Abstract
Clinically useful pre-transplant predictive factors of acute graft-versus-host-disease (aGVHD) after allogeneic hematopoietic stem cell transplantation (allo-SCT) are lacking. We prospectively analyzed HSC graft content in CD34+, NK, conventional T, regulatory T and invariant natural killer T (iNKT) cells in 117 adult patients before allo-SCT. Results were correlated with occurrence of aGVHD and relapse. In univariate analysis, iNKT cells were the only graft cell populations associated with occurrence of aGVHD. In multivariate analysis, CD4− iNKT/T cell frequency could predict grade II-IV aGVHD in bone marrow and peripheral blood stem cell (PBSC) grafts, while CD4− iNKT expansion capacity was predictive in PBSC grafts. Receiver operating characteristic analyses determined the CD4− iNKT expansion factor as the best predictive factor of aGVHD. Incidence of grade II-IV aGVHD was reduced in patients receiving a graft with an expansion factor above versus below 6.83 (9.7 vs 80%, P<0.0001), while relapse incidence at two years was similar (P=0.5).The test reached 94% sensitivity and 100% specificity in the subgroup of patients transplanted with human leukocyte antigen 10/10 PBSCs without active disease. Analysis of this CD4− iNKT expansion capacity test may represent the first diagnostic tool allowing selection of the best donor to avoid severe aGVHD with preserved graft-versus-leukemia effect after peripheral blood allo-SCT.
Similar content being viewed by others
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-SCT) is a curative procedure for malignant haematological disorders, due to the graft-versus-leukemia (GVL) effect, but is however often hampered by the occurrence of a life-threatening acute (a) graft-versus-host disease (GVHD).1 Therefore, one of the main challenges of allo-SCT remains to identify the factors that would allow predicting the risk of aGVHD without impairing the GVL effect. Although several host and donor pre-transplant clinical, immunological and genetic characteristics have been well established as factors correlated to aGVHD,2 none of them can be used to select the best donor or to help deciding to perform or not SCT.
Graft content in T-cell subsets has been correlated to the risk of aGVHD, in particular naive CD45RA+ CD4+ T cells,3 naive and central memory CCR7+ CD4+ T cells,4 or CD8+ effector memory (CD45RA-CD62L−) T cells.5 Immunoregulatory T cells such as CD4+CD25+FoxP3+ CD127low regulatory T lymphocytes (Tregs)6, 7 and invariant natural killer T (iNKT) cells8, 9, 10, 11, 12, 13, 14 represent other cellular targets in order to regulate donor T-cell alloreactivity in experimental mouse models. Some data suggest the involvement of these cells in humans as well.15, 16, 17, 18, 19, 20, 21, 22 We previously reported that enhanced early post-transplant iNKT cell reconstitution from donor cells was correlated to reduced risk of aGVHD without impairment of the GVL effect,23 while Treg cell reconstitution was not. Likewise, graft content in CD4− iNKT cells, but not in Tregs, was associated to the risk of aGVHD in allo-SCT performed with peripheral blood stem cells (PBSCs) from human leukocyte antigen (HLA)-identical siblings.24, 25 Although these studies suggest a role of CD4− iNKT cells for the control and prediction of aGVHD, because of their great variability in terms of numbers, it is currently difficult to define a threshold of graft iNKT cell dose predicting with high specificity and sensitivity the risk of aGVHD. We show here that in vitro donor CD4− iNKT cell expansion capacity represents the best predictive factor of aGVHD without impairment of the GVL effect in allo-SCT performed with PBSC grafts.
Patients and methods
Patients
Between June 2010 and January 2014, 117 adult patients who underwent an allo-SCT in three transplant institutions (Saint Antoine, Necker and Pitié-Salpétrière hospitals, Paris) entered the study. All patients and their sibling donors provided an informed consent for biological research purposes. The study was conducted according to the procedures of the Declaration of Helsinki and the local ethic committee rules. Diagnosis and grading of acute and chronic GVHD were performed as described26, 27 by physicians in charge of the patients who were not aware of the results of laboratory tests.
