Abstract
Chimeric antigen receptors (CARs) have been used to redirect the specificity of autologous T cells against leukemia and lymphoma with promising clinical results. Extending this approach to allogeneic T cells is problematic as they carry a significant risk of graft-versus-host disease (GVHD). Natural killer (NK) cells are highly cytotoxic effectors, killing their targets in a non-antigen-specific manner without causing GVHD. Cord blood (CB) offers an attractive, allogeneic, off-the-self source of NK cells for immunotherapy. We transduced CB-derived NK cells with a retroviral vector incorporating the genes for CAR-CD19, IL-15 and inducible caspase-9-based suicide gene (iC9), and demonstrated efficient killing of CD19-expressing cell lines and primary leukemia cells in vitro, with marked prolongation of survival in a xenograft Raji lymphoma murine model. Interleukin-15 (IL-15) production by the transduced CB-NK cells critically improved their function. Moreover, iC9/CAR.19/IL-15 CB-NK cells were readily eliminated upon pharmacologic activation of the iC9 suicide gene. In conclusion, we have developed a novel approach to immunotherapy using engineered CB-derived NK cells, which are easy to produce, exhibit striking efficacy and incorporate safety measures to limit toxicity. This approach should greatly improve the logistics of delivering this therapy to large numbers of patients, a major limitation to current CAR-T-cell therapies.
Similar content being viewed by others
Introduction
Chimeric antigen receptors (CARs) that redirect the specificity of autologous T cells against lymphoid malignancies have produced striking clinical results.1, 2, 3, 4, 5, 6 Nonetheless, CAR-modified T cells have a number of limitations. The generation of an autologous product for each individual patient is logistically cumbersome and restrictive for widespread clinical use. The manufacturing of CAR-T cells often takes several weeks, making it impractical for patients with rapidly advancing disease. Furthermore, it is not always possible to generate clinically relevant doses of CAR-T cells from heavily pre-treated, often lymphopenic patients. A previously collected allogeneic product could overcome these limitations; however, allogeneic T cells (even if human leukocyte antigen (HLA)-matched) carry a risk of graft-versus-host disease,7 mediated through their native αβ T-cell receptor.
Natural killer (NK) cells provide an attractive alternative to T cells for CAR engineering. NK cells do not cause graft-versus-host disease,8, 9 and thus open opportunities to produce an off-the-shelf product for immediate clinical use. Moreover, as engineered NK cells should also retain their full array of native receptors, they have the potential to exert cytotoxicity10 through mechanisms other than that dictated by the specificity of the CAR, which in principle could reduce the risk of relapse mediated by loss of CAR-targeted antigen, as reported for CAR-T-cell therapy.11
Functional NK cells can be derived from several sources.9, 12, 13 Autologous NK cells can be reproducibly generated in vitro, but have limited activity against autologous tumors,14, 15 which may not be overcome by CAR engineering. Cord blood (CB) is a readily available source of allogeneic NK cells with clear advantages. CB is available as an off-the-shelf frozen product, an advantage that has been bolstered by methods to generate large numbers of highly functional NK cells from frozen CB units ex vivo.16 The generation of CAR-transduced NK cells from frozen CB units stored in large global CB bank inventories holds promise for widespread scalability that cannot be replicated with individual adult donors who require screening and leukapheresis. However, a major disadvantage of NK cells is their lack of persistence after adoptive transfer in the absence of cytokine support.17 Finally, CAR-engineered NK cells may also exert potentially serious toxicity, such as cytokine release syndrome or off-tumor/on-target toxicity, as reported with CAR-T cells.18
Here we present a novel approach to the generation of CAR-CD19+ NK cells that we believe addresses the limitations described above. We genetically modified NK cells with a retroviral vector (iC9/CAR.19/IL-15) that (i) incorporates the gene for CAR.19 to redirect their specificity; (ii) ectopically produces interleukin (IL)-15, to support their survival and proliferation19, 20 and (iii) expresses a suicide gene, inducible caspase-9 (iC9), that can be pharmacologically activated to eliminate transduced cells.21 We investigated whether these genetic modifications would enable CB-NK cells to persist in sufficient numbers to effectively kill B-cell malignancies.
Materials and methods
Cell lines
K562-based feeder cells expressing membrane-bound IL-21 and CD137-ligand (Clone 9.mbIL21)16 were generously provided by Laurence Cooper, MD Anderson Cancer Center (MDACC). Clone 9.mbIL21 cells co-express CD64/FcγRI, CD86/B7-2, CD137L/4-1BBL, truncated CD19 and membrane-bound IL-21 was recently reported to promote peripheral blood and CB-NK cell expansion.16, 22 Raji (Burkitt lymphoma cell line) and K562 (erythroleukemia cell line) were purchased from American Type Culture Collection (Manassa, VA, USA).
Patient details
Primary chronic lymphocytic leukemia (CLL) cells from six patients were used for in vitro studies of NK-CAR cytotoxicity. Patient characteristics are summarized in Supplementary Table 1.
Plasmid construction and retrovirus production
The retroviral vectors encoding iC9.CAR19.CD28-zeta-2A-IL-15 and firefly luciferase have been described.19, 23 Transient retroviral supernatants were produced as previously described.23
Generation of CAR-modified NK cells
CB units for research were provided by the MDACC CB Bank and peripheral blood mononuclear cells were collected from CLL patients following informed consent, under the institutional review board-approved protocols. CB and peripheral blood mononuclear cells were isolated by a density-gradient technique (Ficoll-Histopaque; Sigma, St Louis, MO, USA). CD56+ NK cells, purified with an NK isolation kit (Miltenyi Biotec, Inc., San Diego, CA, USA), were stimulated with irradiated (100 Gy) Clone 9 (2:1 feeder cell:NK ratio) and recombinant human IL-2 (Proleukin, 200 U/ml; Chiron, Emeryville, CA, USA) in complete serum-free stem cell growth medium) (CellGenix GmbH, Freiburg, Germany) on day 0. Activated NK cells were transduced with retroviral supernatants on day +4 in human fibronectin-coated plates (Clontech Laboratories, Inc., Mountain View, CA, USA). Five days later (day +9), NK cells were stimulated again with irradiated Clone 9 and IL-2. On day +14, CAR-transduced NK cells were collected for use.
