaller if losses occurred). This is most likely to be the case for NO3 − which is mobile in soil water, and unlikely for NH4 + which is rapidly sorbed by soil components (Owen and Jones 2001). Second, we applied the tracer to a small volume of soil that prevented us from recovering 13C and/or 15N in bolewood and foliage. In the absence of measurements of tracer recovery in other parts of the tree, we assumed that there was no difference in the translocation of the three different N forms out of the roots. Two recent studies show that the transport of NO3 − out of roots is faster than either amino acid or NH4 + transfer (Persson et al. 2006; Schmidt and Stewart 1999). Consequently, the actual quantity of NO3 − taken up could be greater than that measured (i.e., U15N for NO3 −). Because the relative contributions of glycine, NH4 + and NO3 − uptake depend upon the total quantity of N taken up (i.e., the sum of total glycine, NH4 + and NO3 − uptake), greater losses and faster translocation of NO3 − out of roots may overestimate the relative contribution of glycine and NH4 +. Third, we chose the single amino acid glycine as a tracer for all free amino acids extracted by 2 M KCl, mainly because glycine is in general present in large quantities in soils and easily taken up by plants (Lipson and Näsholm 2001). However, many amino acids are not as available to plants as glycine because of sorption to soil (i.e., amino acids with net positive charge; Jones and Hodge 1999) and because microbes prefer other organic sources of N owing to the low C content of glycine (Schimel and Bennett 2004). Despite these assumptions and methodological limitations, the substantial differences between stands and soil horizons in the form of N uptake suggests that small to moderate bias in our estimate of uptake is unlikely to alter the main conclusion that organic matter chemistry and soil horizon development play important roles in regulating the contribution of glycine to N uptake in temperate forests.
In an attempt to create ecologically realistic conditions, we applied tracer quantities of 98 at.% 13C and/or 15N-enriched forms to the soil system, increasing background pools of N by 1–11%. The advantage to applying tracer-quantities of N is avoiding any fertilization effect that could artificially increase plant capture of amino acid N or saturate microbial uptake thereby leaving more amino acid N for plant uptake (Jones et al. 2005a). Accordingly, the increase in background pools of N in this study are substantially lower than that applied in most studies with enrichments ranging from 20% to greater than 1000% (Harrison et al. 2007; Kielland et al. 2006; McFarland et al. 2002; Näsholm et al. 1998; Nordin et al. 2004; Persson et al. 2003).The drawback to this approach is that the 13C label in the amino acid labeling study was easily swamped by the background 13C in the root.
We found significantly elevated concentrations of 13C in the soluble C fraction of the fine roots in the glycine-treated samples at both sites, and in both horizons after 2 h, and at both sites after 5 h in the mineral soil horizon (Fig. 2). Typically, intact uptake is documented by observing a similar ratio of 13C-excess to 15N-excess in the roots and in the labeled compound (i.e., 2:1 for glycine; Näsholm et al. 1998). A slope with a ratio different than 2:1 would indicate some transformation of glycine before or after its uptake by the roots. The 13C-excess to 15N-excess ratio of the soluble C pool in this study ranged from 2 to 19, much wider than the expected 2:1 ratio, indicating that the soluble C extraction did not recover all the excess 15N in the root. The soluble-C-pool-13C-excess to bulk-root-15N-excess ratio ranged from 0.6 to 3.8, much closer to the expected 2:1 ratio, and similar to that observed in other studies (e.g., McFarland et al. 2002; Nordin et al. 2001), but depends on two different ways of measuring isotopes in the roots (i.e., soluble 13C, bulk root 15N). Losses of 13C occur as a result of root and microbial metabolism (Näsholm et al. 1998; Schmidt and Stewart 1999) where two molecules of glycine are rapidly decarboxylated and converted to serine. The translocation of serine from roots to shoots can also affect the 13C recovery in roots (Schmidt and Stewart 1999). Furthermore, some of the glycine that is taken up by microbes can be deaminated resulting in the release of 15N to the soil solution and the retention or respiration of 13C (Barraclough 1997; Finzi and Berthrong 2005). Alone, or in combination, these processes could have affected isotope recovery in our experiment, but they would tend to decrease the ratio of 13C-excess to 15N-excess in roots, not widen the ratio as was observed here. Despite the uncertainty in the 13C-to-15N ratio of the root, the significant enrichment of 13C in the soluble C pool provides strong support for intact amino acid uptake; there is no other mechanism that we are aware of that could cause such an enrichment of 13C in the root.