Flow cytometry analysis
Analyses were performed on 117 HSC grafts (freshly harvested in 96 and from thawed cells in 21). Among those, 21 freshly harvested sibling donor blood cells drawn before mobilization of HSC by granulocyte colony stimulating factor (G-CSF) were analyzed and compared with the corresponding HSC graft. Graft cell phenotype was performed in all 117 HSC grafts, while ex vivo iNKT expansion tests could only be performed on the 96 freshly harvested grafts. Post-transplant peripheral immune reconstitution was analyzed from 30 recipients on days 30±5, 60±5 and 90±5 days after transplantation (Supplementary Figure S1). In all analyses, mononuclear cells were isolated by density-gradient centrifugation (Ficoll-PaqueTM PLUS, GE Healthcare, Sigma-Aldrich, Saint Quentin Fallavier, France). Cells were first stained with PBS57-loaded or empty-CD1d-tetramers (NIH Tetramer Core Facility) and with the following monoclonal antibodies: anti-CD3, -CD4, -CD8, -CD56, -CD25, -CD127, -CD45RA, -γδTCR, -CD161, -FoxP3, -Ki67 (eBioscience, Paris, France), anti-Vα7.2TCR (Biolegend, London, UK). Data were acquired on a fluorescence-activated cell sorting Canto II flow cytometer using fluorescence-activated cell sorting Diva software (BD, Bioscience, Le Pont de Claix, France), and were analyzed with the FlowJo software (Tree Star Inc., San Diego, CA, USA). Lymphocyte subpopulations were analyzed within the lymphocyte gate on forward and side-scatter plots as described in Supplementary Figure S2. iNKT cells were specifically detected by the co-expression of CD3 and PBS57-loaded CD1d-tetramer, Tregs were analyzed as described by Sakaguchi’s group28 and mucosa-associated invariant T (MAIT) cells were identified as previously reported.29 Results were expressed in absolute numbers or in terms of cell subtype/102 or 103 T lymphocyte ratios (Supplementary Table S1). Since iNKT cells represent a rare population of T lymphocytes, data were considered as reliable when a minimum of 1 × 106 mononuclear cells were stained and at least 5 × 104 CD3+ T cells with a minimum of 20 iNKT CD3+ CD1d Tetramer+ cells acquired. Doses of the different cell subtypes were extrapolated from the multiplication of CD3+ T-cell dose by the corresponding cell subtype/T-cell ratio.
Functional studies of iNKT cells
Mononuclear cells from freshly harvested peripheral blood, bone marrow (BM) or PBSC grafts were cultured in 24-well plates at a density of 1 × 106 cells per well in 2 ml of RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin and streptomycin, 200 mm l-glutamine, and 10 mm HEPES (All from Life Technologies, Villebon sur Yvette, France). Alpha-galactosylceramide (KRN7000, Avanti Polar Lipids, Alabaster, AL, USA) was added at 100 ng/ml at the onset of culture, followed 24 h later by rhIL-2 at 845 U/ml (Immunotools, Friesoythe, Germany). After 2 weeks, cells were collected, washed extensively before surface staining, as described above and represented in Supplementary Figure S3. The expansion factor was calculated by dividing the number of each cell subset obtained on day 15 by the number of cells on day 0.
Statistical analyses
Statistical analyses were performed using the R program.30 Means were compared using the non-parametric Wilcoxon and proportions using the Fisher exact or χ2-tests, as appropriate. Correlations between continuous variables were calculated using the non-parametric Spearman’s rank correlation test. Overall survival (OS) was measured from the date of allo-SCT to the date of death from any cause or of last follow-up. Non-relapse mortality (NRM) was defined as death occurring in continuous complete remission (CR). None of the patients were lost of follow-up and all were censored at date of death or last-follow-up. Median follow up of patients alive was 681 days (range 153–1348).
