Abstract
Several lines of evidence are indicative of a role for immune activation in the pathophysiology of schizophrenia. Nevertheless, studies using positron emission tomography (PET) and radioligands for the translocator protein (TSPO), a marker for glial activation, have yielded inconsistent results. Whereas early studies using a radioligand with low signal-to-noise in small samples showed increases in patients, more recent studies with improved methodology have shown no differences or trend-level decreases. Importantly, all patients investigated thus far have been on antipsychotic medication, and as these compounds may dampen immune cell activity, this factor limits the conclusions that can be drawn. Here, we examined 16 drug-naive, first-episode psychosis patients and 16 healthy controls using PET and the TSPO radioligand [11C]PBR28. Gray matter (GM) volume of distribution (VT) derived from a two-tissue compartmental analysis with arterial input function was the main outcome measure. Statistical analyses were performed controlling for both TSPO genotype, which is known to affect [11C]PBR28 binding, and gender. There was a significant reduction of [11C]PBR28 VT in patients compared with healthy controls in GM as well as in secondary regions of interest. No correlation was observed between GM VT and clinical or cognitive measures after correction for multiple comparisons. The observed decrease in TSPO binding suggests reduced numbers or altered function of immune cells in brain in early-stage schizophrenia.
Similar content being viewed by others
Introduction
Schizophrenia is a severe mental disorder for which currently available treatment is satisfactory only in a minority of cases. Cognitive impairments, such as memory dysfunction and reduced speed of processing, are present already at an early stage of the disease, and are particularly difficult to ameliorate.1 The development of new, improved treatment approaches is presently hampered by a lack of understanding of the pathophysiology of the disease.
Genetic and epidemiological data are indicative of an involvement of the immune system in the development of schizophrenia.2, 3 In patients, an on-going immune activation is suggested by studies showing an elevation of immune markers in blood and cerebrospinal fluid both during long-term illness, as well as in first-episode psychosis.4, 5, 6, 7 Using positron emission tomography (PET) and radioligands for the translocator protein 18kDA (TSPO), which in the brain is expressed primarily in microglia and astrocytes, it is possible to index brain immune cell activation in vivo.8, 9, 10 Initial TSPO PET studies in small samples have shown increased binding in patients with schizophrenia as compared with controls;11, 12 however, a radioligand with low signal-to-noise ratio was used, as well as an outcome measure generally shown to be less reliable.13 More recent studies using novel TSPO radioligands have failed to replicate these findings. Although increases in a relative measure of binding was recently reported in schizophrenia patients and ultra-high-risk (UHR) individuals, there was no absolute TSPO elevation using standard methods of quantification.14 This observation is in line with results from other studies in patients with long-term illness,15, 16 as well as a recent study in patients with recent-onset disease where a trend-level reduction in TSPO was observed.17
A major limitation of the studies published thus far is that all patients have been on treatment with antipsychotic medication. Importantly, several of these compounds have shown to attenuate blood cytokine levels in patients18 as well as decrease binding to TSPO in preclinical studies.19 Although the high-risk individuals investigated by Bloomfield et al.14 were unexposed to antipsychotic medication, on average less than 30% of this group go on to develop a psychotic disorder,20 indicating that the group is heterogeneous and cannot be equated with a prodromal phase of schizophrenia.
The aim of the present study was to overcome the critical drawback of previous investigations by measuring TSPO binding in antipsychotic-naive, first-episode psychotic patients, as compared with age-matched control subjects.
Materials and methods
Patients and control subjects
The study was approved by the Regional Ethics Committee in Stockholm and the Radiation Safety Committee at the Karolinska University Hospital, Stockholm. Subjects were included after providing written informed consent after receiving a complete description of the study.