In previous research, we found that excised roots of the tree species at these sites took up significantly more NH4 + than glycine, and significantly more glycine than NO3 − (Finzi and Berthrong 2005). In contrast to the excised-root study, there was substantially more glycine and NO3 − uptake observed in the field. In particular, NO3 − uptake dominated all other forms of N uptake in the soil in the sugar maple–white ash stand (Fig. 4a), whereas glycine dominated N uptake in the oak–beech–hemlock stand (Fig. 4c). The difference in the form of N capture between the field and lab study may reflect the mobility of the different N forms in the soil. In a temperate grassland soil, Owen and Jones (2001) found that NO3 − diffused through soil water more rapidly (0.282 cm2 day−1) than either glycine (0.0078 cm2 day−1) or NH4 + (0.0023 cm2 day−1), because glycine and NH4 + tended to be sorbed by negatively charged clay particles and soil organic matter (Barber 1995). Consistent with ion mobility as a factor controlling N uptake, we found that the slope of the relationship between N uptake and the concentration of N in the soil was significantly greater for NO3 − (slope = 658.7, r 2 = 0.35, P < 0.01, n = 24) than for glycine (slope = 69.3, r 2 = 0.56, P < 0.0001, n = 24). The steeper slope for NO3 − indicates that uptake is more strongly concentration dependent, presumably because sorption in the soil has little effect on the mobility of this ion. By contrast, the smaller slope for glycine suggests that sorption decreases glycine availability in the soil following depolymerization. Importantly, there was no correlation between NH4 + uptake and the concentration of NH4 + in the soil, suggesting strong physicochemical control over NH4 + availability in temperate forest soils that are known to have a high cation exchange capacity. The interactive effects of production and sorption on the form of N taken up from the soil are an important avenue for future research (Lipson et al. 2001).
Mycorrhizal fungi play a critical role in transferring soil-derived N to plants in return for photoassimilates (Read and Perez-Moreno 2003). To date, much of the emphasis on the capture of organic N has focused on ectomycorrhizal (ECM) fungi because of their known proteolytic capability (Chalot and Brun 1998) and their transfer of N to host plants in culture studies (Abuzinadah and Read 1989; Leake and Read 1990). In contrast to many ECM fungi, arbuscular–mycorrhizal (AM) fungi lack proteolytic capacity, and they are thought to be more important in the capture and transfer of inorganic nutrients to host plants (Smith and Read 1997). In this study, we found intact amino acid uptake in stands dominated by trees associating with both ECM fungi (oak, beech, and hemlock) and AM fungi (sugar maple and white ash), although uptake in the AM trees was much lower (Fig. 4). Several species of grasses and shrubs that associate with AM fungi have also been shown to take up intact amino acids (Näsholm et al. 2000; Nordin et al. 2001; Persson et al. 2003; Rains and Bledsoe 2007). Although these results suggest that AM fungi might play a role in organic N uptake, there is currently no information available to determine which part of the root, the arbuscule within the root or the arbuscule-free portion of the root, was involved in organic N capture.