CAR expression and immunophenotype of transduced cells
Transduced CB-NK cells were stained with Alexa-Fluor647 affinity-purified F(ab')2 fragment goat anti-human IgG (H+L) antibody (CAR Ab) (Jackson ImmunoResearch, West Grove, PA, USA) for CAR expression (see Supplementary Material for details of antibodies used to phenotype NK cells).
IL-15 cytokine secretion
IL-15 production was measured with the human IL-15 Quantikine ELISA kit (R&D, Minneapolis, MN, USA) following the manufacturer’s instructions.
Intracellular cytokine production
On day 14 of culture, control ex vivo-expanded non-transduced (NT) and CAR-transduced CB-NK cells (0.25 × 106 cells/well) were cocultured for 5 h in 96-well plates with purified CLL cells, Raji cells or K562 targets (positive control) at an effector:tumor cell ratio (E:T) of 5:1. CD107a degranulation and intracellular cytokine production were measured as previously described14 (see Supplementary Material for assay details).
NK cell proliferation and cytotoxicity assays
To evaluate for autonomous NK cell growth, we maintained control ex vivo-expanded NT and iC9/CAR.19/IL-15+ NK cells in serum-free stem cell growth medium without stimulation or addition of exogenous cytokines. Cells were cultured for 42 days and counted using trypan blue exclusion every 3 days.
To assess cytotoxicity, CAR-transduced and ex vivo-expanded NT NK cells were cocultured with 51Cr-labeled CLL, Raji and K562 targets (positive control) at multiple E:T ratios; cytotoxicity was measured by 51Cr release as previously described,14 the findings are reported as specific lysis relative to K562 targets.24 For HLA-blocking experiments, the anti-HLA-ABC clone W6/32 (Biolegend, San Diego, CA, USA) was used.
Confocal microscopy and measurement of microtubule-organizing center polarization
Conjugates were imaged by sequential scanning with a Leica TCS SP8 (Buffalo Grove, IL, USA) laser scanning confocal microscope as previously described.25 Details are included in the Supplementary Material.
Xenogeneic lymphoma models
To assess the persistence and antitumor effect of CAR-transduced CB-NK cells in vivo, we used a NOD/SCID IL-2Rγnull (NSG) xenograft model, with the aggressive NK-resistant Raji cell line. Mouse experiments were performed in accordance with NIH recommendations under protocols approved by the Institutional Animal Care and Use Committee.
Antitumor effect of CAR-transduced CB-NK cells
NSG mice (10–12 weeks old; Jackson Laboratories, Bar Harbor, ME, USA) were irradiated with 300 cGy and inoculated intravenously with firefly luciferase-labeled Raji cells (2 × 105) on day 0. Where indicated, 10 × 106 fresh, artificial antigen-presenting cells-expanded NT or CAR-transduced CB-NK cells were injected through the tail vein on days 0 and 7. Mice were subjected to weekly bioluminescence imaging (Xenogen-IVIS 200 Imaging system; Caliper, Waltham, MA, USA). Signal quantitation in photons/second was performed by determining the photon flux rate within standardized regions of interest using Living Image software (Caliper).16 In selected experiments, mice received ex vivo-expanded NT NK cells or CB-NK cells transduced with CAR.19 (lacking IL-15 in the construct) plus low-dose recombinant human IL-15 (Miltenyi Biotech) intraperitoneally at the dose of 0.5 μg/mouse (that is, 2.500 units/mouse) on the day of NK cell infusion and thereafter every 2–3 days for 2 weeks, following established protocols.26
Trafficking, persistence and expansion of transduced vs NT NK cells were measured by flow cytometry.
Activation of suicide gene in vitro and validation in vivo
The small-molecule dimerizer AP1903 (10 nm), generously provided by Bellicum Pharmaceuticals, Inc. (Houston, TX, USA), was added to CB-NK cell cultures for 4 h. The elimination of transduced cells was evaluated by Annexin-V/7-AAD staining. The efficacy of the suicide gene was tested in vivo by treating tumor-bearing mice that had received iC9/CAR.19/IL-15+ NK cells with two doses of AP1903 (50 μg each) intraperitoneally, 2 days apart.19
Karyotyping and single-nucleotide polymorphism microarray analysis
Standard karyotyping and single-nucleotide polymorphism analysis were performed in the MDACC Cytogenetics Laboratory (see Supplementary Material for details).
Pathologic analysis
Mice were killed and necropsied 10 months after the first treatment, at the age of 13 months. Blood samples were collected immediately after euthanasia and analyzed with Advia 120 (Malvern, PA, USA). Formalin-fixed tissues were embedded in paraffin blocks, cut into 4 μm-thick sections, hematoxyin and eosin-stained and examined microscopically by a trained pathologist.
Statistical analysis
Student’s t-test was used to compare quantitative differences (mean±s.d.) between samples; P-values were two-sided and P<0.05 was considered significant. For all bioluminescence experiments, intensity signals were summarized as means±s.d. at baseline and at multiple subsequent time points for each group of mice.16 Probabilities of survival were calculated using the Kaplan–Meier method.
Results
CB-NK cells can be stably transduced with a retroviral vector to express iC9/CAR.CD19/IL-15
Two million NK cells were isolated from banked CB units and cultured with Clone 9 and IL-2 for 14 days (NT control) or transduced on day +4 with a retroviral vector expressing iC9/CAR.19/IL-15 and cultured for an additional 10 days (see Materials and Methods). NK cell viability on day 4 after transduction was ⩾95% in all cases. The median CAR-NK transduction efficiency on day 14 of culture was 66.6% (range, 47.8–87.4%; n=18; Supplementary Figure 1A). Table 1 summarizes data on fold expansion and absolute CAR-NK counts from five different CB units. After 14 days of culture, the median NK cell expansion was 2222-fold (range 564–7370).
We studied the stability of CAR expression over time by culturing CAR-transduced CB-NK cells from six different CB units for a total of 7 weeks. CAR expression remained stable over this interval, as determined by flow cytometry every 10–14 days. In addition, there was no significant difference in the expression level of CAR when NK cells were transduced with iC9/CAR.19/IL-15 or CAR19 (lacking IL-15). Representative fluorescence-activated cell sorting plots and a summary of the data are presented in Supplementary Figures 1B and C.