Univariate and multivariate Cox proportional hazards models were analyzed using the survival package.31 OS curves were established using the Kaplan–Meier method and statistical differences in survival distributions according to baseline parameters and the expansion factor of CD4− iNKT cells were assessed using the logrank test. Cox proportional hazard model was used for multivariate analysis. CD4− iNKT/103 T frequency and CD4− iNKT expansion factor covariates were log transformed to ensure a normal distribution. The martingale residuals versus the log-transformed covariates were well distributed around the 0 ordinate line, with no trend or particular shape observed. Acute GVHD occurred in all patients before day 100 and was analyzed as a time-dependent covariate in the Cox model. Stepwise regression consisting of alternating forward and backward elimination steps was used to find the most parsimonious set of significant covariates using the Bayesian Information criterion. The internal validation of the multivariate analysis was assessed using a bootstrap method.
To assess the ability of the expansion factor of CD4− iNKT cells to predict aGVHD, the value of that factor and actual development of aGVHD were subjected to receiver operating characteristic (ROC) curve analysis32 using the ROCR package.33 A bootstrap method was used to calculate the 95% confidence interval of the area under the curve (AUC) of ROC curves.
A P-value of 0.05 was considered statistically significant for all analyses, except for univariate analysis including multiple biological parameters associated with the occurrence of acute GVHD in which an adjusted P-value of 0.0017, corresponding to 0.05/30 factors, was applied. Two-sided tests were used in all analyses.
Results
Patients, treatment assignments and transplant outcomes
Clinical characteristics for the 117 patients included in the study are summarized in Table 1. Median age at transplantation was 49.7 years for recipients and 42.4 years for donors. A majority of patients (66%) received an allo-SCT for acute leukemia. Sixty nine (59%) received an allograft from an HLA-identical sibling, 42 (36%) from a matched unrelated donor and six (5%) from a mismatched unrelated donor. HSC were obtained from unmanipulated BM in 33 (28%) or PBSC in 84 (72%) transplanted patients. The choice of conditioning was dependent on the type of underlying disease, recipient’s age and co-morbidities: myeloablative conditioning (MAC) for patients in CR under 50 years of age and without co-morbidities (42%), conventional reduced intensity conditioning (RIC) for patients above 50 years or presenting co-morbidities (47%), and sequential-based RIC regimen34 for refractory diseases (11%). Altogether, 63 (54%) patients received an in vivo T-cell depletion with ATG-Thymoglobuline (total dose 5 mg/kg), mainly with unrelated PBSCs or with fludarabine-busulfan RIC.
With a median follow-up of 15 months (range 1–45), cumulative incidence of grade II-IV aGVHD was 25% (95% confidence interval (CI), 14–38%) at day 100. At 2 years, cumulative incidence of NRM was 14.5% (95% CI, 3–35%), cumulative incidence of chronic GVHD was in 57% (95% CI, 47–66%), relapse incidence was 35% (95% CI, 22–48%) and OS was 64% (95% CI, 53–73%).
Graft cell content and iNKT subsets’ expansion capacities of donor grafts
In all 117 HSC grafts, we measured graft cell content in CD34, CD56 NK, CD3 T, total CD4 T, CD45RA+ and CD45RA− CD4 T, CD8 T, γδ T, mucosa-associated invariant T, total, naive and memory Tregs, and total iNKT cells, as well as CD4+ and CD4− iNKT cell subsets. CD4+ and CD4− iNKT cell expansion was carried out in 96 freshly harvested grafts (77 from PB and 19 from BM stem cells; Supplementary Table S1). The frequencies of total iNKT and CD4− iNKT cells among T cells were similar between PBSC and BM grafts, while the frequency of the minoritary CD4+ iNKT cell subtype was slightly higher in BM than in PBSC grafts (P=0.02; Supplementary Table S2). Because PBSC contained one log higher number of T cells than BM grafts, all iNKT cell subset doses were increased in PBSCs (P<0.0001; Supplementary Table S2). Ex vivo expansion capacities of both CD4+ and CD4− iNKT cells were higher in PBSC as compared with BM grafts (P<0.05; Supplementary Table S2). Age of donor had a negative impact on the frequency and dose of CD4+ iNKT cells (P=0.049), but not on CD4− iNKT cells (Supplementary Table S3).