Sixteen first-episode psychotic patients (11 male, 5 female, mean age 28.5 (s.d. 8.4)) were recruited from psychiatric emergency wards and out-patient clinics in Stockholm. At the time of investigation, all patients were naive to antipsychotic treatment and met the diagnostic criteria for schizophrenia (n=4), schizophreniform psychosis (n=7), psychosis NOS (n=4) or brief psychosis (n=1), according to DSM-IV. Exclusion criteria were neurologic or severe somatic illness, current use or history of abuse of illegal drugs (including cannabis) and autism-spectrum disorder. Absence of major brain abnormalities was confirmed using magnetic resonance imaging. Occasional medication with sedatives and anxiolytics, including benzodiazepines, were allowed during the course of the study, as well as medication with antidepressants. In cases where diazepam was used, the daily dose was significantly lower than equivalent concentrations of this drug shown to affect TSPO binding according to in vitro data.21
Sixteen control subjects (7 male, 9 female, age 26.4 (8.4)) were recruited by advertisement. They were healthy according to medical history, clinical examination, routine laboratory blood and urine tests as well as a brain magnetic resonance imaging (MRI) examination. The Mini International Neuropsychiatric Interview was used to exclude previous or current psychiatric illness. Further exclusion criteria were previous or current use of illegal drugs and first-degree relatives with psychotic illness. None of the control subjects were on any medication at the time of the study.
The TSPO genotype was assessed in all subjects as described previously22 using DNA extracted either from whole blood or saliva. There were eight high-affinity binders (HABs) and eight mixed-affinity binders (MABs) among the patients and nine HABs and seven MABs in the control group (Table 1).
Behavioral measures
In patients, psychotic symptoms were assessed using the Positive and Negative Syndrome Scale for Schizophrenia (PANSS) and the Clinical Global Impression (CGI) scale. Cognitive functioning was assessed in all subjects using tests from the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB),23, 24 which is designed specifically to measure cognition in research on schizophrenia.1 The cognitive domains assessed were speed of processing (using the tests Category Fluency: Animal Naming, Trail Making Test: Part A), attention/vigilance (Continuous Performance Test-Identical Pairs), working memory (Wechsler Memory Scale: Spatial Span), verbal learning (Hopkins Verbal Learning Test-Revised) and visual learning (Brief Visuospatial Memory Test-Revised). We also performed the Wisconsin Card Sorting Test and included categories completed and percent errors as outcome measures.
Magnetic resonance imaging
Brain MRI was performed at the MR Centre, Karolinska University Hospital, using a 3-T General Electric Discovery MR750 system (GE, Milwaukee, WI, USA). T2-weighted images were acquired for evaluation regarding pathology by a neuroradiologist, and T1-weighted images were acquired for definition of regions of interest (ROIs).
PET procedures
PET measurements were performed at the PET Centre at the Karolinska University Hospital, Stockholm, Sweden, using a high-resolution research tomograph (HRRT, Siemens Molecular Imaging, Knoxville, TN, USA). Individualized plaster helmets were made for each subject and used together with a head fixation system to minimize movement artifacts. A 6-min transmission scan using a 137Cs source was performed for attenuation correction. [11C]PBR28 was prepared as described previously25 and injected as a bolus over ~10 s into the cubital vein. Emission data were acquired in list mode for up to 91 min, except for one patient (85 min) and two control subjects (77 min). The inclusion of data from these subjects was supported by analyses of a previous data set22 where 63 min two-tissue compartment model (2TCM) for gray matter (GM) showed high correlation to that of 91 min (n=12, r=0.997, P=8 × 10−13). PET images were reconstructed using ordered subset expectation maximization with modeling of the point-spread function, and subsequently corrected for head movement using a frame-by-frame realignment process as described previously.26, 27
An automated blood-sampling system (ABSS, Allogg, Mariefred, Sweden) was used during the first 5 min of each PET measurement. Discrete arterial blood samples (2–4 ml) were drawn manually at 1, 3, 5, 7, 9, 10.5, 20, 30, 40, 50, 60, 70, 80 and 90 min, except for one patient, for whom the samples were drawn at 1, 2, 3, 4, 5, 6, 7, 8, 9, 10.5, 20, 30, 40, 50, 60, 70, 80 and 90 min, and two control subjects with sampling times of 2, 4, 6, 8, 10, 15, 20, 25, 30, 40, 50, 60 and 72 min. The radioactivity was measured immediately after sampling in a well counter cross-calibrated with the PET system. After centrifugation, 0.7–1.5 ml plasma was pipetted and the radioactivity was measured in the same well counter.