Transduction with iC9/CAR.19/IL-15 enhances CB-NK cytotoxicity against CD19+ tumor targets in vitro
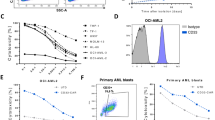
We tested whether engineering CB-NK cells to express iC9/CAR.19/IL-15 enhanced their cytotoxicity against CD19-expressing tumors compared to expanded NT CB-NK cells. iC9/CAR.19/IL-15 CB-NK cells and NT CB-NK cells cultured for 14 days were co-incubated with Raji (n=18; Figure 1a) or primary CLL cells (n=6; Figure 1b) at different E:T ratios and their cytotoxicity was tested using a standard 51Cr-release assay. Across all E:T ratio, CAR-transduced CB-NK cells exerted superior killing of Raji and CLL cells compared to ex vivo-expanded NT NK cells. CAR-transduced NK cells were equally efficient as NT NK cells in killing K562 targets (Figures 1a and b), indicating that the enhanced killing of CD19 targets by the transduced cells is mediated by the CAR receptor and not related to a nonspecific enhancement in NK cytotoxicity.
Antitumor function of CB-NK cells transduced with the iC9/CAR.19/IL-15 vector. (a, b) The cytotoxic activity of iC9/CAR.19/IL-15-transduced CB-NK cells (CAR, solid lines) vs ex vivo-expanded NT CB-NK cells (broken lines), as measured by 51Cr-release assay, against Raji (n=18) (a) and primary CLL cells (n=6) (b). Data are presented as specific lysis relative to K562 targets20 to correct for inter-donor variability in killing. (c) Cytokine production and CD107a degranulation by flow cytometry in gated CAR-positive vs the CAR-negative fraction of the CAR-NK product in response to stimulation with the CD19-expressing Raji cell line or primary CLL cells or K562 targets (as control) in eight independent experiments.
To confirm that the enhanced cytotoxic activity against CD19 targets by CAR-CB-NK cells is derived from the iC9/CAR.19/IL-15-expressing fraction of the product, we measured CD107a degranulation and interferon-γ and tumor necrosis factor-α (TNF-α) response to Raji and CLL targets (n=8). As shown in Figure 1c, the CAR-positive NK cell fraction was the main source of interferon-γ, tumor necrosis factor-α and CD107a in response to CD19+ targets, compared to the CAR-negative fraction, while the CAR-positive and CAR-negative fractions showed similar effector function against K562 cells.
CB-derived iC9/CAR.19/IL-15-transduced NK cells have superior cytotoxicity against primary CLL targets, compared to CLL patient-derived NK cells transduced with the same vector
We generated both NT and iC9/CAR.19/IL-15-transduced NK cells from 2 CLL patients using the methodology described above and tested their ability to lyse autologous CLL cells in three independent experiments. We also compared their cytotoxicity with those of CB-derived iC9/CAR.19/IL-15-transduced and NT NK cells. Expanded NT NK cells from CLL patients and CB were equally poorly cytotoxic against CLL targets (Supplementary Figure 2A). However, whereas CAR-transduced CB-NK cells could efficiently kill CLL cells, expression of the same vector by NK cells from patients only modestly increased their cytotoxicity against autologous CLL targets, suggesting that transduced NK cells from CLL patients will be less-effective immunotherapy than healthy CAR-transduced CB cells. To investigate whether an inhibitory effect of killer immunoglobulin receptor/self-HLA interaction could have impaired the cytotoxicity of patient-derived CAR-NK cells against autologous CLL cells, we repeated the experiments in the presence or absence of HLA class-I blocking (Supplementary Figure 2B). This intervention only partially improved the cytotoxicity of patient-derived CAR-transduced NK cells against autologous targets. CLL cells express high levels of HLA-E (Supplementary Figure 2C), the ligand for the inhibitory receptor NKG2 A. Thus, to determine whether an inhibitory effect of NKG2A/HLA-E interaction could influence patient-derived CAR-NK killing of autologous CLL cells or CB CAR-NK mediated killing of CLL targets, we repeated the experiments in the presence or absence of an NKG2A-blocking antibody (clone Z199, Beckman Coulter, Indianapolis, IN, USA). As shown in Supplementary Figures 2D and E, NKG2A blocking significantly improved the ability of both NT NK and CAR.19-transduced CLL-NK and CB-NK cells to recognize and kill primary CLL cells, without significantly influencing their cytotoxicity against K562 cells (Supplementary Figure 2D). Taken together, these data suggest that multiple mechanisms likely contribute to the relative inability of CAR-transduced patient-derived NK cells to kill autologous targets and that strategies to block NKG2A may further improve the efficacy of this approach.
iC9/CAR.19/IL-15-transduced CB-NK cells form a stronger immunologic synapse with CLL targets compared to CB-NK cells transduced with CAR.CD19 (without IL-15) or patient-derived iC9/CAR.19/IL-15-transduced NK cells
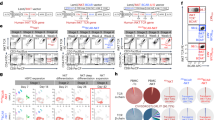
To gain insight into the specific mechanisms by which iC9/CAR.19/IL-15-transduced CB-NK cells mediate superior cytotoxicity and the contribution of IL-15 to this effect, we performed qualitative and quantitative assessments of immunologic synapse (IS) formation in human NK cells. We first asked whether CAR molecules accumulate at the IS between iC9/CAR.19/IL-15-transduced CB-NK cells and CLL targets and whether this recruitment was CD19 antigen-specific. Using confocal microscopy, we observed significantly higher accumulation of CAR molecules at the IS formed between CAR.19/IL-15 CB-NK cells and CLL targets (Figures 2a–c) compared to the diffuse presence of CARs at the IS with K562 targets (Figures 2b and c), indicating that CAR molecules participate in IS formation in a CD19-dependent manner.
