Abstract
Translocation (11;14) on interphase fluorescent in situ hybridization in plasma cells is regarded as a standard risk prognostic marker in multiple myeloma based on studies conducted before introduction of current therapies. We identified 365 patients with t(11;14), and 730 matched controls:132 patients with non-(11;14) translocations and 598 patients with no chromosomal translocation. The median progression-free survival for the three groups were 23.0 (95% confidence interval (CI), 20.8–27.6), 19.0 (95% CI, 15.8–22.7) and 28.3 (95% CI, 25.7–30.6) months, respectively (P<0.01). The median overall survival (OS) for t(11;14), non-(11;14) translocation and no-translocation groups were 74.4 (95% CI, 64.8–89.3), 49.8 (95% CI, 40.0–60.6) and 103.6 (95% CI, 85.2–112.3) months, respectively (P<0.01). Excluding those with 17p abnormality, the median OS in the three groups were 81.7 (95% CI, 67.0–90.7), 58.2 (95% CI, 47.0–76.4) and 108.3 (95% CI, 92.4–140.1) months, respectively (P<0.01). The above relationship held true in patients with age <65 years, international staging system (ISS) I/II stage or those who received novel agent-based induction. Advanced age (hazard ratio (HR): 1.98), 17p abnormality (HR: 2.2) and ISS III stage (HR: 1.59) at diagnosis predicted reduced OS in patients with t(11;14). These results suggest that outcomes of t(11;14) MM are inferior to other standard risk patients.
Similar content being viewed by others
Introduction
Multiple myeloma (MM) is a plasma cell (PC) malignancy characterized by well-defined genetic abnormalities, which are major drivers of the outcomes.1 Interphase fluorescent in situ hybridization (FISH) of bone marrow PCs identifies one or more abnormalities in virtually all patients with MM. These are classified into primary abnormalities (translocations and trisomies), which occur early in the evolution of PC dyscrasias, and secondary abnormalities (deletion of 17p, 1q gain), which occur later. Recurrent translocations involving the immunoglobulin heavy chain region include t(11;14), t(4;14), t(14;16), t(14;20) and t(6;14), with t(11;14) being the most common, having an estimated prevalence of 16–24%.2, 3, 4, 5, 6 Translocation (11;14) has been associated with a characteristic lymphoplasmacytic morphology, light chain (LC) MM and rarer variants of MM (IgD, IgM and non-secretory forms), and expression of CD20 on the surface of PCs.2, 7, 8, 9, 10
Increased use of high-dose therapy with autologous stem cell transplant (ASCT) and the introduction of novel agents including immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs) have improved the survival in MM.11, 12 Patients with t(11;14) have traditionally been classified as having standard risk MM, based on studies conducted before novel agents were available. Recent observations suggest that patients with t(11;14) may have unfavorable outcomes when compared with other standard risk patients, casting doubt on the traditional view.13, 14, 15 In this context, we retrospectively reviewed our patient database to assess the survival outcomes of t(11;14) MM patients in the current era.
Patients and methods
Patients
We identified 372 patients with MM diagnosed between January 2004 and November 2014 with t(11;14) detected on FISH before or within 12 months of starting treatment for MM. We excluded patients who had MM with an amyloid related systemic syndrome, or PC leukemia diagnosed before the index FISH. FISH done before starting treatment was used whenever available, and in the remaining patients the earliest FISH report within 12 months of starting therapy was used. After excluding seven patients for whom details of induction therapy were not available, 365 patients with t(11;14) (t(11;14) group) were included in the analysis. To assess the impact of t(11;14) on outcomes, we identified two MM patients matched for age and year of diagnosis, for each patient with t(11;14) from our database applying the same exclusion and inclusion criteria as the study group. The control group could have normal cytogenetics or any abnormality other than t(11;14) detected by FISH. We further subdivided the non-t(11;14) group into those with a defined non-(11;14) translocation such as t(4;14), t(6;14), t(14;16) or t(14;20) (non-(11;14) translocation group) (n=132) and those without any defined translocation (no-translocation group) (n=598). The Mayo Clinic Institutional Review Board approved the study, and it was conducted in accordance with the Declaration of Helsinki and the Health Insurance Portability and Accountability Act guidelines of 1996.
