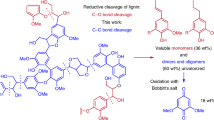
Abstract
Lignin is a heterogeneous aromatic biopolymer that accounts for nearly 30% of the organic carbon on Earth1 and is one of the few renewable sources of aromatic chemicals2. As the most recalcitrant of the three components of lignocellulosic biomass (cellulose, hemicellulose and lignin)3, lignin has been treated as a waste product in the pulp and paper industry, where it is burned to supply energy and recover pulping chemicals in the operation of paper mills4. Extraction of higher value from lignin is increasingly recognized as being crucial to the economic viability of integrated biorefineries5,6. Depolymerization is an important starting point for many lignin valorization strategies, because it could generate valuable aromatic chemicals and/or provide a source of low-molecular-mass feedstocks suitable for downstream processing7. Commercial precedents show that certain types of lignin (lignosulphonates) may be converted into vanillin and other marketable products8,9, but new technologies are needed to enhance the lignin value chain. The complex, irregular structure of lignin complicates chemical conversion efforts, and known depolymerization methods typically afford ill-defined products in low yields (that is, less than 10–20wt%)2,10,11. Here we describe a method for the depolymerization of oxidized lignin under mild conditions in aqueous formic acid that results in more than 60wt% yield of low-molecular-mass aromatics. We present the discovery of this facile C–O cleavage method, its application to aspen lignin depolymerization, and mechanistic insights into the reaction. The broader implications of these results for lignin conversion and biomass refining are also considered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Main
Lignin is biosynthesized from a relatively small number of phenylpropanoid building blocks that contribute to recurring structural elements in this otherwise complex polymeric material. One of the most common motifs is the ‘β-O-4’ alkyl–aryl ether linkage between aromatic rings2, which features a secondary benzylic alcohol at the Cα position and a primary aliphatic alcohol at the Cγ position (Fig. 1). Fundamental studies of wood pulp delignification in the context of paper production have shown that oxidation of the Cα alcohol to a ketone (for example by using stoichiometric Mn or Cr oxide reagents) facilitates the removal of lignin from cellulose, apparently by promoting cleavage of the β-O-4 linkage12,13. This concept has not been applied to the production of low-molecular-mass aromatics from lignin. Several groups, including our own, have probed the reactions of oxidized lignin model compounds, such as 2 (Fig. 1b), with varying degrees of success12,14,15,16,17,18,19,20,21. The recent development of a method for the chemoselective aerobic oxidation of Cα alcohols to ketones in native lignin, which achieved ∼90% conversion to ‘ligninox’14, enables direct investigation of the reactivity of oxidized lignin. (Oxidative transformations of lignin and lignin model compounds have been the focus of extensive study and are summarized in refs 2, 20, 21. Primary references associated with this work are compiled in Supplementary Information.)
a, Representative structure of a fragment of poplar (including aspen) lignin, highlighting a β-O-4 unit, together with the strategy for lignin conversion to low-molecular-mass aromatics by chemoselective alcohol oxidation, followed by C–C and/or C–O cleavage. b, Structure of β-O-4 model compounds 1 and 2.
We observed previously that the oxidized lignin model 2 reacts with alkaline hydrogen peroxide to yield the aromatic monomers veratric acid (88% yield) and guaiacol (42%)14. The instability of the guaiacol under the reaction conditions prompted us to consider reductive cleavage methods. The reactivity of 2 was tested in the presence of different reducing metals, including zinc, aluminium, magnesium, iron and manganese, in aqueous formic acid at 110 °C (Fig. 2a). Four different major products were identified from these reaction conditions. In the presence of zinc, 2 afforded small amounts of the O-formylated product 3 (6%), together with good yields of the aryl ethyl ketone reductive cleavage product 4 (76%) and guaiacol (69%) (Fig. 2a, entry 1). When the reaction was performed with metal sources other than Zn (Fig. 2a, entries 2–5), the diketone product 5 was observed instead of 4. Good yields of 5 (74%) and guaiacol (63%) were obtained from the reaction with manganese. Numerous other conditions were tested with these metal sources (see Supplementary Table 1); however, no further improvement in the product yields was observed. For example, substantially lower yields were obtained with organic solvents (ethanol, γ-valerolactone or toluene) or on replacement of formic acid with another acid source (acetic acid, HCl or H2SO4).
a, Cleavage products obtained from the treatment of ligninox model compound 2 in formic acid with and without reducing metals (M). Yields shown in the table were determined by 1H NMR spectroscopy, using hexamethyldisiloxane as the internal standard. b, Cleavage of different ligninox model compounds in the presence of formic acid and sodium formate. c, Control reaction with the non-oxidized lignin model compound 1 under the formic acid/formate cleavage conditions.