Assessments of immunologic synapse formation and function in human iC9/CAR.19/IL-15-transduced CB-NK cells. (a) Confocal microscopy showing representative synapse images of CB-NK cells (transduced with iC9/CAR.19/IL-15) conjugated to primary CLL cells. Conjugates were stained with anti-perforin (green), phalloidin-F-actin (red) and anti-CD19-CAR (yellow). Note formation of immunological synapse (black arrow; left panels). (b, top panel) Confocal representative images (original magnification × 100) demonstrating that CD19-specific CAR on NK cells preferentially accumulates at the CLL (target) cell synapse and not at the K562 (non-target) cell synapse. Cells were imaged in Z stacks covering the entire volume of the immunological synapse. Imaging was performed on a Leica TCS SP8 confocal microscope using a × 100 oil objective. Images were acquired with Imaris software (Bitplane, Belfast, UK). Transmitted light (TL) overlay, single-color anti-CD19 CAR (blue), anti-perforin (red) and an overlay of all stains are shown. (b, bottom panel) Data on the accumulation of CD19-specific CARs at the immunologic synapse between CB-NK cells transduced with iC9/CAR.19/IL-15 vector with CLL cells (CD19-positive target) vs K562 targets (CD19-negative); *P=0.02. (c) iC9/CAR.19/IL-15-transduced CB-NK cells, CAR.19-transduced CB-NK cells (without IL-15) and CLL patient-derived NK cells transduced with iC9/CAR.19/IL-15 were assessed and compared with NT NK cells for their ability to polarize lytic granules and MTOC to CLL targets (left panel) vs K562 cells (right) (measured by distance from the MTOC to the immune synapse). Results from two independent experiments are shown; each data point represents a single immunologic synapse. Cells were imaged as a Z stack on a Leica TCS SP8 laser scanning microscope. Images were acquired with Volocity software (Perkin Elmer, Waltham, PA, USA). The asterisk indicates statistical significance (P<0.05 by Student’s t-test) vs the control or another CAR construct.
Increased polarization of the microtubule-organizing center (MTOC) is an essential step in the final stages of NK cell-mediated cytotoxicity and exocytosis of lytic granules at the IS.27 Thus, we used confocal microscopy to assess the MTOC polarization of ex vivo-expanded NT CB-NK cells, iC9/CAR.19/IL-15+ CB-NK, CLL patient-derived iC9/CAR.19/IL-15+ NK cells and CAR.19-transduced CB-NK (lacking IL-15) in experiments with CLL targets. MTOC polarization was quantified by measuring the distance between the pericentrin-defined MTOC to the IS for at least 15 conjugates, including all four groups of NK cells and CLL targets. MTOC polarization against K562 targets was used as control as NK cells form a ‘natural’ synapse with K562 targets through multiple activating receptors on their surface. As shown in Figure 2c and Supplementary Figure 3, MTOC was significantly closer to the IS in iC9/CAR.19/IL-15-transduced CB-NK cells compared to any of the remaining groups (Figure 2c, left panel). CLL patient-derived iC9/CAR.19/IL-15 NK cells did show improved polarization compared to NT CB-NK cells, but this gain was still significantly less than that seen with CB-NK cells transduced with iC9/CAR.19/IL-15. No differences in MTOC polarization were observed among the NK cell groups in the presence of a non-CD19-presenting K562 target (Figure 2c, right panel). These findings provide a mechanistic basis for the enhanced antitumor activity of iC9/CAR.19/IL-15-transduced CB-NK cells; namely, engagement of CARs on transduced NK cells with CD19 on target cells results in increased polarization of the MTOC, and superior killing.
IL-15 by iC9/CAR.19/IL-15+ CB-NK cells is produced predominantly in response to CD19+ targets and does not induce NK cell anergy
NT CB-NK and iC9/CAR.19/IL-15+ CB-NK lymphocytes expanded for 14 days were cultured with or without CLL cells, and IL-15 release was measured at 24, 48 and 72 h. IL-15 was undetectable in supernatants collected from NT CB-NK cells cultured alone or with CLL targets. By contrast, iC9/CAR.19/IL-15+ CB-NK cells produced small amounts of IL-15 in the absence of antigen stimulation (mean 15.05 pg/ml per 106 cells, range 6.2–23.47), which significantly increased with antigen stimulation (mean 27.61 pg/ml per 106 cells, range 15.82–38.18; Figure 3a), in keeping with enhanced proliferation of iC9/CAR.19/IL-15+ CB-NK cells in response to CLL cells in culture (Supplementary Figure 4).
IL-15 production and phenotype of iC9/CAR.19/IL-15-transduced CB-NK cells. (a) IL-15 production by ex vivo-expanded NT NK cells or CAR-transduced NK cells cultured in the presence or absence of CLL targets for 24, 48 or 72 h in four independent experiments. (b) NK cell phenotype based on the average expression of 25 markers, including NK cell receptors, transcription factors, adaptor molecules, homing receptors and markers of exhaustion, in triplicate experiments. Mean fluoresence intensity or the percentages of positive cells were submitted to a hierarchical clustering program to generate a global view of marker expression in iC9/CAR.19/IL-15-transduced CB-NK vs NT CB-NK cells vs resting CB-NK cells (non-expanded; n=3 independent NK expansion and transduction experiments using different CB units). (c) Proliferative capacity of CAR-transduced vs NT CB-NK expansion in response to in vitro stimulation with clone 9 and IL-2 (200 iU/ml; starting from 2 × 106 CB-NK cells; N=5).
To investigate the potential of IL-15 to induce NK anergy, we comprehensively characterized the NK cell phenotype, including expression of activating and inhibitory receptors, exhaustion markers, chemokine receptors and transcription factors on expanded NK products by multiparameter flow cytometry. The heatmap in Figure 3b summarizes the average expression levels of markers from three independent CB-NK expansion and transduction experiments. Compared to resting CB-NK cells (before expansion), ex vivo expansion drove the maturation of both NT NK and iC9/CAR.19/IL-15+ as evidenced by expression of CD16 and killer immunoglobulin receptor (Figure 3b; Supplementary Figure 5) with no selectivity in the subsets of NK cell transduced with the CAR vector. iC9/CAR.19/IL-15+ CB-NK cells expanded for 2 weeks showed no signs of exhaustion, such as downregulation of eomesodermin and T-bet,28 or upregulation of KLRG1, and in fact exhibited a phenotype similar to that of ex vivo-expanded NT NK cells. Moreover, in contrast to a previous report in murine NK cells that sustained stimulation with IL-15/IL-15 R-α complexes induces dysfunction,29 human iC9/CAR.19/IL-15-transduced CB-NK cells proliferated as efficiently as NT CB-NK cells in culture and followed a similar kinetic of in vitro expansion (Figure 3c).