Data regarding demographic characteristics, pre-treatment laboratory parameters, treatment administered, response to induction, disease progression and survival status at final data cutoff were collected from our database and by review of the patients’ electronic medical records. The cutoff date for follow-up was 31st January 2017. Diagnosis of MM was made based on the 2003 International Myeloma Working Group criteria, which was in use during the defined period.16 We risk stratified the patients at diagnosis according to the international staging system (ISS), where the required laboratory parameters were available.1 We assessed the response to treatment and disease progression using the International Myeloma Working Group uniform response criteria.17, 18 We used the designation of ‘early SCT’ when a patient received SCT within 12 months of starting treatment for MM.
Outcome measures
Progression-free survival (PFS), time to next treatment (TTNT) and overall survival (OS) were the primary outcome measures.19 PFS was defined as the duration from the initiation of treatment to first progression or death, whichever was earlier. TTNT was defined as the duration from starting treatment to the date of starting second line therapy or death, whichever was earlier. Initiation of second line of therapy was defined using current guidelines as discontinuation (for reasons including end of planned therapy, toxicity, progression or lack of response) of the first regimen and start of another regimen, or unplanned addition or substitution of one or more drugs in the first regimen, irrespective of the reason for change.20 OS was defined as the duration from the date of diagnosis of MM to the date of death or date of last follow-up at which patient was known to be alive, with those alive censored at the date of last contact.
Cytoplasmic immunoglobulin FISH (cIgFISH)
Bone marrow aspirate samples enriched for mononuclear cells by the Ficoll method were used for preparing cytospin slides, and concurrent staining for cytoplasmic immunoglobulin LCs improved the specificity. cIgFISH analysis was performed as described previously from our institution using the following probes: 3cen (D3Z1), 7cen (D7Z1), 9cen (D9Z1), 15cen (D15Z4), 11q13 (CCND1-XT), 14q32 (IGH-XT), 13q14 (RB1), 13q34 (LAMP1), 14q32 (5′IGH,3′IGH), 17p13.1 (p53), and 17cen (D17Z1).21 The cutoffs used for cytogenetic abnormalities detected on FISH were: abnormalities involving immunoglobulin heavy chain locus–5%, chromosomal translocations–3%, del (17p)–7%, monosomy 17–9%, del (13q)–9%, monosomy 13–6%, trisomy/tetrasomy 3–13%, trisomy/tetrasomy 7–10%, trisomy/tetrasomy 9–14%, trisomy/tetrasomy 11–10%, and trisomy/tetrasomy 15 and 16–10%.
Statistical analysis
We performed statistical analysis using JMP Pro 22.0 software package (SAS Institute Inc., Cary, NC, USA). We summarized categorical variables as proportions and continuous variables as median (range). Fisher’s exact test was used to compare categorical variables. Continuous variables were compared using the non-parametric Kruskal–Wallis test. PFS, TTNT and OS were estimated using the Kaplan–Meier method and compared between groups using log-rank test. We used Cox proportional hazards model to identify factors affecting PFS, TTNT and OS. A two-tailed P-value <0.05 was considered significant for all statistical tests.
Results
The baseline characteristics of the three groups are summarized in Table 1. Median age at diagnosis was similar across the groups. Pathological fractures at diagnosis were more common in the t(11;14) group (20.3%). Median M-spike at diagnosis was slightly higher in the non-(11;14) translocation group. Within the t(11;14) group, 128 (35.1%) patients had LC–MM; among them 90 (70.3%) were kappa restricted and 38 (29.7%) were lambda restricted. Proportion of patients with ISS III stage was higher in the non-(11;14) translocation group. The cytogenetic profiles of the three groups of patients are shown in Table 2. The proportions of patients with abnormalities of chromosome 13 and 17p were higher in the non-(11;14) translocation group (P<0.001 and P=0.002 respectively).