Graft parameter and pre-transplant factors associated with the occurrence of grade II-IV aGVHD
In univariate analysis, in the total cohort of patients, those who developed grade II-IV aGVHD received a graft with higher frequencies of CD45RA+ CD4+ T cells (P=0.045), lower frequencies of total, CD4+ and CD4− iNKT cells (P=0.001, P=0.021 and P=0.0005, respectively), and lower doses of total and CD4− iNKT cells (P=0.027 and 0.025, respectively; Table 2 and Supplementary Figure S4). Functional analyses of iNKT cell subsets showed significantly reduced expansion capacities of the CD4− iNKT cells in patients developing grade II-IV aGVHD (P=0.0007; Table 2 and Supplementary Figure S4). None of the other graft parameters analyzed including total, naive and memory Tregs were associated with the occurrence of acute GVHD. The only pre-transplant parameter associated with a higher risk of developing grade II-IV aGVHD was the use of an unrelated donor (P=0.00004; Table 2).
Cumulative incidence of grade II-IV aGVHD at day 100 in patients receiving a graft with an iNKT cell dose below or above the median for total iNKT cells was 33 versus 17% (P=0.028). However, when P-value was adjusted to the multiple tests performed in univariate analysis, the remaining significant factors associated to the occurrence of aGVHD were frequencies of total iNKT cells, frequency and expansion capacity of CD4− iNKT cells and type of donor (P⩽0.00017).
Analyses performed separately between patients transplanted with BM and PB confirmed the correlation between frequencies of total and CD4− iNKT cells and occurrence of aGVHD in both subgroups of stem cell source, but the association with the CD4− iNKT expansion factor was only observed in the PBSC group (Supplementary Tables S4 and S5).
In multivariate analysis, among all the graft parameters analyzed, the only two associated with an independent reduced risk of developing grade II-IV aGVHD were the frequency of CD4− iNKT cells (odd ratio=0.56, 95% CI=0.38–0.84, P=0.0048) and the expansion factor of CD4− iNKT cells (odd ratio=0.72, 95% CI= 0.55–0.82, P<0.001) (Table 3, Model 1). The use of matched unrelated donors and progressive disease (PD) status at transplant were the other pre-transplant factors associated with an increased risk of grade II-IV aGVHD (odd ratio=8.16, 95% CI=3–22.1, P<0.0001 for matched unrelated donor and odd ratio=10, 95% CI=3–33.5, P=0.00017 for PD) (Table 3, Model 1). Predictive value of these factors on the occurrence of severe aGVHD was confirmed in an internal validation test by using the bootstrap method (Table 3).
In a Cox model taking into account the stem cell source and after adjusting to donor type and disease status at transplantation, the graft CD4− iNKT/T frequency could significantly predict the occurrence of grade II-IV aGVHD in both PB and BM transplants, but the CD4− iNKT expansion factor was only significant in PBSC grafts (Table 3, Model 2).
Determination of the best graft factor and threshold predicting aGVHD occurrence
Using ROC analyses, the predictive value in the global population of patients with available iNKT expansion tests of the whole model including CD4− iNKT frequency and expansion factor, donor type and disease status at SCT was high with an AUC of 0.927 (95% CI: 0.86–0.994; Figure 1a). However, because such model remains complex for clinical application, we sought to determine which of the graft CD4− iNKT frequency or expansion factor might be the most useful. In this objective, we compared the sensitivity and specificity of these two parameters to predict the occurrence of grade II-IV aGVHD in the total population of patients and in the subgroups of patients transplanted with BM or PB stem cells (Table 4a). Both factors were highly sensitive (>90%) but the CD4− iNKT expansion factor provided the best specificity in all subpopulations of patients analyzed.