The plasma radioactivity curve for the first 5 min was generated by combining the ABSS data with an interpolated curve from the manual samples, and then multiplied with the blood-to-plasma ratio obtained from the plasma samples to create an arterial plasma curve. The time curve for parent fraction of the radioligand was determined using high-performance liquid chromatograhpy (HPLC), interpolated and multiplied with the plasma curve to generate the arterial input function. The area under the curve (AUC) of the standardized uptake value (SUV) for the metabolite-corrected plasma curves was calculated for all subjects from 0 to 72 min. To assess the impact of protein binding, the free fraction of radioligand was analyzed using an ultrafiltration method. For additional details on these procedures see Collste et al.25and Kanegawa et al.28
Quantification of [11C]PBR28 binding
MR images were realigned, segmented and co-registered to PET images using SPM5 in MATLAB R2007b (Wellcome Trust Centre for Neuroimaging, London, UK; The Mathworks, Natick, MA, USA).25 ROIs were defined using an automated procedure based on the FreeSurfer software (version 5.0.0, http://surfer.nmr.mgh.harvard.edu/), previously validated for PET data analysis.29 GM was selected as the primary ROI. In addition, we examined white matter, frontal cortex, temporal cortex and hippocampus on account of their central interest for research in schizophrenia as well as to enable comparison with previous TSPO PET studies.12, 14 Furthermore, a FreeSurfer whole-brain (WB) ROI was created for calculation of distribution volume ratios (DVRs), as described previously.14 Volumetric data for all ROIs used in the PET analysis were extracted from FreeSurfer, as well as for the total intracranial volume.
The main outcome measure of TSPO binding was calculated using the 2TCM with a metabolite-corrected arterial plasma curve as input function. Binding was expressed as the total volume of distribution (VT), which corresponds to the ratio between the sum of specific and non-displaceable concentrations of radioligand in the target region to the concentration in plasma at equilibrium. As a secondary analysis to allow for comparison with Bloomfield et al.,14 data were analyzed using the 2TCM-1K model that includes an additional compartment with irreversible uptake hypothesized to account for endothelial binding in blood vessels.30 All kinetic analyses were performed using MATLAB R2007b. Finally, DVRs were calculated as the ratio between GM VT and WB VT for both 2TCM and 2TCM-1K to enable additional comparisons with previously published data.14, 17
Statistics
Normality of all demographic data, clinical and cognitive variables, radiochemical data and uptake outcome measures were evaluated using Kolmogorov–Smirnov tests as well as ocular inspection of histograms. Independent sample two-tailed t-tests were performed to examine potential group differences in demographic data, measurements of cognitive function, radiochemical data, ROI volumes, free fraction of radioligand in plasma as well as differences in plasma SUV AUC values. For variables that were not normally distributed, a Mann–Whitney U-test was performed to examine group differences. Group differences in categorical variables were assessed using a χ2-test.
Effects of genotype, sex and age on GM VT were examined using independent sample t-tests (two-tailed) and Pearson’s correlation. Multivariate analysis of covariance with all ROIs as dependent variables was deemed invalid because of high multicollinearity between the outcome measures. For the main statistical analysis, the difference between patients and controls in [11C]PBR28 binding in GM was, therefore, examined using a univariate analysis of covariance (ANCOVA). GM VT derived from the standard 2TCM model was used as dependent variable, group (patients versus control subjects) as independent variable and gender and genotype as covariates. An analysis was also performed excluding the five patients on benzodiazepine treatment. Furthermore, effect sizes for GM binding was estimated for MABs and HABs separately, using partial eta-squared values obtained from ANCOVAs with gender as covariate.