The estimated median duration of follow-up for all the patients was 66.2 months (95% confidence interval (CI), 63.4–70.4) from diagnosis. For the t(11;14), non-(11;14) translocation and no-translocation groups, the estimated median follow-up periods were 70.7 (95% CI, 63.6–77.3), 54.9 (95% CI, 47.0–71.5) and 65.9 (95% CI, 63.0–70.6) months, respectively (P=0.225). At data cutoff, 170 (46.6%), 76 (57.6) and 220 (36.8%) patients, respectively, in the three groups had died.
Induction therapy and response to induction
The induction therapy received by the three groups of patients is shown in Figure 1a. Most patients received a novel agent (PI or IMiD)-based induction, whereas a smaller proportion received combinations containing a PI and an IMiD. Less than 10% of patients in each group received ‘other’ therapies, which included high-dose steroids and alkylating agents. There was no statistically significant difference in the proportion of patients receiving different classes of induction therapies between the three groups. Response to induction was not evaluable in 27 (7.4%), 4 (3.0%) and 51 (8.5%) patients, respectively, in the t(11;14), non-(11;14) translocation and no-translocation groups. The proportion of patients with complete response, very good partial response, partial response, stable disease and progressive disease as best response to induction are shown in Figure 1b. A partial response or better (overall response rate) was attained by 71.4%, 82.2% and 85.4% patients, respectively, in the three groups (P<0.001). However, there was no difference in the proportion of patients who attained very good partial response or better in the three groups (42.6 vs 40.6 vs 39.8% and P=0.720). The lower overall response rate in the t(11;14) group was due to stable disease being the best response in 24.8% patients. Among patients with t(11;14) who received a PI+IMiD combination, overall response rate was 91.5% compared to 68% in patients who received a single novel agent or conventional therapy (P<0.001). Rates of complete response were 34% in the PI+IMiD group, and 15.8% in the other patients (P=0.003).
Induction therapy and response to induction in the three groups. (a) Proportion of patients receiving proteasome inhibitor (PI) based, immunomodulatory drug (IMiD) based, PI+ IMiD-based and other induction therapies in patients with (i) translocation t(11;14), (ii) non-(11;14) translocation and (iii) no-translocation. (b) Best response to induction therapy in patients with translocation t(11;14), non-(11;14) translocation and no-translocation, expressed as percentage. CR, complete response, VGPR, very good partial response, SD, stable disease and PD progressive disease.
Survival outcomes
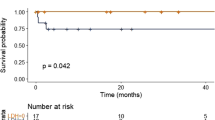
The estimated median PFS for t(11;14), non-(11;14) translocation and no-translocation groups were 23.0 (95% CI, 20.8–27.6), 19.0 (95% CI, 15.8–22.7) and 28.3 (95% CI, 25.7–30.6) months, respectively (P=0.01 for both t(11;14) vs non-(11;14) translocation and t(11;14) vs no-translocation). The estimated median TTNT for the three groups were 20.8 (95% CI, 17.0–24.0), 18.2 (95% CI, 15.1–22.0) and 27.0 (95% CI, 23.6–29.5) months, respectively (P=0.064 for t(11;14) vs non-(11;14) translocation and P=0.01 for t(11;14) vs no-translocation). The estimated median OS for the three groups were 74.4 (95% CI, 64.8–89.3), 49.8 (95% CI, 40.0–60.6) and 103.6 (95% CI, 85.2–112.3) months, respectively (P<0.001 for t(11;14) vs non-(11;14) translocation and P=0.003 for t(11;14) vs no-translocation). The survival curves for above comparisons are shown in Figure 2. The estimated proportions of patients surviving in the three groups at 5 years were 57.8%, 41.7% and 68.1%, respectively.