The conversion of 2 into the diketone product 5 is a redox-neutral process. We therefore tested the reaction in the absence of a reducing metal, and products 5 and 6 were obtained in yields comparable to the best results obtained with a stoichiometric metal source (Fig. 2a, entry 6). Even better yields of 5 and 6 resulted (96% and 87%, respectively) when 3 equivalents of sodium formate were included in the reaction mixture (Fig. 2a, entry 7). These conditions proved to be effective with several other ligninox model compounds, including those derived from p-hydroxyphenyl (H)-, guaiacyl (G)- and syringyl (S)-type lignin units 7–9 (Fig. 2b). The Cα ketone is crucial to the success of the reaction: no cleavage products were observed when the non-oxidized model compound 1 was subjected to the formic acid/formate reaction conditions (Fig. 2c).
These results provided the basis for testing the reactivity of authentic lignin polymer. Aspen is a representative hardwood, and a sample of native aspen lignin was isolated and determined to have a ratio of S:G subunits of 2.2:1 on the basis of two-dimensional heteronuclear single quantum coherence NMR analysis (see Supplementary Fig. 1)22. After treatment of this material under the aerobic conditions described previously14, the oxidized lignin was subjected to the formic acid/sodium formate reaction conditions at 110 °C. The amount of sodium formate added to the reaction mixture was estimated from the mass of the lignin sample to provide about 3 equivalents of formate per S/G aromatic subunit. The formic acid was evaporated after 24 h, and the residue was extracted with ethyl acetate. A soluble fraction, corresponding to 61.2wt% of the original lignin, was obtained, together with an insoluble fraction that accounted for 29.7wt% of the lignin (Fig. 3a). Acetylation of the insoluble material and analysis by gel-permeation chromatography revealed a significant decrease in molecular mass relative to the initial lignin polymer (Fig. 3b). The soluble fraction, containing the majority of the lignin-derived mass, was analysed by high-resolution mass spectrometry coupled with liquid chromatography (LC–MS) to identify and quantify the products of the reaction (Fig. 3c and Supplementary Table 2). Product identities were confirmed by comparison with authentic samples obtained commercially or synthesized independently (see Supplementary Information for details).
a, Depolymerization of oxidized aspen lignin. b, Gel-permeation chromatography of the insoluble fraction from depolymerization (29.7wt%) (blue trace) compared with that of native lignin (red trace). For the insoluble fraction of depolymerized lignin, Mw/Mn = 1.08 and Mn = 4,600 Da; for native lignin, Mw/Mn = 1.18 and Mn = 10,800 Da. c, Identification, quantification and distribution of products obtained from the depolymerization of oxidized lignin (upper left) compared with those from native lignin (upper right). Bottom: major depolymerization products from oxidized lignin. For a full product listing see Supplementary Table 2.
Overall, more than 52% of the original lignin was converted to well-defined aromatic compounds (Fig. 3c). Syringyl and guaiacyl-derived diketones, directly analogous to those observed in the model study (compare Fig. 2b), are two of the major depolymerization products (19.8%). The S:G diketone ratio (2:1) is very similar to the S:G monomer composition in the lignin. Additional syringyl and guaiacyl-derived aromatics (for example, syringaldehyde and vanillin) account for most of the remaining product mass, together with p-hydroxybenzoic acid (4%), which is probably derived from p-hydroxybenzoic esters present in aspen (and other poplar) lignins23. A full listing of characterized products is provided in Supplementary Table 2.