Secondary, to examine group differences in binding in frontal cortex, temporal cortex, hippocampus and white matter, we performed four additional univariate ANCOVAs, each having sex and genotype as covariates. To allow for comparison with previously published results, the same set of univariate ANCOVAs was performed using regional VT values derived from 2TCM-1K, as well as DVR values based on both 2TCM and 2TCM-1K methods as dependent variables.14 For the main analysis of GM VT differences, alpha was set to 0.05.
The relationships between GM VT and symptom levels (PANSS), global functioning (CGI) and duration of illness were assessed using partial correlation controlling for genotype and gender. For correlations between GM VT and cognitive measures, age was added as a covariate and the threshold for significance was Bonferroni-corrected for multiple comparisons (alpha=0.05/8=0.00625). All statistical analyses were performed in SPSS 23 (IBM, Armonk, NY, USA) or R (version 3.2.4 'Very Secure Dishes').
Results
Patient and control groups did not differ significantly in age, sex distribution, body mass index or genotype distribution (Table 1). Duration of illness was 7.9 (9.6) months and total PANSS score 77.4 (18.3). None of the patients had been exposed to antipsychotic medication at the time of investigation. Nine patients received anxiolytics or sedatives, of which five patients received benzodiazepines (diazepam (5 mg per day) in one case and oxazepam (10–30 mg per day) in four cases; Supplementary Table 1). One patient was prescribed paroxetin 20 mg per day. Two of the patients and none of the controls were cigarette smokers. Regional brain volumes did not differ significantly between patients and controls (Table 2) also after normalizing with intracranial volume (ST2); therefore, correction for partial volume effects was not performed for analyses of [11C]PBR28 binding.
Group effects on regional [11C]PBR28 binding
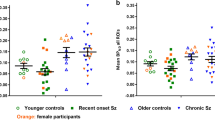
There was a significant effect of both genotype and sex on GM VT (t=3.5, degree of freedom (df)=30, P=0.001 and t=2.1, df=30, P=0.043, respectively), whereas no correlation was observed between age and GM VT (r=−0.09, P=0.62). Hence, only sex and genotype were included as covariates in the ANCOVA. A decrease in [11C]PBR28 GM VT was observed in patients as compared with controls, with a significant effect of diagnostic group obtained in the ANCOVA (F=6.19, df=1.28, P=0.019). The decrease was more prominent in HAB patients, with an effect size of 0.38 (partial eta square) compared with 0.02 for the MAB subgroup (Figure 1). The effect remained when excluding the five patients on benzodiazepine treatment (F=6.71, df=1.23, P=0.016). In the regional analysis, significant group effects were observed for frontal cortex (F=6.08, df=1.28, P=0.020), temporal cortex (F=6.51, df=1.28, P=0.016) and hippocampus (F=5.49, df=1.28, P=0.026), but not for white matter F=1.54, df=1.28, P=0.23; ST3. There was also a significant effect of group for GM VT derived using 2TCM-1k (F=6.66, df=1.28, P=0.015), whereas no difference was found when analyzing DVR values calculated either using 2TCM or 2TCM-1K VT (ST3). No statistically significant difference between patients and control subjects was observed for plasma SUV AUC (mean=2.53 × 103 (s.d.=1.0 × 103) versus 2.20 × 103 (0.66 × 103); t=−1.1, df=30, P=0.28) or free fraction (8.2 (3.1) versus 9.0 (2.8); t=−0.74, df=30, P=0.46).
Regional VT values in first-episode psychosis patients (Pat) and healthy control subjects (HC) estimated using 2TCM. FC, frontal cortex; GM, gray matter; HAB, high-affinity binder; HIP, hippocampus; MAB, mixed-affinity binder; TC, temporal cortex; WM, white matter; VT, volume of distribution.