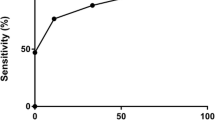
ROC analysis and according to the expansion factor of CD4− iNKT cells. (a) ROC analysis of the whole model including donor type, disease status at transplantation, graft CD4− iNKT/T frequency and expansion factor in predicting the occurrence of grade II-IV acute GVHD in the total population of patients (b) ROC analysis of the expansion factor of CD4− iNKT cells in predicting the occurrence of grade II-IV acute GVHD in the total population of patients. Choice of the 6.83 cutoff point determining the best sensitivity and specificity (94% true-positive rate and 36% false-positive rate. Cumulative incidence of (c) grade II-IV acute GVHD, (d) relapse, (e) chronic GVHD, (f) NRM and (g) probability of OS according to the CD4− iNKT expansion factor (<6.83 in dashed line and ⩾6.83 in continuous line) in the total population of patients. Comparisons are performed with the log-rank test.
In the total population of 96 patients with available functional iNKT tests, the CD4− iNKT expansion factor could discriminate the risk of grade II-IV aGVHD at day 100, with an AUC of 0.767 (Table 4a and Figure 1b). A CD4− iNKT expansion factor cutoff of 6.83 provided the best balance between sensitivity and specificity, with 94% true and 36% false-positive rates. Pre-transplant characteristics of patients transplanted with a graft providing a CD4− iNKT expansion factor below or above the 6.83 threshold were comparable except for increased siblings in the good expander group (73.5 vs 30%, P=0.00037; Supplementary Table S6). Using this cutoff point, 16 patients out of 20 (80%) with a CD4− iNKT expansion factor below 6.83 experienced grade II-IV aGVHD, while only 9 out of 76 (12%) with CD4− iNKT expansion factor above 6.83 did so (Figure 1c). In contrast, relapse incidence at 2 years was comparable between the two groups (31 vs 35%, P=0.50) (Figure 1d), as well as incidence of chronic GVHD (CI at 2 years of 68 vs 55%, P=0.45; Figure 1e). In this series of patients, 25 developed a grade II and four a grade III-IV aGVHD, of whom only four patients died of acute and two of chronic GVHD. At 2 years, NRM and OS therefore were comparable between the two groups of patients (17.2 versus 10.4%, P=0.53, and 67 versus 63%, P=0.58, respectively) (Figures 1f and g, respectively).
In the subgroup of patients transplanted with PBSC grafts with available iNKT expansion tests (n=77), the AUC of the expansion test was 0.772 (Table 4a). Using an expansion factor cutoff of 8.7, 12 out of 15 (80%) patients with an expansion factor below 8.7 developed grade II-IV aGVHD compared with six out of 62 (10%) patients above the threshold (P<0.001). These two subgroups of patients had otherwise comparable relapse risk, NRM and OS (data not shown).
Correlation between graft CD4− iNKT expansion factor, other graft characteristics and post-transplant iNKT reconstitution
We compared the different graft characteristics in patients who received a graft with a CD4− iNKT cell expansion factor above (n=76) or below (n=20) the previously determined threshold of 6.83 in the total population of patients. The CD4+ iNKT cell expansion factor was also higher in grafts with a CD4− iNKT cell expansion factor ⩾6.83 in comparison with those below that threshold (P=0.03; Figure 2a). Grafts with a higher expansion capacity of CD4− iNKT cells contained similar doses of CD34+ HSC, NK cells, CD3+, CD4+, CD8+, γδ+ and regulatory T cells than grafts with a lower expansion capacity but higher doses of mucosa-associated invariant T cells (P=0.01; Figure 2b). Grafts with enhanced CD4− iNKT expansion capacity had also significantly higher frequencies and doses of total and CD4− iNKT cells (P<0.05 for all; Figures 2c and d). Patients who received a graft with a CD4− iNKT cell expansion factor above 6.83 (n=21) had higher levels of total PB iNKT cells on days 30, 60 and 90 (P<0.05 for all) post transplantation than those with a CD4− iNKT expansion factor<6.83 (n=9; Figure 2e).