An attempt to use unoxidized lignin in the reaction resulted in only 7.2wt% yield of low-molecular-mass aromatics. The products that are observed probably arise from the small amount of Cα ketones present in native lignin (evident from two-dimensional NMR spectroscopy). This result demonstrates the importance of Cα oxidation in promoting lignin depolymerization and provides strong motivation to develop improved lignin oxidation methods suitable for large-scale application. The Cα benzylic alcohol in lignin is activated electronically relative to the Cγ primary alcohol, and our previous study showed that numerous classes of oxidants promote the chemoselective oxidation of the Cα alcohol14. These observations bode well for the development of improved oxidation methods.
Mechanistic insights into the depolymerization process were obtained from additional studies of model compound 2. Reaction time-course data, obtained by 1H NMR spectroscopy, revealed that 2 converts quickly into the alcohol formylation product 3, followed by a slower conversion of 3 into products 5 and 6 (Fig. 4a, b). Conversion of 2 into 3 is rapid, even at room temperature (Fig. 4c). Direct investigation of the reaction of 3 revealed a small steady-state concentration of the aryl vinyl ether intermediate 13 during the formation of diketone product 5 (Fig. 4d). Independent testing of 13 confirmed that this intermediate is consumed more rapidly than the overall conversion of 3 into 5 (Fig. 4e). A large deuterium kinetic isotope effect, observed when the independent reaction rates of 2 and 2-d1 were compared (kH/kD = 9.3 ± 0.2; Fig. 4f), indicates that the elimination of formic acid from 3 is the rate-limiting step in the transformation. This kinetic isotope effect is larger than the semi-classical limit associated with the cleavage of a C–H bond, but it aligns with a previous study providing evidence for proton tunnelling in concerted E2 elimination reactions24.
a, b, Different intermediates (a) and overall time course of the reaction 2→3→[13]→5 at 110 °C (b). c–e, Time courses of formylation of 2 at 25 °C (c), elimination of formic acid from 3 at 110 °C (d), and hydrolysis of aryl vinyl ether 13 at 110 °C (e). f, Kinetic isotope effect for cleavage of the oxidized model compound 2. g, Proposed mechanism for C–O cleavage.
Overall, the sequence is a redox-neutral process that results in no net consumption of formic acid (Fig. 4g). This feature distinguishes the present approach from other lignin conversion methods that employ formic acid as a source of H2 in transfer hydrogenation/hydrogenolysis reactions with heterogeneous catalysts (see, for example, refs 25, 26). According to the mechanism in Fig. 4g, the beneficial effect of lignin oxidation may be attributed to the ability of the benzylic carbonyl group to polarize the C–H bond and lower the barrier for the rate-limiting E2 elimination reaction. Both the increased C–H acidity and orbital overlap between the carbonyl C = O π system and the developing π bond of the alkene are expected to contribute to a lower barrier for this step. The mechanism also accounts for the beneficial effect of using a ‘buffered’ reaction medium, containing both formate and formic acid (compare Fig. 2a, entries 6 and 7). The rate-limiting elimination step in Fig. 4g is proposed to involve both a base (formate) to remove the proton and an acid (formic acid) to assist in the loss of the formate as a leaving group.
The overall yield of structurally identified, monomeric aromatics obtained here is the highest reported so far for lignin depolymerization2,10,11. Although some of the products obtained from this process have direct commercial value (for example vanillin and syringaldehyde)27,28, the more important result may be the generation of a stream of soluble aromatic feedstocks for further upgrading. For example, low-molecular-mass feedstocks should reduce coking and char formation, enhance conversions and facilitate product separations in processes with heterogeneous catalysts (such as hydrogenation, hydrogenolysis, decarbonylation and decarboxylation). The syringyl, guaiacyl and p-hydroxyphenyl aromatic products arise directly from the monomeric composition of the lignin, and their yields correlate closely with the quantity of β-O-4 linkages present in the original lignin. These observations highlight the importance of developing chemical conversion technologies for S-, G- and H-derived aromatics and suggest that plants containing lignin with high β-O-4 content (as much as 85% has been observed29) could be particularly appealing feedstocks for biomass valorization. This work further draws attention to biomass separation methods. Since the inception of the pulp and paper industry, biomass separation methods have emphasized the production of high-purity cellulose. Most existing process conditions, ranging from the classic kraft pulping to modern organosolvolysis methods, significantly modify or damage the lignin and lead to a significant decrease or complete loss of β-O-4 structural units. Biomass separation methods that deliver both high-purity sugar and native-type lignin streams, such as a recent γ-valerolactone-based process30, could gain a competitive advantage in biorefining applications.