iC9/CAR.19/IL-15-tranduced CB-NK cells exert enhanced antitumor activity in vivo
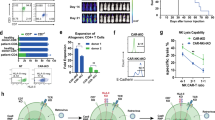
We used our Raji xenograft model to study the in vivo antitumor activity of iC9/CAR.19/IL-15-transduced CB-NK cells and the contribution of IL-15 to this effect. Mice received one intravenous infusion (10 × 106/mouse) of control ex vivo-expanded NT CB-NK cells, iC9/CAR.19/IL-15-transduced CB-NK cells or CAR.19 CB-NK cells (lacking IL-15 in the construct; 5 mice per group). Tumor growth was monitored by measuring changes in tumor bioluminescence over time. Tumor bioluminescence increased rapidly in mice treated with control NT CB-NK cells (Figures 4a and b). By contrast, infusion of either CAR.19+ or iC9/CAR.19/IL-15+ CB-NK cells led to improved tumor control and significant prolongation of survival compared to NT CB-NK (P=0.006 and 0.001, respectively; Figure 4c). Notably, iC9/CAR.19/IL-15+ CB-NK cells controlled tumor expansion (Figure 4a) and prolonged survival (Figure 4c) better than the CB-NK cells transduced with CAR.CD19 without IL-15 (P=0.044), underscoring the vital contribution of IL-15 to enhanced antitumor activity. We also asked whether intraperitoneal administration of low doses of recombinant human IL-15 could support the survival potential and antitumor efficacy of CAR.19 CB-NK cells (lacking IL-15 in the construct). A dose of 0.5 μg/mouse (that is, 2.500 units/mouse) administered on the day of NK cell infusion and every 2–3 days thereafter resulted in significant expansion of CAR.19 NK cells and control of tumor progression (Figures 4g and h; Supplementary Figures 6A and B); however, it was associated with significant toxicity, and early mortality and treatment-related mortality (defined as death before day 21) when compared with CAR19/IL-15 NK cells (P=0.038).
In vivo homing, proliferation and antitumor activity of iC9/CAR.19/IL-15-transduced CB-NK cells in NSG Raji mouse model. (a) Bioluminescence imaging was used to monitor the growth of firefly luciferase-labeled Raji tumor cells in NSG mice. The plot summarizes the bioluminescence data from four groups of mice treated with Raji alone, or Raji plus one dose (10 × 106) of iC9/CAR.19/IL-15 CB-NK cells, CAR.19 (no IL-15) CB-NK cells or ex vivo-expanded NT CB-NK cells (5 mice per group). (b) Bioluminescence imaging (BLI) figures of the experiments described in a. Colors indicate intensity of luminescence (red, highest; blue, lowest). (c) Kaplan–Meier plots showing the probability of survival for the four groups of mice described in a (5 mice per group). Mice treated with a single dose of 10 × 106 iC9/CAR.19/IL-15-transduced CB-NK cells (blue line) had significantly better survival than mice receiving CB-NK cells that were either not transduced (green line; P=0.001) or transduced with a CAR.CD19 construct lacking IL-15 (pink line; P=0.044). (d) Kaplan–Meier plots of NSG mice treated with Raji cells alone (n=9) or Raji plus two doses (10 × 106 each, 5–7 days apart) of iC9/CAR.19/IL-15 CB-NK cells (n=10), CAR.19 (no IL-15) CB-NK cells(n=5) or ex vivo-expanded NT CB-NK cells (n=9). (e) NSG mice were treated with Raji cells alone or Raji plus two doses (10 × 106 each, 5–7 days apart) of iC9/CAR.19/IL-15 CB-NK cells, CAR.19 (no IL-15) CB-NK cells or NT CB-NK cells (n=5 mice per group). Mice were killed on day +21 post infusion and peripheral blood, bone marrow (BM), liver and spleen were collected and analyzed by flow cytometry for expression of human (h)CD45, hCD19, hCD56 and CAR. Representative fluorescence-activated cell sorting (FACS) plots and pooled data (including medians and interquartile ranges—75th–25th percentiles) are presented. (f) Mice that received Raji cells plus two doses (10 × 106 each, 5–7 days apart) of iC9/CAR.19/IL-15 CB-NK cells were monitored over time by weekly blood collection for expansion of CAR-expressing NK cells. Serial measurement of CAR-expressing NK cells in the peripheral blood of mice by flow cytometry. Representative FACS plots and pooled data (including medians and interquartile ranges—75th–25th percentiles) are presented. (g, h) Comparison of Raji tumor control as measured by BLI imaging (g), and survival (h) of NSG mice receiving Raji alone, Raji plus one dose (10 × 106) of iC9/CAR.19/IL-15 CB-NK cells or Raji plus CAR.19+ CB-NK cells (lacking IL-15 in the construct) plus or minus exogenous low-dose recombinant human IL-15 (Miltenyi Biotech) administered intraperitoneally at a dose of 0.5 μg/mouse (that is, 2.500 units/mouse) on the day of NK cell infusion and thereafter every 2–3 days for 2 weeks (n=5 mice per group).
We next asked whether increasing the dose of CB-NK cells could enhance antitumor activity by administering two intravenous infusions (10 × 106 cells each, 5–7 days apart) of control ex vivo-expanded NT CB-NK cells or CAR.19 CB-NK cells (lacking IL-15) or iC9/CAR.19/IL-15+ NK cells (5–10 mice per group). None of the mice receiving iC9/CAR.19/IL-15+ CB-NK cells died of lymphoma (Figure 4d). However, four mice died on days 11, 12, 14 and 16 after infusion of the cells from complications related to the release of inflammatory cytokines, including high levels of tumor necrosis factor-α (median 999.2 pg/ml), IL-1β (median 1271.4 pg/ml) and IL-18 (5570 pg/ml) detected in serum at the time of death. These data indicate that activated NK cells transduced with iC9/CAR.19/IL-15 may also cause cytokine release syndrome similar to that of CAR-T cells, with the potential to cause toxicity in humans.