Research conducted in the northeastern US has figured prominently in the development of the classic model for soil N cycling in which microbial activity controls N availability and plants take up only inorganic N (e.g., Melillo 1981; Nadelhoffer et al. 1985). Positive correlations between the rate of net N mineralization and net primary production, the flux of N in litterfall, the rate of N uptake from the soil, and N-use efficiency have reinforced the idea that inorganic N alone accurately reflects N availability and the form of N uptake (Nadelhoffer et al. 1985; Pastor et al. 1984; Reich et al. 1997). Based on these observations, biogeochemical models have been parameterized with an inorganic N economy for the N nutrition of trees (Aber et al. 1997; Comins and McMurtrie 1993; Melillo et al. 1993; Parton and Rasmussen 1994; Rastetter et al. 1997). This study shows that temperate forest trees take up the intact amino acid glycine, in direct competition with the microbial community, with variability in the degree of uptake resulting from differences in root biomass (Fig. 1), rate of production (Finzi and Berthrong 2005) soil-horizon development (Fig. 4), and ion mobility. To accurately characterize the availability of a key, growth-limiting resource in northern hardwood–conifer forests, future empirical and modeling studies should be reformulated to allow organic N uptake from the soil.
References
Aber JD, Melillo JM (1982) Nitrogen immobilization in decaying hardwood leaf litter as a function of initial nitrogen and lignin content. Can J Bot 60:2263–2269
Aber JD, Ollinger SV, Driscoll CT (1997) Modeling nitrogen saturation in forest ecosystems in response to land use and atmospheric deposition. Ecol Modell 101:61–78
Abuzinadah RA, Read DJ (1989) The role of proteins in the nitrogen nutrition of ectomycorrhizal plants. IV. The utilization of peptides by birch (Betula pendula L.) infected with different mycorrhizal fungi. New Phytol 112:55–60
Barber SA (1995) Soil nutrient bioavailability: a mechanistic approach, 2nd edn. Wiley, New York
Barraclough D (1997) The direct or MIT route for nitrogen immobilization: a 15N mirror image study with leucine and glycine. Soil Biol Biochem 29:101–108
Bennett JN, Prescott CE (2004) Organic and inorganic nitrogen nutrition of western red cedar, western hemlock and salal in mineral N-limited cedar-hemlock forests. Oecologia 141:468–476
Berthrong ST, Finzi AC (2006) Amino acid cycling in three cold-temperate forests of the northeastern USA. Soil Biol Biochem 38:861–869
Binkley D, Aber J, Pastor J, Nadelhoffer K (1986) Nitrogen availability in some Wisconsin forests: comparisons of resin bags and on-site incubations. Biol Fertil Soils 2:77–82
Chalot M, Brun A (1998) Physiology of organic nitrogen acquisition by ectomycorrhizal fungi and ectomycorrhizas. FEMS Microbiol Rev 22:21–44
Chapin FS, Matson P, Mooney H (2002) Principles of terrestrial ecosystem ecology. Springer, New York
Comins HN, McMurtrie RE (1993) Long-term response of nutrient-limited forests to CO2 enrichment; equilibrium behavior of plant-soil models. Ecol Appl 3:666–681
Finzi AC, Berthrong ST (2005) The uptake of amino acids by microbes and trees in three cold-temperate forests. Ecology 86:3345–3353
Finzi A, Canham C (1998) Non-additive effects of litter mixtures on net N mineralization in a southern New England forest. For Ecol Manage 105:129–136
Finzi A, Canham C, van Breemen N (1998a) Canopy tree-soil interactions within temperate forests: species effects on pH and cations. Ecol Appl 8:447–454
Finzi A, Van Breemen N, Canham C (1998b) Canopy tree-soil interactions within temperate forests: species effects on soil carbon and nitrogen. Ecol Appl 8:440–446