References
Ralph, J., Brunow, G. & Boerjan, W. Lignins. eLShttp://dx.doi.org/10.1002/9780470015902.a0020104 (2007)
Zakzeski, J., Bruijnincx, P. C. A., Jongerius, A. L. & Weckhuysen, B. M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 110, 3552–3599 (2010)
Sanderson, K. Lignocellulose: a chewy problem. Nature 474, S12–S14 (2011)
Lin, S. Y. & Lin, I. S. in Ullmann’s Encyclopedia of Industrial Chemistry 5th edn (eds Elvers, B., Hawkins, S., Schulz, G. ) Vol. A15 305–315 (VCH, 1985)
Tuck, C. O., Pérez, E., Horváth, I. T., Sheldon, R. A. & Poliakoff, M. Valorization of biomass: deriving more value from waste. Science 337, 695–699 (2012)
Holladay, J. E., Bozell, J. J., White, J. F. & Johnson, D. Top Value-added Chemicals from Biomass Vol. 2 http://www1.eere.energy.gov/bioenergy/pdfs/pnnl-16983.pdf (United States Department of Energy, 2007)
Ragauskas, A. J. et al. Lignin valorization: improving lignin processing in the biorefinery. Science 344, 1246843 (2014)
Hocking, M. B. Vanillin: synthetic flavoring from spent sulfite liquor. J. Chem. Educ. 74, 1055–1059 (1997)
Business Areas. Borregaard http://www.borregaard.com/Business-Areas (20 June 2014)
Pandey, M. P. & Kim, C. S. Lignin depolymerization and conversion: a review of thermochemical methods. Chem. Eng. Technol. 34, 29–41 (2011)
Wang, H., Tucker, M. & Ji, Y. Recent development in chemical depolymerization of lignin: a review. J. Appl. Chem 2013, 1–9 (2013)
Gierer, J. & Norén, I. Oxidative pretreatment of pine wood to facilitate delignification during kraft pulping. Holzforschung 36, 123–130 (1982)
Ljunggren, S. & Olsson, A. The specificity in oxidation of some lignin and carbohydrate models and pine wood shavings with permanganate and pyridinum dichloromate before the kraft pulping process. Holzforschung 38, 91–99 (1984)
Rahimi, A., Azarpira, A., Kim, H., Ralph, J. & Stahl, S. S. Chemoselective metal-free aerobic oxidation in lignin. J. Am. Chem. Soc. 135, 6415–6418 (2013)
Crestini, C. & D’Auria, M. Singlet oxygen in the photodegradation of lignin models. Tetrahedron 53, 7877–7888 (1997)
Fukagawa, N. & Ishizu, A. Photoreaction of phenacyl aryl ether type lignols. J. Wood Chem. Technol. 11, 263–289 (1991)
Argyropoulos, D. S. & Sun, Y. Photochemically induced solid-state degradation, condensation, and rearrangement reactions in lignin model compounds and milled wood lignin. Photochem. Photobiol. 64, 510–517 (1996)
Vanucci, C., De Violet, P. F., Bouas-Laurent, H. & Castellan, A. Photodegradation of lignin: a photophysical and photochemical study of a non-phenolic α-carbonyl β-O-4 lignin model dimer, 3,4-dimethoxy-α-(2′-methoxyphenoxy) acetophenone. J. Photochem. Photobiol. Chem. 41, 251–265 (1988)
Nguyen, J. D., Matsuura, B. S. & Stephenson, C. R. J. A photochemical strategy for lignin degradation at room temperature. J. Am. Chem. Soc. 136, 1218–1221 (2014)
Collinson, S. R. & Thielemans, W. The catalytic oxidation of biomass to new materials focusing on starch, cellulose and lignin. Coord. Chem. Rev. 254, 1854–1870 (2010)
Chatel, G. & Rogers, R. D. Oxidation of lignin using ionic liquids—an innovative strategy to produce renewable chemicals. ACS Sustainable Chem. Eng. 2, 322–339 (2014)
Chang, H., Cowling, E. B. & Brown, W. Comparative studies on cellulolytic enzyme lignin and milled wood lignin of sweetgum and spruce. Holzforschung 29, 153–159 (1975)
Ralph, J. & Landucci, L. L. in Lignin and Lignans; Advances in Chemistry (eds Heitner, C., Dimmel, D. R. & Schmidt, J. A. ) 137–234 (CRC Press, 2010)