Graft content and post-transplant iNKT recovery according to the graft CD4− iNKT expansion factor. Graft characteristics and post-transplant iNKT reconstitution were analyzed in two groups according to their graft CD4− iNKT expansion factor (exp.fact.): ⩾6.83 (grey) or<6.83 (white). (a) Graft CD4+ iNKT expansion factor, (b) graft cell subset frequencies, (c) graft iNKT cell subset frequencies and (d) graft iNKT cell subset doses. Results are presented as mean±s.e.m. (e) Recipient peripheral blood levels of total iNKT cells on days 30, 60 and 90 post transplantation according to CD4− iNKT expansion factor. Means were compared with unpaired t-test. ns, non significant.
Determination of the most appropriate allo-SCT context in which the CD4− iNKT expansion test can predict aGVHD
Since occurrence of grade II-IV aGVHD was also associated with PD status at transplant (Table 3), we performed ROC analyses in the 79 patients allografted in non-progressive disease with an HLA 10/10 matched donor. In comparison to the whole population, the value of the graft CD4− iNKT expansion factor AUC in the prediction of grade II-IV aGVHD was improved in this subgroup of patients (AUC=0.90) (Table 4b). In this context, the predictive value of the test was confirmed to be limited for BM grafts (AUC=0.679) but optimal for PBSC grafts (AUC=0.986), with 94% sensitivity and 100% specificity (Table 4b and Figure 3). In the predominant group of patients transplanted with HLA compatible PBSCs and non-progressive disease at transplant (n=60), 10 out of the 14 patients (71%) who received a graft with a CD4− iNKT expansion factor below the threshold of 8.7 developed grade II-IV aGVHD, while none of the 46 patients having received a graft with a CD4− iNKT expansion factor above that threshold did so (P<0.0001) (Figure 3).
ROC analysis and cumulative incidence of grade II-IV aGVHD in the subgroup of patients allografted at non-progressive disease with an HLA 10/10 PBSC graft. (a) ROC analysis of the expansion factor of CD4− iNKT cells in predicting the occurrence of grade II-IV acute GVHD in the subpopulation of patients allografted with non-progressive disease with an HLA 10/10 PBSC graft (n=60, AUC=0.986) Determination of the 8.7 cutoff point providing the best sensitivity and specificity (94% true-positive rate and 0% false-positive rate). (b) Cumulative incidence of grade II-IV acute GVHD according to the CD4− iNKT expansion factor in this subgroup of patients (<8.7 in dashed line and ⩾8.7 in continuous line). Comparisons are performed with the Log-rank test.
We finally compared the CD4− iNKT cell expansion factor from the HSC graft with that obtained from peripheral blood mononuclear cells from the same donor before the administration of G-CSF (Supplementary Table S7). Although the expansion factor was often higher in the graft, the two tests could similarly predict the risk of occurrence of aGVHD in all 21 cases tested.