Correlations between [11C]PBR28 binding and behavioral measures
In patients, there was no significant association between GM VT and either positive, negative, general or total PANSS scores, CGI or duration of psychosis. The patients performed more poorly than controls in all the cognitive outcome measures (Table 1). However, there were no significant correlations between GM VT and cognitive performance in patients after correction for multiple comparisons (Table 3).
Discussion
The results of the present study are novel in two important respects. First, this is, to our knowledge, the only study thus far to investigate TSPO binding in drug-naive patients with psychosis. The finding of reduced TSPO levels suggests that the lack of increases, or even trend-level decreases in VT reported in recent studies employing novel TSPO radioligands14, 15, 17 is not explained by the effects of antipsychotic medication. Second, patients had a mean duration of illness of less than 8 months, which is significantly shorter than in previous studies, including the average of 2.2 years reported in a recently published report in recent-onset schizophrenia.17 Hence, the results imply that there is no TSPO elevation also in very early stages of the disease. Instead, first-episode psychosis patients may even be characterized by having decreased glial cell activation.
TSPO is present in myeloid and astrocytic cells throughout the whole brain, also at physiological conditions.31, 32 Therefore, quantification of radioligand binding to TSPO requires a metabolite-corrected plasma input function as reference. To reduce the variability induced by plasma measurements, relative outcome measures of binding such as SUV ratio or DVR have been suggested instead of the widely used VT values.33 In the study by Bloomfield et al.,14 a nonsignificant reduction of ~10% in regional VT was found in patients and UHR individuals, whereas VT values normalized to WB binding showed an apparent increase in both groups. However, the normalization approach has been criticized.34 Importantly, the WB values used for the normalization procedure were ~20% lower both for the UHR and patient groups as compared with controls, a difference that in patients appeared to be driven by significant decreases in white matter. Moreover, DVR values were obtained using WB as a covariate in the statistical analysis rather than dividing the ROI by WB. As a high degree of correlation is expected between WB and GM regions, this method may reduce variability significantly, resulting in increased effect sizes. In the present study, the observed reduction of GM TSPO in patients did not remain when using WB-normalized VT (DVR, ratio method) as outcome measure. The effect of using DVR instead of VT was thus in the same direction as for Bloomfield et al.35 The lack of significant increases in our case may be explained by the statistical DVR procedure as well as the use of different WB templates, which could influence WB VT values. Finally, we observed no group difference in the metabolite-corrected input function or free plasma fraction of radioligand in our data, providing support for the validity of our primary outcome measures.
Evidence for a heightened immune response in schizophrenia patients comes primarily from biomolecular studies, showing increases in pro-inflammatory markers both in long-term illness and in early psychosis.4, 5, 6, 7 With regard to the role of brain immune cells, some post-mortem studies have shown increased levels of microglia in patients; however, no differences or even decreases have also been reported.36 For instance, two autoradiography studies assessing TSPO have yielded contrasting results, reporting reductions in [3H]PK11195 and increases in [3H]PBR28 binding, respectively.37, 38 Whereas initial in vivo PET studies using [11C]PK11195 in small samples have shown increased TSPO binding in antipsychotic drug-treated schizophrenia patients, this finding has not been replicated using more recently developed TSPO radioligands with higher sensitivity. Importantly, preclinical studies combining PET with post-mortem immunohistochemistry have shown a close correspondence between TSPO levels and glial cell markers.8, 9 Consequently, although the high variability observed in human TSPO PET studies may limit the sensitivity,15, 25, 37 a plausible interpretation of both in vitro and in vivo TSPO studies across different disease stages is that glial upregulation is not a robust feature of schizophrenia.