IL-15 enhances the proliferation, persistence and homing of CAR.CD19-transduced CB-NK cells in a xenograft NSG mouse model of Raji lymphoma
To examine the contribution of IL-15 to the proliferation, persistence and homing of CAR-CB-NK cells, NSG mice engrafted with Raji lymphoma were treated with two intravenous infusions (10 × 106 cells each, 5–7 days apart) of NT CB-NK cells (control), CB-NK cells transduced with iC9/CAR.19/IL-15 or CAR.19 (lacking IL-15) as described in Materials and Methods (five mice per group). On day +21 post-NK infusion, mice were killed. High frequencies of CAR-expressing NK cell were identified in blood, bone marrow, liver and spleen of mice treated with iC9/CAR.19/IL-15 (Figure 4e), indicating proliferation and successful homing of CAR-NK cells to sites of disease. Notably, there was no evidence of human CD19+ cells in any of the organs examined, consistent with efficient control of tumor by the iC9/CAR.19/IL-15 CB-NK cells. By contrast, in mice treated with either CAR.19 CB-NK cells (lacking IL-15) or ex vivo-expanded NT CB-NK cells, proliferation or homing to sites of disease was more limited. Furthermore, CD19+ tumor cells were detected at high frequencies in blood and organs of the mice, suggesting that CAR.19 CB-NK cells lacking IL-15 are capable of controlling the tumor for only a short period of time. Moreover, in mice receiving two infusions of iC9/CAR.19/IL-15+ CB-NK cells, the cells expanded over time and could be detected up to 68 days post infusion, after which their numbers receded (Figure 4f). These data indicate that IL-15 in the CAR construct endows CB-NK cells with the capacity to proliferate and persist in vivo.
iC9/CAR.19/IL-15-transduced CB-NK cells do not show signs of dysregulated growth either in vitro or in vivo
To investigate the possibility that the IL-15 gene in the vector may result in autonomous or dysregulated growth of transduced CB-NK cells, we cultured iC9/CAR.19/IL-15-transduced CB-NK cells in media without the addition of exogenous IL-2 or clone 9.mbIL21 for 42 days (n=5). Cultured iC9/CAR.19/IL-15-transduced CB-NK cells did not show any signs of abnormal growth over 6 weeks (Figure 5a), after which the cells stopped expanding. Karyotyping and single-nucleotide polymorphism microarray analyses of CAR-transduced NK cells after up to 22 weeks of culture (n=7) did not reveal any chromosomal alterations or evidence of genetic instability (data not shown).
IL-15-transduced CB-NK cells lack signs of autonomous or dysregulated growth. (a) iC9/CAR.19/IL-15-transduced CB-NK and NT CB-NK cells were put in culture without cytokines or exogenous stimulation to assess their growth over time. (b) NSG mice 10 months after treatment with CB-NK cells transduced with iC9/CAR.19/IL-15 or CAR.19 (no IL-15) were killed and examined for evidence of NK dysregulated growth or leukemia/lymphoma. Photomicrographs of mesenteric lymph nodes show vestigial lymphoid tissue with no lymphocytic infiltration. Images of the spleen show rudimentary periarteriolar lymphoid tissue devoid of lymphocytes (black arrows) and is surrounded by hematopoietic tissue comprising of erythroid and myeloid series cells in different stages of development, including megakaryocytes and hemosiderin-laden macrophages. Bone marrow contains normal hematopoietic cells and no abnormal lymphocytes. Hematoxylin and eosin stain, magnification × 200. The micrographs are from two representative groups of NSG mice treated with iC9/CAR.19/IL-15-transduced CB-NK cells.
Nine mice treated with CB-NK cells transduced with iC9/CAR.19/IL-15 (n=5) or CAR.CD19 (lacking IL-15; n=4) were followed for at least 10 months and then killed. The hematologic parameters were within normal ranges (Supplementary Table 2), with no evidence of lymphocytic leukemia in either treatment group (Figure 5b).
iC9/CAR.19/IL-15+ CB-NK cells are eliminated by activation of the suicide gene with a small-molecule dimerizer
To counteract excessive toxicity mediated by the release of inflammatory cytokines by transduced CB-NK cells, we incorporated a suicide gene (iC9) into our construct.21 The addition of as little as 10 nm of the small-molecule dimerizer AP1903 to cultures of iC9/CAR.19/IL-15-transduced CB-NK cells induced apoptosis/necrosis of transduced NK cells within 4 h but had no effect on the viability of NT CB-NK cells (Figures 6a and b). The suicide gene was also effective in vivo. Mice engrafted with Raji tumor received iC9/CAR.19/IL-15-transduced CB-NK cells. Mice were then either treated with the dimerizer or not (n=5 mice per group) and were killed 3 days later. Administration of the small-molecule dimerizer resulted in a striking reduction in iC9/CAR.19/IL-15-transduced CB-NK cells in the blood and tissues of the treated mice (Figure 6c).
Activation of iC9 suicide gene eliminates iC9/CAR.19/IL-15+ CB-NK cells. (a) Addition of 10 nm of AP1903 to cultures of iC9-CAR-IL-15+ CB-NK cells induced apoptosis/necrosis of transgenic cells within 4 h as assessed by Annexin-V-7-AAD staining in four independent experiments. The dimerizer did not induce apoptosis in NT NK cells. (b) A representative fluorescence-activated cell sorting (FACS) plot of the experiment described in a is presented. (c) NSG mice engrafted with Raji cells and infused with iC9/CAR.19/IL-15+ CB-NK cells were treated 10–14 days later with two doses of the AP1903 dimerizer (50 μg) intraperitoneally 2 days apart. FACS plots from a representative experiment measuring the frequencies of CAR-positive NK cells in blood, bone marrow, spleen and liver of animals by flow cytometry are presented.
Discussion
We have developed a novel approach to engineering NK cells with potent antitumor activity by transducing CB-derived NK cells with a retroviral vector encoding a CAR against CD19, IL-15 (a cytokine crucial for NK cell persistence) and the iC9 suicide gene. NK cells transduced with this vector form strong immunologic synapses with CD19-positive targets and effectively kill CD19-expressing leukemia/lymphoma cell lines as well as primary CLL cells. Moreover, when the iC9/CAR.19/IL-15-transduced CB-NK cells were infused into an NSG mouse model of Raji lymphoma, they proliferated rapidly in vivo and homed to sites of disease, where they mediated potent antitumor responses.
We chose CD19 as the target for our studies, as proof of principle, based on the striking clinical efficacy shown by CAR.CD19+ T cells against B-lineage cancers.1, 2, 3, 4, 5, 6 The signaling domain of our construct contains CD28 and CD3ζ. CD3ζ is crucial for both T- and NK cell signaling and activation.30, 31 While CD28 is well recognized as an important costimulatory molecule for T-cell activation,32 its role in NK cell activation is less clear; however, human fetal NK cells and a number of NK cell lines express CD28 and can kill CD80/CD86-expressing tumor targets, supporting a role for CD28 in the activation of NK cells.33, 34, 35 Further, CD28 ligation in NK cells enhances NK cell killing of its target by phosphorylating ERK2.27 In our study, NK cells transduced with a CAR incorporating CD28 showed marked antitumor activity, both in vitro and in vivo, although other costimulatory domains, such as 4-1BB,17 may improve results further.