Discussion
This study confirms the value of iNKT cells as predictors of aGVHD after allo-SCT in humans. As reported in the context of allo-SCT performed with PBSC from HLA-identical sibling donors without in vivo T cell depletion,25 our study confirms, that the HSC graft content in total and CD4− iNKT cells represents the only graft cell subtype associated with the occurrence of grade II-IV aGVHD. In multivariate analyses, we show that higher graft CD4− iNKT T frequencies are associated with reduced risk of aGVHD in BM grafts, while both graft CD4− iNKT frequency and expansion factor could independently predict the occurrence of aGVHD in PBSC grafts. ROC curves show that the ex vivo expansion factor of CD4− iNKT cells represents the best predictive factor for the occurrence of aGVHD in allo-SCT performed with PBSCs. This iNKT functional test reaches its optimal predictive capacity with 100% specificity in allo-SCT performed with HLA 10/10 matched PBSC grafts for non-progressive hematological malignant diseases, which represents the majority of the indications of allogeneic SCT. Similar predictive value was also observed when the test was performed from donor’s peripheral blood before G-CSF mobilization. Thus, this test could allow to easily select the best donor if different siblings or unrelated donors are available before PB allo-SCT.
In line with the study of Chaidos et al. who reported, in allo-SCT performed without ATG, a reduced incidence of aGVHD at day 180 post-transplant in the group of patients receiving a dose of total iNKT cells above the median (31 versus 61%, P=0.014),25 we observed reduced incidence of aGVHD at day 100 post-transplant in patients receiving a total dose of iNKT cells above the median (17 versus 33%, P=0.028). These observations contrast with the study of Malard et al. who did not find a significant correlation between iNKT cell dose and aGVHD incidence in a series of 80 RIC allo-SCT performed with PBSC and ATG (20 versus 25%, above and below the total iNKT median dose, respectively, P=0.53).35 In contrast with other T cells, iNKT cells are spared by the use of total lymphoid irradiation (TLI) and ATG in mice and humans.36, 37, 38 Thus, iNKT/T cell frequencies seem more meaningful to be analyzed, in particular in the context of clinical use of ATG. Unfortunately, such analysis has not been performed in Malard’s study.
As described by others,39 the proportions of iNKT cells after G-CSF mobilization were similar between PBSC and BM grafts. However, iNKT cell doses were significantly increased in PBSC, and G-CSF mobilized iNKT cells were more prompt to expand in response to rhIL-2 and α-GalCer, possibly due to reduced antigen presenting cell numbers in BM versus PB. This may explain the absence of predictive value of the CD4− iNKT cell expansion factor from BM grafts on the occurrence of aGVHD. We also observed that frequencies, doses and expansion capacity of CD4− iNKT cells were not correlated with donor age, whereas numbers of CD4+ iNKT cells declined with donor’s age, a phenomenon explained by the dependence of CD4+ iNKT cells on thymic output, while CD4− iNKT cells can undergo peripheral homeostatic expansion.40, 41
Although the frequency of iNKT cells and their ex vivo expansion capacity in the presence of IL-2 and α-GalCer are known to be very variable between individuals,40, 41, 42 the reasons are poorly understood. Croudace et al.42 showed that poor proliferative responses of iNKT cells to IL-2 and α-GalCer were not dependent on the type and concentration of the CD1d ligand used, nor to the disappearance of iNKT cells during the 14-day culture period, but could be partially salvaged by the addition of IL-4. The expansion factor of CD4+ iNKT cells was higher in grafts with a CD4− iNKT expansion factor above 6.83 in comparison to those below that threshold. Thus, the difference of CD4− iNKT cell expansion in the presence of rhIL-2 and α-GalCer in healthy donors might be due to distinct CD4+ iNKT cells response to IL-2, which might subsequently, directly or indirectly impact on the expansion of to the CD4− iNKT cell subset. It has been shown that murine iNKT cells undergo a bystander expansion and activation depending on IL-2 produced by alloreactive T cells after allo-SCT.43 We observed an enhanced early post-transplant iNKT cell recovery in patients who received a graft with higher ex vivo CD4− iNKT cell expansion capacities. These data confirm our previous report correlating post-transplant iNKT cell recovery with the occurrence of aGVHD,23 and suggest that the ex vivo expansion test of CD4− iNKT cells can reflect and predict the in vivo expansion capacities of these cells.