Our findings of reduced TSPO levels in drug-naive patients with psychosis are congruent with the numerically lower VT values reported in antipsychotic drug-treated patients with long-term schizophrenia,14, 15 as well as the trend-level reductions shown in recent-onset patients.17 It may be hypothesized that reduced glial activation in early-stage psychosis reflects a failure of the immune system to adapt to other pathological processes associated with being in a psychotic state, thus promoting the development of the disorder. Importantly, both microglia and astrocytes also have neuroprotective and pro-inflammatory roles in the brain,39 and the immune hypothesis in schizophrenia may thus be thought of as an imbalance rather than a one-dimensional activation. A limitation is that TSPO radioligands cannot differentiate between pro- and anti-inflammatory cell phenotypes, and there is thus a need for immune cell markers with improved functional specificity. This view is corroborated by the lack of significant correlations between TSPO VT values and symptom levels or cognitive functioning observed in our study as well as in previous reports.15, 17
In the present study the decrease in TSPO binding was more prominent in the group of HAB individuals. Although the lack of statistical power limits the conclusions that can be drawn when examining the genetic groups separately, this effect may partly be explained by the higher ratio of specific to non-displaceable binding in HABs compared with MABs.31, 40 This pattern needs confirmation in larger samples or as part of a meta-analysis.
An alternative hypothesis that allows for reconciliation of the lack of TSPO elevation at least with genetic and epidemiological data2, 3 is that pro-inflammatory microglial activation exerts its major influence preceding the onset of manifest disease. For instance, both human and animal research has suggested a link between immune activation and excessive synaptic pruning,41, 42 which may correspond to the cortical thinning observed already in early stages of schizophrenia.43 Consequently, although all patients in the present study were examined during their first psychotic episode, it may be speculated that this was still too late to detect a hypothesized increase in immune cell activation.
Conclusion
In this study we found a decrease in TSPO binding in antipsychotic-naive first-episode psychosis patients compared with control subjects, indicating lower number or altered function of immune cells in brain in early-stage schizophrenia. Further studies combining molecular and structural brain imaging with detailed characterization of pro- and anti-inflammatory immune markers over time are needed to clarify the role of the immune system during the different disease stages of the disorder.
References
Fatouros-Bergman H, Cervenka S, Flyckt L, Edman G, Farde L . Meta-analysis of cognitive performance in drug-naïve patients with schizophrenia. Schizophr Res 2014; 158: 156–162.
Arias I, Sorlozano A, Villegas E, de Dios Luna J, McKenney K, Cervilla J et al. Infectious agents associated with schizophrenia: a meta-analysis. Schizophr Res 2012; 136: 128–136.
Ripke S, Neale BM, Corvin A, Walters JTR, Farh K-H, Holmans PA et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014; 511: 421–427.
Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B . Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 2011; 70: 663–671.
Upthegrove R, Manzanares-Teson N, Barnes NM . Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res 2014; 155: 101–108.
Söderlund J, Schröder J, Nordin C, Samuelsson M, Walther-Jallow L, Karlsson H et al. Activation of brain interleukin-1beta in schizophrenia. Mol Psychiatry 2009; 14: 1069–1071.
Schwieler L, Larsson MK, Skogh E, Kegel ME, Orhan F, Abdelmoaty S et al. Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia—significance for activation of the kynurenine pathway. J Psychiatry Neurosci 2015; 40: 126–133.
Toth M, Little P, Arnberg F, Mulder J, Halldin C, Ha J et al. Acute neuroinflammation in a clinically relevant focal cortical ischemic stroke model in rat: longitudinal positron emission tomography and immunofluorescent tracking. Brain Struct Funct 2016; 221: 1279–1290.
Ory D, Planas A, Dresselaers T, Gsell W, Postnov A, Celen S et al. PET imaging of TSPO in a rat model of local neuroinflammation induced by intracerebral injection of lipopolysaccharide. Nucl Med Biol 2015; 42: 753–761.
Venneti S, Lopresti BJ, Wiley CA . Molecular imaging of microglia/macrophages in the brain. Glia 2013; 61: 10–23.
van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry 2008; 64: 820–822.
Doorduin J, de Vries EFJ, Willemsen ATM, de Groot JC, Dierckx RA, Klein HC . Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med 2009; 50: 1801–1807.
Varnäs K, Varrone A, Farde L . Modeling of PET data in CNS drug discovery and development. J Pharmacokinet Pharmacodyn 2013; 40: 267–279.
Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR et al. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [ 11 C]PBR28 PET brain imaging study. Am J Psychiatry 2015; 173: 44–52.
Kenk M, Selvanathan T, Rao N, Suridjan I, Rusjan P, Remington G et al. Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo pet study with [ 18 F ] -FEPPA. Schizophr Bull 2014; 41: 85–93.
Takano A, Arakawa R, Ito H, Tateno A, Takahashi H, Matsumoto R et al. Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. Int J Neuropsychopharmacol 2010; 13: 943–950.
Coughlin JM, Wang Y, Ambinder EB, Ward RE, Minn I, Vranesic M et al. In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [11C]DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry 2016; 6: e777.
Drzyzga L, Obuchowicz E, Marcinowska A, Herman ZS . Cytokines in schizophrenia and the effects of antipsychotic drugs. Brain Behav Immun 2006; 20: 532–545.
Danovich L, Veenman L, Leschiner S, Lahav M, Shuster V, Weizman A et al. The influence of clozapine treatment and other antipsychotics on the 18 kDa translocator protein, formerly named the peripheral-type benzodiazepine receptor, and steroid production. Eur Neuropsychopharmacol 2008; 18: 24–33.
Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L et al. Predicting psychosis. Arch Gen Psychiatry 2012; 69: 220–229.
Kalk NJ, Owen DR, Tyacke RJ, Reynolds R, Rabiner EA, Lingford-Hughes AR et al. Are prescribed benzodiazepines likely to affect the availability of the 18 kDa translocator protein (TSPO) in PET studies? Synapse 2013; 67: 909–912.
Collste K, Forsberg A, Varrone A, Amini N, Aeinehband S, Yakushev I et al. Test–retest reproducibility of [11C]PBR28 binding to TSPO in healthy control subjects. Eur J Nucl Med Mol Imaging 2015; 43: 173–183.
Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD et al. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiatry 2008; 165: 203–213.
Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM et al. The MATRICS consensus cognitive battery, part 2: co-norming and standardization. Am J Psychiatry 2008; 165: 214–220.
Collste K, Forsberg A, Varrone A, Amini N, Aeinehband S, Yakushev I et al. Test–retest reproducibility of [11C]PBR28 binding to TSPO in healthy control subjects. Eur J Nucl Med Mol Imaging 2016; 43: 173–183.
Varrone A, Sjöholm N, Eriksson L, Gulyás B, Halldin C, Farde L . Advancement in PET quantification using 3D-OP-OSEM point spread function reconstruction with the HRRT. Eur J Nucl Med Mol Imaging 2009; 36: 1639–1650.
Schain M, Tóth M, Cselényi Z, Stenkrona P, Halldin C, Farde L et al. Quantification of serotonin transporter availability with [ 11C]MADAM - a comparison between the ECAT HRRT and HR systems. Neuroimage 2012; 60: 800–807.
Kanegawa N, Collste K, Forsberg A, Schain M, Arakawa R, Jucaite A et al. In vivo evidence of a functional association between immune cells in blood and brain in healthy human subjects. Brain Behav Immun 2016; 54: 149–157.
Schain M, Varnäs K, Cselényi Z, Halldin C, Farde L, Varrone A . Evaluation of two automated methods for PET region of interest analysis. Neuroinformatics 2014; 12: 551–562.
Rizzo G, Veronese M, Tonietto M, Zanotti-Fregonara P, Turkheimer FE, Bertoldo A . Kinetic modeling without accounting for the vascular component impairs the quantification of [(11)C]PBR28 brain PET data. J Cereb Blood Flow Metab 2014; 34: 1060–1069.
Owen DR, Guo Q, Kalk NJ, Colasanti A, Kalogiannopoulou D, Dimber R et al. Determination of [(11)C]PBR28 binding potential in vivo: a first human TSPO blocking study. J Cereb Blood Flow Metab 2014; 34: 989–994.