CAR-NK cells also exert cytotoxicity that is non-CAR.CD19-mediated, as demonstrated by the modest killing of tumor targets by NT NK cells. This could represent an advantage for NK cells over T cells in CAR-driven immunotherapy, as the intrinsic capacity of NK cells to recognize and target tumor cells remains intact, making disease escape through downregulation of the CAR target antigen less likely than it is with CAR-T cells. One could potentially exploit this property by selecting donors for NK-CAR production based on killer immunoglobulin receptor-ligand mismatch with the recipient, or haplotype B killer immunoglobulin receptor gene content, as shown in the setting of stem cell transplantation.8, 13, 36, 37, 38 Using readily accessible CB units and GMP-compliant procedures for robust expansion,19 it is feasible to generate multiple clinical doses of CAR-NK cells from a single CB unit (Table 1).
Mature NK cells have a short lifespan with poor in vivo persistence both in humans and in mice,39, 40 although recent data support the existence of a subset of long-lived memory NK cells in mice41, 42 and adaptive/memory NK cells in humans.43, 44, 45, 46, 47 In vivo persistence of effector cells are crucial for sustained clinical responses.48 We therefore incorporated in our construct the gene encoding IL-15, a cytokine that drives NK cell expansion and persistence.19, 20, 49 This modification led to ectopic production of IL-15, which was predominantly antigen-driven, and to more robust activation of NK cells with enhanced in vivo proliferation, persistence and antitumor activity than that seen with CAR.19-transduced NK cells lacking IL-15. Although the latter could mediate an antitumor response, the effect was only transient, further emphasizing the importance of in vivo persistence of CAR-expressing NK cells for effective and durable antitumor immunity. We also examined whether exogenous administration of IL-15 could support the in vivo proliferation and antitumor activity of CAR19-transduced NK cells, thus, overcoming the requirement to include IL-15 in the construct. However, IL-15, even when administered at a low dose of 0.5 μg/mouse every 2–3 days,26 was associated with significant toxicity when administered in combination with CAR.CD19-transduced CB-NK cells (but not NT NK cells), supporting our strategy to include IL-15 in the construct. It is conceivable that ectopic IL-15 production could lead to abnormal NK cell proliferation or leukemia transformation.50 In the present study only picogram quantities of IL-15 were produced by our CAR-transduced CB-NK cells, without evidence of autonomous growth in vitro or leukemic transformation in vivo.
Severe toxicity, including on-target/off-tumor effects and cytokine release syndrome is a major clinical limitation of CAR-T-cell therapy.51 These concerns may also be relevant to CAR-NK cells. Human NK cells predominantly produce interferon-γ, IL-3 and granulocyte–macrophage colony-stimulating factor,50 which may result in a different pattern and kinetic of cytokine release syndrome. In our study infusion of a higher number of iC9/CAR.19/IL-15-transduced CB-NK cells was associated with a systemic inflammatory response and toxic death in a number of mice. To counteract these potential toxicities, we equipped CAR-modified NK cells with an inducible suicide gene21 and showed that pharmacologic activation of this molecule could rapidly and efficiently eliminate the gene-modified NK cells.
In conclusion, we have developed a novel approach to immunotherapy using engineered CB-derived NK cells. The iC9/CAR.19/IL-15-transduced CB-NK cells are relatively easy to produce, show striking efficacy both in vitro and in vivo, and incorporate safety measures that are designed to limit toxicity. Clinical trials of these CAR-NK cells have begun at our center.
References
Sadelain M, Riviere I, Brentjens R . Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer 2003; 3: 35–45.
Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME . Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 2008; 8: 299–308.
June CH, Blazar BR, Riley JL . Engineering lymphocyte subsets: tools, trials and tribulations. Nat Rev Immunol 2009; 9: 704–716.
Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013; 5: 177ra38.
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013; 368: 1509–1518.
Porter DL, Levine BL, Kalos M, Bagg A, June CH . Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011; 365: 725–733.
Goulmy E . Human minor histocompatibility antigens: new concepts for marrow transplantation and adoptive immunotherapy. Immunol Rev 1997; 157: 125–140.
Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002; 295: 2097–2100.
Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol 2010; 28: 955–959.
Caligiuri MA, Velardi A, Scheinberg DA, Borrello IM . Immunotherapeutic approaches for hematologic malignancies. Hematology Am Soc Hematol Educ Program 2004, 337–353.
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015; 385: 517–528.
Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005; 105: 3051–3057.
Curti A, Ruggeri L, D'Addio A, Bontadini A, Dan E, Motta MR et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood 2011; 118: 3273–3279.
Rouce RH, Shaim H, Sekine T, Weber G, Ballard B, Ku S et al. The TGF-beta/SMAD pathway is an important mechanism for NK cell immune evasion in childhood B-acute lymphoblastic leukemia. Leukemia 2016; 30: 800–811.
Stringaris K, Sekine T, Khoder A, Alsuliman A, Razzaghi B, Sargeant R et al. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica 2014; 99: 836–847.
Shah N, Martin-Antonio B, Yang H, Ku S, Lee DA, Cooper LJ et al. Antigen presenting cell-mediated expansion of human umbilical cord blood yields log-scale expansion of natural killer cells with anti-myeloma activity. PLoS One 2013; 8: e76781.
Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res 2009; 69: 4010–4017.
Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ . Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics 2016; 3: 16011.
Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia 2010; 24: 1160–1170.
Tagaya Y, Bamford RN, DeFilippis AP, Waldmann TA . IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity 1996; 4: 329–336.
Di SA, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 2011; 365: 1673–1683.
Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE 2012; 7: e30264.
Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood 2006; 108: 3890–3897.
Kruse V, Hamann C, Monecke S, Cyganek L, Elsner L, Hubscher D et al. Human induced pluripotent stem cells are targets for allogeneic and autologous natural killer (NK) cells and killing is partly mediated by the activating NK receptor DNAM-1. PLoS ONE 2015; 10: e0125544.