Survival outcomes in the three groups. Kaplan–Meier curves showing comparison of (a) progression-free survival, (b) time to next treatment and (c) overall survival between patients with translocation t(11;14), non-(11;14) translocation and no-translocation. *P-value for translocation t(11;14) vs non-(11;14) translocation comparison, **P-value for translocation t(11;14) vs no-translocation comparison.
Use of SCT
Among patients with t(11;14), non-(11;14) translocation and no-translocation, 223 (61.1%), 77 (58.3%) and 353 (59.0%) patients, respectively, underwent SCT at any time after starting therapy (P=0.776). Within the three groups, 28 (7.7%), 11 (8.3%) and 34 (5.7%) patients, respectively, received >1 SCT and 5 (1.4%), 5 (3.8%) and 10 (1.7%) patients, respectively, had an allogeneic SCT. Among 588 patients whose age was <65 years at diagnosis, 161 (81.7%), 58 (78.4%) and 239 (75.4%) patients belonging to t(11;14), non-(11;14) translocation and no-translocation groups, respectively, received SCT (P=0.242). An early SCT was associated with improved OS in all three groups. The median OS in patients receiving early vs delayed SCT in the three groups were 88.4 (95% CI, 68.8–124.7) vs 58.1 (95% CI, 50.0–81.7) months, 51.9 (95% CI, 45.2–not reached) vs 40.0 (95% CI, 30.1–60.6) months and 112.3 (95% CI, 103.6–not reached) vs 73.7 (95% CI, 66.9–97.0) months, respectively (P=0.002, P=0.022 and P<0.001, respectively, for the three groups).
Survival outcomes in patients with no 17p abnormality
Since loss of p53 (17p abnormality) is a poor prognostic marker, we separately studied patients with no 17p abnormality. Of 1095 patients, 951 (86.8%) had no 17p abnormality, which included 326 (89.3%), 102 (77.3%) and 523 (87.4%) patients, respectively, within t(11;14), non-(11;14) translocation and no-translocation groups. The results are summarized in Table 3 and Kaplan–Meier curves for the above analyses are shown in Figure 3. The TTNT for the t(11;14) group was worse when compared with no-translocation group. OS of the t(11;14) group, even though better than the non-(11;14) translocation group, was worse when compared with the no-translocation group. Since age <65 years (a surrogate marker for transplant eligibility), ISS I/II stages at diagnosis and novel agent-based induction are associated with improved outcomes, we further analyzed the outcomes of patients with no 17p abnormality stratified by the above factors. The results are shown in Table 3 and in Supplementary Appendix. Survival outcomes in patients with 17p abnormality are given in the Supplementary Appendix. There was no difference in OS between t(11;14) and no-translocation groups in the presence of 17p abnormality (P=0.245).
Survival outcomes in the three groups with no 17p abnormality. Kaplan–Meier curves showing comparison of (a) progression-free survival, (b) time to next treatment and (c) overall survival between patients with translocation t(11;14), non-(11;14) translocation and no-translocation. *P-value for translocation t(11;14) vs non-(11;14) translocation comparison, **P-value for translocation t(11;14) vs no-translocation comparison.
We then identified patients with t(11;14) (n=321) and those with hyperdiploidy (trisomy or tetrasomy of ⩾1 chromosomes) (HRD) (n=394), who did not have 17p abnormality, 1q gain or 1p deletion, and compared their outcomes. The estimated median PFS for the t(11;14) and HRD groups were 26.5 months (95% CI, 21.4–29.3) and 29.6 months (95% CI, 25.8–32.3), respectively (P=0.08). The estimated median TTNT for the two groups were 22.3 months (95% CI, 18.8–27.9) and 29.0 months (95% CI, 25.5–32.6), respectively (P=0.019). The respective estimated median OS were 81.7 months (95% CI, 67.0–90.7) and 108.3 months (95% CI, 88.8–not reached) (P=0.005). We further compared the TTNT and OS between t(11;14) and HRD patients stratified by age, ISS stage and type of induction therapy. The details of these analyses are given in Supplementary Appendix.