Doble A, Malgouris C, Daniel M, Daniel N, Imbault F, Basbaum A et al. Labelling of peripheral-type benzodiazepine binding sites in human brain with [3H]PK 11195: anatomical and subcellular distribution. Brain Res Bull 1987; 18: 49–61.
Lyoo CH, Ikawa M, Liow J-S, Zoghbi SS, Morse C, Pike VW et al. Cerebellum can serve as a pseudo-reference region in Alzheimer’s disease to detect neuroinflammation measured with PET radioligand binding to translocator protein(TSPO). J Nucl Med 2015; 56: 701–707.
Narendran R, Frankle WG . Comment on analyses and conclusions of ‘microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [11C]PBR28 PET brain imaging study’. Am J Psychiatry 2016; 173: 536–537.
Bloomfield PS, Howes OD, Turkheimer F, Selvaraj S, Veronese M . Response to Narendran and Frankle: the Interpretation of PET Microglial Imaging in Schizophrenia. Am J Psychiatry 2016; 173: 537–538.
Laskaris LE, Di Biase MA, Everall I, Chana G, Christopoulos A, Skafidas E et al. Microglial activation and progressive brain changes in schizophrenia. Br J Pharmacol 2015; 173: 666–680.
Kreisl WC, Jenko KJ, Hines CS, Hyoung Lyoo C, Corona W, Morse CL et al. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab 2013; 33: 53–58.
Kurumaji A, Wakai T, Toru M . Decreases in peripheral-type benzodiazepine receptors in postmortem brains of chronic schizophrenics. J Neural Transm 1997; 104: 1361–1370.
Hohlfeld R, Kerschensteiner M, Meinl E . Dual role of inflammation in CNS disease. Neurology 2007; 68: S58–63–6.
Kreisl WC, Jenko KJ, Hines CS, Hyoung Lyoo C, Corona W, Morse CL et al. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab 2013; 33: 53–58.
Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N et al. Schizophrenia risk from complex variation of complement component 4. Nature 2016; 530: 177–183.
Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci 2014; 17: 400–406.
Cannon TD . How schizophrenia develops: cognitive and brain mechanisms underlying onset of psychosis. Trends Cogn Sci 2015; 19: 744–756.
Acknowledgements
The Swedish Research Council (09114 (LFA); 523-2014-3467 (SC); 2009-7053; 2013-2838 (SE)), Stockholm County Council (ALF; LFA, LF, SC), Swedish Society of Medicine (SLS-332411(SC)), PRIMA Barn-och Vuxenpsykiatri AB (KC), Torsten Söderbergs Stiftelse, Söderström Königska fonden, the European Union’s Seventh Framework Programme (FP7/2007–2013) under grant agreement no. HEALTH-F2-2011-278850 (INMIND; CH), Centre for Psychiatry Research (HFB). We thank Joachim Eckerström, Martin Szabo and other personnel of KaSP for their help with recruitment of subjects, as well as all members of the PET group at the Karolinska Institutet for their close assistance during this study. We also express our gratitude toward the patients and the healthy volunteers for their participation.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
LFa is an employee of AstraZeneca and affiliated with KI. SC has received grant support from AstraZeneca as a co-investigator, and has served as a one-off speaker for Roche and Otsuka Pharmaceuticals. SE has received grant support from AstraZeneca as the principal investigator, has served as a one-off speaker for Roche pharmaceuticals and participated in workshops organized by Otsuka Pharmaceuticals. The remaining authors declare no conflict of interest.
Additional information
Karolinska Schizophrenia Project (KaSP) consortium
Supplementary Information accompanies the paper on the Molecular Psychiatry website .
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Collste, K., Plavén-Sigray, P., Fatouros-Bergman, H. et al. Lower levels of the glial cell marker TSPO in drug-naive first-episode psychosis patients as measured using PET and [11C]PBR28. Mol Psychiatry 22, 850–856 (2017). https://doi.org/10.1038/mp.2016.247
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2016.247
- Springer Nature Limited