Fouad, F. M. & Farrell, P. G. Primary and secondary kinetic isotope effects in E2 elimination reactions. Tetrahedr. Lett. 19, 4735–4738 (1978)
Xu, W., Miller, S. J., Agrawal, P. K. & Jones, C. W. Depolymerization and hydrodeoxygenation of switchgrass lignin with formic acid. ChemSusChem 5, 667–675 (2012)
Toledano, A. et al. Fractionation of organosolv lignin from olive tree clippings and its valorization to simple phenolic compounds. ChemSusChem 6, 529–536 (2013)
Bauer, K., Garber, D. & Surburg, H. in Ullmann’s Encyclopedia of Industrial Chemistry 5th edn (eds Elvers, B., Rounsaville, J. F. & Schulz, G. ) Vol. A11 199–200 (VCH, 1985)
Bjørsvik, H.-R. & Liguori, L. Organic processes to pharmaceutical chemicals based on fine chemicals from lignosulfonates. Org. Process Res. Dev. 6, 279–290 (2002)
Martínez, Á. T. et al. Monolignol acylation and lignin structure in some nonwoody plants: a 2D NMR study. Phytochemistry 69, 2831–2843 (2008)
Luterbacher, J. S. et al. Nonenzymatic sugar production from biomass using biomass-derived γ-valerolactone. Science 343, 277–280 (2014)
Acknowledgements
We thank J. Ralph for numerous helpful discussions, H. Kim and A. Azarpira for assistance with the purification and NMR characterization of aspen lignin, and S. Chakraborty for assistance with gel-permeation chromatographic analysis of lignin samples. Financial support for this project was provided by the Great Lakes Bioenergy Research Center (Department of Energy Biological and Environmental Research Office of Science DE-FC02-07ER64494). The NMR facility was partly supported by the National Science Foundation (CHE-9208463) and the National Institutes of Health (S10 RR08389).
Author information
Authors and Affiliations
Contributions
A.R. and S.S.S. conceived the idea for the lignin depolymerisation. A.R. performed the chemical reactions of lignin and lignin model compounds, including NMR spectroscopic characterization of the products. A.U. and J.J.C. designed the analytical approach to characterize the lignin depolymerization products. A.U. performed LC/MS analysis. A.R. and S.S.S. wrote the manuscript in consultation with A.U. and J.J.C.
Corresponding author
Ethics declarations
Competing interests
A patent application based on the results reported here has been submitted.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data, Supplementary Figures 1-6 and Supplementary Tables 1-2. (PDF 3606 kb)
Rights and permissions
About this article
Cite this article
Rahimi, A., Ulbrich, A., Coon, J. et al. Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature 515, 249–252 (2014). https://doi.org/10.1038/nature13867
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13867
- Springer Nature Limited
This article is cited by
-
Selective lignin arylation for biomass fractionation and benign bisphenols
Nature (2024)
-
Carbon–carbon bond cleavage for a lignin refinery
Nature Chemical Engineering (2024)
-
Aqueous amine enables sustainable monosaccharide, monophenol, and pyridine base coproduction in lignocellulosic biorefineries
Nature Communications (2024)
-
An overview of lignin pathways of valorization: from isolation to refining and conversion into value-added products
Biomass Conversion and Biorefinery (2024)
-
Lignin Degradation by Isolated Lignolytic Acinetobacter baumanii S2, Aspergillus niger SF4 and Rhodotorula glutinis and Profiling Products from Bio-Valorization Perspective
Waste and Biomass Valorization (2024)