Predictors of outcome in patients with t(11;14)
We performed univariate analysis with age ⩾65 vs <65 years, serum creatinine >2 vs ⩽2 mg/dl, bone marrow PC percentage ⩾50 vs <50, LC-only MM vs others, ISS III vs I/II stage, elevated vs normal lactate dehydrogenase, abnormal vs normal 17p, abnormal vs normal chromosome 13, presence vs absence of HRD and novel agent-based vs conventional induction therapy as independent variables to determine their association with PFS and OS. Variables with a P-value <0.1 on univariate analysis were included as predictor variables in multivariate Cox proportional hazards model. The results of the analysis are shown in Table 4. Age ⩾65 years, ISS III stage and presence of 17p abnormality were associated with reduced OS. Type of induction therapy had no impact on OS. ISS III stage, presence of 17p abnormality and conventional induction therapy predicted reduced PFS.
Discussion
Our study is the largest to specifically examine the clinical characteristics, treatment profiles and outcomes of a large cohort of MM patients with t(11;14). Although the clinical characteristics such as the higher proportion of LC–MM, IgM or IgD myeloma and the spectrum of bone disease are all consistent with the prior studies, we identified unique characteristics such as poorer response to induction therapy, and inferior PFS and OS compared with the rest of the patients considered to have standard risk MM.3
Translocation (11;14) places the proto-oncogene cyclin D1 under the transcriptional control of the immunoglobulin heavy chain enhancer, with resultant dysregulation of cyclin D1, which accelerates the G1 to S-phase transition in PCs.22, 23, 24 Translocations involving the immunoglobulin heavy chain locus in MM commonly occur due to errors in physiologic DNA recombination during isotype switching. However, t(11;14) may also originate due to errors of somatic hypermutation, an event which occurs earlier in the development of PCs.3, 25, 26 Initial studies using standard metaphase cytogenetics showed that patients with t(11;14) had worse OS.27, 28 Early studies that classified t(11;14) on FISH as a standard risk marker included patients enrolled in clinical trials and receiving conventional chemotherapy as induction.3, 5, 8 Fonseca et al. reported a median OS of 49.6 months and PFS of 33.0 months in 53 patients with t(11;14) treated initially with VBMCP (vincristine+carmustine+melphalan+cyclophosphamide+prednisolone), which were comparable to 283 patients without t(11;14) who received similar treatment. The authors suggested that t(11;14) detected on standard cytogenetics is probably a surrogate for high proliferative rate, which explained the poor outcomes previously noted.3 Avet-Loiseau et al.5 reported an estimated 80% OS and an event-free survival (EFS) of 35 months for patients with t(11;14), which was comparable to the HRD group (82% OS at 41 months and EFS of 37 months). This study included patients younger than 66 years, who were treated with VAD (vincristine+doxorubicin+dexamethasone) induction and double SCT, which is not representative of the current practice. The follow-up period was short for a meaningful comparison between the t(11;14) and HRD groups (median OS was not reached in both groups after 41 months of median follow-up).
A few studies have assessed the outcomes of t(11;14) MM after the introduction of novel agents as frontline therapy for MM. An et al. in a retrospective review from China, reported a median PFS close to 2 years in t(11;14) patients who received thalidomide or bortezomib-based induction, which is comparable to the PFS observed in our population.6 The OS in thalidomide and bortezomib treated groups were 30 and 54 months, respectively. Leiba et al.29 reported a median OS of 63.3 months in a group of 212 patients treated with thalidomide-based or bortezomib-based induction. The study by Sasaki et al.,13 which compared the PFS and OS in 27 patients with t(11;14), with high-risk patients and normal controls was limited by a small cohort and the fact that it contained a heterogeneous mix of patients with t(11;14) detected on conventional cytogenetics as well as FISH.