Sanborn KB, Rak GD, Mentlik AN, Banerjee PP, Orange JS . Analysis of the NK cell immunological synapse. Methods Mol Biol 2010; 612: 127–148.
Cany J, van der Waart AB, Tordoir M, Franssen GM, Hangalapura BN, de Vries J et al. Natural killer cells generated from cord blood hematopoietic progenitor cells efficiently target bone marrow-residing human leukemia cells in NOD/SCID/IL2Rg(null) mice. PLoS One 2013; 8: e64384.
Chen X, Allan DS, Krzewski K, Ge B, Kopcow H, Strominger JL . CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells. Proc Natl Acad Sci USA 2006; 103: 10346–10351.
Gill S, Vasey AE, De SA, Baker J, Smith AT, Kohrt HE et al. Rapid development of exhaustion and down-regulation of eomesodermin limit the antitumor activity of adoptively transferred murine natural killer cells. Blood 2012; 119: 5758–5768.
Elpek KG, Rubinstein MP, Bellemare-Pelletier A, Goldrath AW, Turley SJ . Mature natural killer cells with phenotypic and functional alterations accumulate upon sustained stimulation with IL-15/IL-15Ralpha complexes. Proc Natl Acad Sci USA 2010; 107: 21647–21652.
Lanier LL . Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 2008; 9: 495–502.
Lanier LL . On guard—activating NK cell receptors. Nat Immunol 2001; 2: 23–27.
Schwartz RH . Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell 1992; 71: 1065–1068.
Lanier LL, O'Fallon S, Somoza C, Phillips JH, Linsley PS, Okumura K et al. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J Immunol 1995; 154: 97–105.
Azuma M, Cayabyab M, Buck D, Phillips JH, Lanier LL . Involvement of CD28 in MHC-unrestricted cytotoxicity mediated by a human natural killer leukemia cell line. J Immunol 1992; 149: 1115–1123.
Galea-Lauri J, Darling D, Gan SU, Krivochtchapov L, Kuiper M, Gaken J et al. Expression of a variant of CD28 on a subpopulation of human NK cells: implications for B7-mediated stimulation of NK cells. J Immunol 1999; 163: 62–70.
Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Le CT et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood 2010; 116: 2411–2419.
Cooley S, Trachtenberg E, Bergemann TL, Saeteurn K, Klein J, Le CT et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood 2009; 113: 726–732.
Sekine T, Marin D, Cao K, Li L, Mehta P, Shaim H et al. Specific combinations of donor and recipient KIR-HLA genotypes predict for large differences in outcome after cord blood transplantation. Blood 2016; 128: 297–312.
Wang JW, Howson JM, Ghansah T, Desponts C, Ninos JM, May SL et al. Influence of SHIP on the NK repertoire and allogeneic bone marrow transplantation. Science 2002; 295: 2094–2097.
Zhang Y, Wallace DL, de Lara CM, Ghattas H, Asquith B, Worth A et al. in vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology 2007; 121: 258–265.
Sun JC, Beilke JN, Lanier LL . Adaptive immune features of natural killer cells. Nature 2009; 457: 557–561.
Sun JC, Beilke JN, Bezman NA, Lanier LL . Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J Exp Med 2011; 208: 357–368.
Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood 2010; 116: 3865–3874.
Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK et al. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol 2012; 189: 5082–5088.
Sun JC, Lopez-Verges S, Kim CC, DeRisi JL, Lanier LL . NK cells and immune ‘memory’. J Immunol 2011; 186: 1891–1897.
Corat MA, Schlums H, Wu C, Theorell J, Espinoza DA, Sellers SE et al. Acquired somatic mutations in PNH reveal long-term maintenance of adaptive NK cells independent of HSPCs. Blood 2017; 129: 1940–1946.
Tesi B, Davidsson J, Voss M, Rahikkala E, Holmes TD, Chiang SCC et al. Gain-of-function SAMD9L mutations cause a syndrome of cytopenia, immunodeficiency, MDS, and neurological symptoms. Blood 2017; 129: 2266–2279.
Dudley ME, Rosenberg SA . Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer 2003; 3: 666–675.
Sahm C, Schonfeld K, Wels WS . Expression of IL-15 in NK cells results in rapid enrichment and selective cytotoxicity of gene-modified effectors that carry a tumor-specific antigen receptor. Cancer Immunol Immunother 2012; 61: 1451–1461.
Mishra A, Liu S, Sams GH, Curphey DP, Santhanam R, Rush LJ et al. Aberrant overexpression of IL-15 initiates large granular lymphocyte leukemia through chromosomal instability and DNA hypermethylation. Cancer Cell 2012; 22: 645–655.
Nellan A, Lee DW . Paving the road ahead for CD19 CAR T-cell therapy. Curr Opin Hematol 2015; 22: 516–520.
Acknowledgements
This work was funded in part by LLS 6470-15, ACS RSG-15-218-01-LIB and the generous philanthropic contributions to The University of Texas MD Anderson Moon Shots Program. The flow studies were performed in the Flow Cytometry & Cellular Imaging Facility, which is supported in part by the National Institutes of Health through MD Anderson Cancer Center Support Grant CA016672.
Author contributions
EL designed and performed experiments, interpreted the data and wrote the manuscript, YT, MaM, HS, XL, AR, MG, LL, MHB, XW, RC, RB and PB performed experiments and commented on the manuscript. GP, BS, MuM, JO, MK, DM, WW, RC and EJS provided advice on experiments and commented on the manuscript. KR designed and directed the study and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Liu, E., Tong, Y., Dotti, G. et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 32, 520–531 (2018). https://doi.org/10.1038/leu.2017.226
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.226
- Springer Nature Limited
This article is cited by
-
Current and future immunotherapeutic approaches in pancreatic cancer treatment
Journal of Hematology & Oncology (2024)
-
Enhanced cellular therapy: revolutionizing adoptive cellular therapy
Experimental Hematology & Oncology (2024)
-
Proteasome inhibition enhances the anti-leukemic efficacy of chimeric antigen receptor (CAR) expressing NK cells against acute myeloid leukemia
Journal of Hematology & Oncology (2024)
-
Beyond CAR-T: The rise of CAR-NK cell therapy in asthma immunotherapy
Journal of Translational Medicine (2024)
-
Natural killer cell therapies
Nature (2024)