Harrison KA, Bol R, Bardgett RD (2007) Preferences for different nitrogen forms by coexisting plant species and soil microbes. Ecology 88:989–999
Hodge A, Robinson D, Fitter A (2000) Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci 5:304–308
Hofmockel KS, Schlesinger WH, Jackson RB (2007) Effects of elevated atmospheric carbon dioxide on amino acid and NH4 +-N cycling in a temperate pine ecosystem. Glob Chang Biol 13:1950–1959
Jones DL, Hodge A (1999) Biodegradation kinetics and sorption reactions of three differently charged amino acids in soil and their effects on plant organic nitrogen availability. Soil Biol Biochem 31:1331–1342
Jones DL, Healey J, Willett V, Farrar J, Hodge A (2005a) Dissolved organic nitrogen uptake by plants—an important N uptake pathway? Soil Biol Biochem 37:413–423
Jones DL, Shannon D, Junvee-Fortune T, Farrar JF (2005b) Plant capture of free amino acids is maximized under high soil amino acid concentrations. Soil Biol Biochem 37:179–181
Kielland K, McFarland J, Olson K (2006) Amino acid uptake in deciduous and coniferous taiga ecosystems. Plant Soil 288:297–307
Lajtha K (1994) Nutrient uptake in eastern deciduous tree seedlings. Plant Soil 160:193–199
Leake JR, Read DJ (1990) Proteinase activity in mycorrhizal fungi. II. The effects of mineral and organic nitrogen sources on induction of extracellular proteinase in Hymenoscyphus ericae (Read) Korf and Kernan. New Phytol 116:123–128
Lipson D, Näsholm T (2001) The unexpected versatility of plants: organic nitrogen use and availability in terrestrial ecosystems. Oecologia 128:305–316
Lipson D, Raab T, Schmidt S, Monson R (2001) An empirical model of amino acid transformations in an alpine soil. Soil Biol Biochem 33:189–198
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London, p 889
Matson PA, Vitousek PM (1981) Nitrogen mineralization and nitrification potentials following clearcutting in the Hoosier National Forest, Indiana. For Sci 27:781–791
McFarland JW, Ruess RW, Kielland K, Doyle AP (2002) Cycling dynamics of NH4 + and amino acid nitrogen in soils of a deciduous boreal forest ecosystem. Ecosystems 5:775–788
McKane RB et al (2002) Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 415:68–71
Melillo JM (1981) Nitrogen cycling in deciduous forests. Ecol Bull 33:427–442
Melillo JM, McGuire AD, Kicklighter DW, Moore B, Vorosmarty CJ, Schloss AL (1993) Global climate change and terrestrial net primary production. Nature 363:234–240
Nadelhoffer K, Aber J, Melillo J (1984) Seasonal patterns of ammonium and nitrate uptake in nine temperate forest ecosystems. Plant Soil 80:321–335
Nadelhoffer KJ, Aber JD, Melillo JM (1985) Fine roots, net primary production, and soil nitrogen availability: a new hypothesis. Ecology 66:1377–1390
Näsholm T, Ekblad A, Nordin A, Giesler R, Högberg M, Högberg P (1998) Boreal forest plants take up organic nitrogen. Nature 392:914–916
Näsholm T, Huss-Danell K, Högberg P (2000) Uptake of organic nitrogen in the field by four agriculturally important plant species. Ecology 81:1155–1161
Nordin A, Högberg P, Näsholm T (2001) Soil nitrogen form and plant nitrogen uptake along a boreal forest productivity gradient. Oecologia 129:125–132
Nordin A, Schmidt IK, Shaver GR (2004) Nitrogen uptake by arctic soil microbes and plants in relation to soil nitrogen supply. Ecology 85:955–962
Northup RR, Yu Z, Dahlgren RA, Vogt KA (1995) Polyphenol control of nitrogen release from pine litter. Nature 377:227–229
Owen AG, Jones DL (2001) Competition for amino acids between wheat roots and rhizosphere and the role of amino acids in plant N acquisition. Soil Biol Biochem 33:651–657
Parton WJ, Rasmussen PE (1994) Long-term effects of crop management in wheat-fallow. 2. CENTURY model simulations. Soil Sci Soc Am J 58:530–536