Our group has shown before that after an early SCT, patients with t(11;14) fare worse compared to those with standard risk MM with regard to OS.15 Results of the current study indicate that clinical outcomes including PFS, TTNT and OS in the t(11;14) group are better than the high-risk-translocation group, but worse than the no-translocation group, which include those with trisomies or a normal FISH. The difference was not observed in the presence of 17p abnormality, a reflection of the profound adverse prognostic impact associated with this abnormality across all patient groups. Among those without 17p abnormality, OS in the t(11;14) group was worse than the no-translocation group in patients younger than 65 years, those with ISS I/II stage disease at diagnosis, and those who received a novel agent-based induction therapy. However, the lack of difference between the two groups in those who were 65 years or older, those with ISS III stage, or those who received conventional induction therapy may reflect the dilution of the prognostic impact of t(11;14) by other predictors of poor outcomes. The worse PFS in patients with t(11;14) compared with the no-translocation group in the entire cohort of patients and absence of such a difference after removing patients with 17p abnormality is consistent with our prior finding that HRD may ameliorate the adverse impact of 17p abnormality to some extent.30
The lower overall response rate observed in the t(11;14) group was due to a higher proportion of patients who obtained only stable disease as the best response to initial induction. A PI+IMiD-based induction overcame this effect with higher rates of complete response, indicating that a single novel agent is probably insufficient for induction in t(11;14) patients, and lending weight to the argument that the biology of t(11;14) may entail a higher-than-standard risk associated with it.
The principal strength of our study lies in the large group of patients with t(11;14). We had a long follow-up and details of initial therapy were available for most patients. All patients in the t(11;14) and comparator groups had FISH done before or within a year of starting therapy, making it more likely that the secondary abnormalities were all present at diagnosis. Selecting matched controls diagnosed and treated at the same time as cases make it more likely that they received similar treatments that were the standard-of-care at the particular times. The retrospective design of the study with its attendant susceptibility to incomplete data (for example: baseline characteristics in many patients) is an important limitation.
In conclusion, our study details the natural history of t(11;14) MM in the current era. Based on our findings of lower response rate, and reduced PFS and OS compared with patients with other traditional standard risk markers, it is appropriate to consider t(11;14) to have a higher-than-standard risk even in the absence of other adverse prognostic factors. Venetoclax, a bcl2 inhibitor, has shown significant single agent activity in patients with t(11;14) abnormality and may improve outcomes in patients with t(11;14).31
References
Rajkumar SV . Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol 2016; 91: 719–734.
Avet-Loiseau H, Facon T, Grosbois B, Magrangeas F, Rapp M-J, Harousseau J-L et al. Oncogenesis of multiple myeloma: 14q32 and 13q chromosomal abnormalities are not randomly distributed, but correlate with natural history, immunological features, and clinical presentation. Blood 2002; 99: 2185–2191.
Fonseca R, Blood EA, Oken MM, Kyle RA, Dewald GW, Bailey RJ et al. Myeloma and the t(11;14)(q13;q32); evidence for a biologically defined unique subset of patients. Blood 2002; 99: 3735–3741.
Segges P, Braggio E, Minnicelli C, Hassan R, Zalcberg IR, Maiolino A . Genetic aberrations in multiple myeloma characterized by cIg-FISH: a Brazilian context. Braz J Med Biol Res 2016; 49: e5034.
Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood 2007; 109: 3489–3495.
An G, Xu Y, Shi L, Zou D, Deng S, Sui W et al. t(11;14) multiple myeloma: a subtype associated with distinct immunological features, immunophenotypic characteristics but divergent outcome. Leuk Res 2013; 37: 1251–1257.
Avet-Loiseau H, Garand R, Lode L, Harousseau JL, Bataille R . Translocation t(11;14)(q13;q32) is the hallmark of IgM, IgE, and nonsecretory multiple myeloma variants. Blood 2003; 101: 1570–1571.
Moreau P, Facon T, Leleu X, Morineau N, Huyghe P, Harousseau JL et al. Recurrent 14q32 translocations determine the prognosis of multiple myeloma, especially in patients receiving intensive chemotherapy. Blood 2002; 100: 1579–1583.
Garand R, Avet-Loiseau H, Accard F, Moreau P, Harousseau JL, Bataille R . t(11;14) and t(4;14) translocations correlated with mature lymphoplasmacytoid and immature morphology, respectively, in multiple myeloma. Leukemia 2003; 17: 2032–2035.
Robillard N, Avet-Loiseau H, Garand R, Moreau P, Pineau D, Rapp MJ et al. CD20 is associated with a small mature plasma cell morphology and t(11;14) in multiple myeloma. Blood 2003; 102: 1070–1071.
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008; 111: 2516–2520.
Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 2014; 28: 1122–1128.
Sasaki K, Lu G, Saliba RM, Bashir Q, Hosing C, Popat U et al. Impact of t(11;14)(q13;q32) on the outcome of autologous hematopoietic cell transplantation in multiple myeloma. Biol Blood Marrow Transplant 2013; 19: 1227–1232.
Shin HJ, Kim K, Lee JJ, Song MK, Lee EY, Park SH et al. The t(11;14)(q13;q32) translocation as a poor prognostic parameter for autologous stem cell transplantation in myeloma patients with extramedullary plasmacytoma. Clin Lymphoma Myeloma Leuk 2015; 15: 227–235.
Kaufman GP, Gertz MA, Dispenzieri A, Lacy MQ, Buadi FK, Dingli D et al. Impact of cytogenetic classification on outcomes following early high-dose therapy in multiple myeloma. Leukemia 2016; 30: 633–639.
International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Bri J Haematol 2003; 121: 749–757.
Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K et al. International uniform response criteria for multiple myeloma. Leukemia 2006; 20: 1467–1473.
Kyle RA, Rajkumar SV . Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 2009; 23: 3–9.
Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood 2011; 117: 4691–4695.
Rajkumar SV, Richardson P, San Miguel JF . Guidelines for determination of the number of prior lines of therapy in multiple myeloma. Blood 2015; 126: 921–922.
Fonseca R, Blood E, Rue M, Harrington D, Oken MM, Kyle RA et al. Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood 2003; 101: 4569–4575.
Chesi M, Bergsagel PL, Brents LA, Smith CM, Gerhard DS, Kuehl WM . Dysregulation of cyclin D1 by translocation into an IgH gamma switch region in two multiple myeloma cell lines. Blood 1996; 88: 674–681.
Gabrea A, Bergsagel PL, Chesi M, Shou Y, Kuehl WM . Insertion of excised IgH switch sequences causes overexpression of cyclin D1 in a myeloma tumor cell. Mol Cell 1999; 3: 119–123.
Specht K, Haralambieva E, Bink K, Kremer M, Mandl-Weber S, Koch I et al. Different mechanisms of cyclin D1 overexpression in multiple myeloma revealed by fluorescence in situ hybridization and quantitative analysis of mRNA levels. Blood 2004; 104: 1120–1126.
Bergsagel PL, Chesi M, Nardini E, Brents LA, Kirby SL, Kuehl WM . Promiscuous translocations into immunoglobulin heavy chain switch regions in multiple myeloma. Proc Natl Acad Sci USA 1996; 93: 13931–13936.
Chesi M, Bergsagel P, Brents L, Smith C, Gerhard D, Kuehl W . Dysregulation of cyclin D1 by translocation into an IgH gamma switch region in two multiple myeloma cell lines [see comments]. Blood 1996; 88: 674–681.