Pastor J, Aber J, McClaugherty C, Melillo J (1984) Aboveground production and N and P cycling along a nitrogen mineralization gradient on Blackhawk Island, Wisconsin. Ecology 65:256–268
Persson J, Högberg P, Ekblad A, Högberg M, Nordgren A, Näsholm T (2003) Nitrogen acquisition from inorganic and organic sources by boreal forest plants in the field. Oecologia 137:252–257
Persson J, Gardestrom P, Nasholm T (2006) Uptake, metabolism and distribution of organic and inorganic nitrogen sources by Pinus sylvestris. J Exp Bot 57:2651–2659
Rains K, Bledsoe C (2007) Rapid uptake of 15N-ammonium and glycine-13C, 15N by arbuscular and ericoid mycorrhizal plants native to a Northern California coastal pygmy forest. Soil Biol Biochem 39:1078–1086
Rastetter EB, Agren GI, Shaver GR (1997) Responses of N-limited ecosystems to increased CO2: a balanced-nutrition, coupled-element-cycles model. Ecol Appl 7:444–460
Read DJ, Perez-Moreno J (2003) Mycorrhizas and nutrient cycling in ecosystems—a journey towards relevance? New Phytol 157:475–492
Reich P, Grigal D, Aber J, Gower S (1997) Nitrogen mineralization and productivity in 50 hardwood and conifer stands on diverse soils. Ecology 78:335–347
Rosen H (1957) A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys 67:10–15
SAS (2006) SAS/STAT guide for personal computers. Version 9. SAS Institute, Cary
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602
Schmidt S, Stewart GR (1999) Glycine metabolism by plant roots and its occurrence in Australian plant communities. Funct Plant Biol 26:253–264
Shepherd M, Bhogal A, Barrett G, Dyer C (2001) Dissolved organic nitrogen in agricultural soils: effects of sample preparation on measured values. Commun Soil Sci Plant Anal 32:1523–1542
Smith SE, Read DJ (1997) Mycorrhizal symbiosis, 2nd edn. Academic Press, New York
Talbot J, Finzi A (2008) Differential effects of sugar maple, red oak, and hemlock tannins on carbon and nitrogen cycling in temperate forest soils. Oecologia 155:583–592
Templer PH, Dawson TE (2004) Nitrogen uptake by four species of the Catskill Mountains, New York: implications for forest N dynamics. Plant Soil 262:251–261
Turnbull MH, Schmidt S, Erskine PD, Richards S, Stewart GR (1996) Root adaptation and nitrogen source acquisition in natural ecosystems. Tree Physiol 16:941–948
Warren CR, Adams PR (2007) Uptake of nitrate, ammonium and glycine by plants of Tasmanian wet eucalypt forests. Tree Physiol 27:413–419
Zerihun A, Bassirirad H (2001) Interspecies variation in nitrogen uptake kinetic responses of temperate forest species to elevated CO2: potential causes and consequences. Glob Chang Biol 7:211–222
Acknowledgements
We would like to thank the Great Mountain Forest Corporation, Jody Bronson and Frank Christinat for providing logistical support at the Great Mountain Forest; the state of Connecticut Department of Environmental Protection, Natural Area Preserves Program for granting us access to the forests on the Canaan Mountain; Sinlan Poo for laboratory assistance. This research complies with the current laws of United States and was supported by a grant from the United States Department of Agriculture to A. C. F. A. G. B. was a collaborator, via a fellowship under the OECD Co-operative Research Programme: Biological Resource Management for Sustainable Agriculture Systems.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jason Kaye.
Rights and permissions
About this article
Cite this article
Gallet-Budynek, A., Brzostek, E., Rodgers, V.L. et al. Intact amino acid uptake by northern hardwood and conifer trees. Oecologia 160, 129–138 (2009). https://doi.org/10.1007/s00442-009-1284-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-009-1284-2