Fonseca R, Witzig TE, Gertz MA, Kyle RA, Hoyer JD, Jalal SM et al. Multiple myeloma and the translocation t(11;14)(q13;q32): a report on 13 cases. Br J Haematol 1998; 101: 296–301.
Fonseca R, Hoyer JD, Aguayo P, Jalal SM, Ahmann GJ, Rajkumar SV et al. Clinical Significance of the Translocation (11;14)(q13;q32) in Multiple Myeloma. Leuk Lymphoma 1999; 35: 599–605.
Leiba M, Duek A, Amariglio N, Avigdor A, Benyamini N, Hardan I et al. Translocation t(11;14) in newly diagnosed patients with multiple myeloma: Is it always favorable? Genes Chromosomes Cancer 2016; 55: 710–718.
Kumar S, Fonseca R, Ketterling RP, Dispenzieri A, Lacy MQ, Gertz MA et al. Trisomies in multiple myeloma: impact on survival in patients with high-risk cytogenetics. Blood 2012; 119: 2100–2105.
Kumar S, Vij R, Kaufman JL, Mikhael J, Facon T, Pegourie B et al. Venetoclax Monotherapy for Relapsed/Refractory Multiple Myeloma: Safety and Efficacy Results from a Phase I Study. Am Soc Hematology 2016. Annual meeting of American Society of Hematology, San Diego, CA, December 4, 2016.
Acknowledgements
Author contributions
AL and MAM collected and analyzed the data, wrote the first draft and approved the final version of the manuscript; SVR, AD, MAG, FKB, MQL, DD, ALF, SRH, MAH, WIG, YLH, PK, NL, RSG, YL, TVK, JAL, SJR and SRZ performed patient management, revised the manuscript critically and approved the final version of the manuscript; RAK performed patients’ follow-up, revised the manuscript critically, and participated in final data analysis and approval of the final version of the manuscript; and SKK designed the study, analyzed the data, wrote the first draft, approved the final version of the manuscript and performed patient management.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
AD received research funding from Celgene, Millennium, Pfizer and Janssen and travel grant from Pfizer. MAG received consulting fees from Millenium and honoraria from Celgene, Millenium, Onyx, Novartis, Smith Kline, Prothena, Ionis and Amgen. MQL received research funding from Celgene. PK received research funding from Takeda, Celgene and Amgen. SKK received consulting fees from Celgene, Millennium, Onyx, Janssen and BMS and research funding from Celgene, Millennium, Novartis, Onyx AbbVie, Janssen and BMS. The remaining authors declare no competing financial interest.
Additional information
Presented as oral presentation at the 16th International Myeloma Workshop, New Delhi, India, 3 March 2017 and as poster at the annual meeting of American Society of Clinical Oncology, Chicago, IL, USA, 5 June 2017.
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Lakshman, A., Alhaj Moustafa, M., Rajkumar, S. et al. Natural history of t(11;14) multiple myeloma. Leukemia 32, 131–138 (2018). https://doi.org/10.1038/leu.2017.204
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.204
- Springer Nature Limited
This article is cited by
-
Which factors are associated with adverse prognosis in multiple myeloma patients after surgery? - preliminary establishment and validation of the nomogram
World Journal of Surgical Oncology (2024)
-
Multiple myeloma with t(11;14): impact of novel agents on outcome
Blood Cancer Journal (2023)
-
Combination venetoclax and selinexor effective in relapsed refractory multiple myeloma with translocation t(11;14)
npj Precision Oncology (2022)
-
High-risk disease in newly diagnosed multiple myeloma: beyond the R-ISS and IMWG definitions
Blood Cancer Journal (2022)
-
Treatment patterns and outcomes according to cytogenetic risk stratification in patients with multiple myeloma: a real-world analysis
Blood Cancer Journal (2022)