In our study, grade II-IV aGVHD occurred in 25 patients and four of them developed grade III-IV aGVHD, which was lethal in two cases. Although the CD4− iNKT cell expansion factor could highly predict the occurrence of aGVHD, no impact on NRM and OS was observed. Despite a very low risk of aGVHD, patients who received a graft with a CD4− iNKT cell expansion factor above 6.83 had preserved GVL effect without increased relapse risk, possibly due to the unaffected development of chronic GVHD. This observation was in line with preserved GVL effect observed in patients with higher iNKT cell recovery after allo-SCT in other studies.21, 23
The mechanisms by which human CD4− iNKT cells can control GVHD while preserving the GVL effect remain to be determined. By comparison to CD4+ iNKT cells, their CD4− counterparts kill dendritic cells and reduce the maturation of monocyte-derived DCs.25, 44 Whether they can induce Tregs or modulate the cytokine orientation of allogeneic T-cell responses, as reported for murine iNKT cells in mouse models of GVHD,37, 45 will require further studies.
In conclusion, despite the limitations due to the heterogeneity of our series of patients and the need for prospective validation studies, we describe a clinically useful and relevant tool that could predict at the individual level the occurrence of aGVHD without impairment of the GVL effect after allo-SCT performed with PBSC grafts. Therefore, this test could easily be used to select the best donor before transplantation and/or to adapt post-transplant immunosuppressive treatments in the recipient.
References
Ferrara JL, Levine JE, Reddy P, Holler E . Graft-versus-host disease. Lancet 2009; 373: 1550–1561.
Nash RA, Storb R . Graft-versus-host effect after allogeneic hematopoietic stem cell transplantation: GVHD and GVL. Curr Opin Immunol 1996; 8: 674–680.
Chang YJ, Zhao XY, Huo MR, Huang XJ . Expression of CD62L on donor CD4(+) T cells in allografts: correlation with graft-versus-host disease after unmanipulated allogeneic blood and marrow transplantation. J Clin Immunol 2009; 29: 696–704.
Yakoub-Agha I, Saule P, Depil S, Micol JB, Grutzmacher C, Boulanger-Villard F et al. A high proportion of donor CD4+ T cells expressing the lymph node-homing chemokine receptor CCR7 increases incidence and severity of acute graft-versus-host disease in patients undergoing allogeneic stem cell transplantation for hematological malignancy. Leukemia 2006; 20: 1557–1565.
Loschi M, Porcher R, Peffault de Latour R, Vanneaux V, Robin M, Xhaard A et al. High number of memory T cells is associated with higher risk of acute graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2015; 21: 569–574.
Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med 2003; 9: 1144–1150.
Trenado A, Sudres M, Tang Q, Maury S, Charlotte F, Gregoire S et al. Ex vivo-expanded CD4+CD25+ immunoregulatory T cells prevent graft-versus-host-disease by inhibiting activation/differentiation of pathogenic T cells. J Immunol 2006; 176: 1266–1273.
Hashimoto D, Asakura S, Miyake S, Yamamura T, Van Kaer L, Liu C et al. Stimulation of host NKT cells by synthetic glycolipid regulates acute graft-versus-host disease by inducing Th2 polarization of donor T cells. J Immunol 2005; 174: 551–556.
Kuwatani M, Ikarashi Y, Iizuka A, Kawakami C, Quinn G, Heike Y et al. Modulation of acute graft-versus-host disease and chimerism after adoptive transfer of in vitro-expanded invariant Valpha14 natural killer T cells. Immunol Lett 2006; 106: 82–90.
Morris ES, MacDonald KP, Rowe V, Banovic T, Kuns RD, Don AL et al. NKT cell-dependent leukemia eradication following stem cell mobilization with potent G-CSF analogs. J Clin Invest 2005; 115: 3093–3103.
Yang J, Gao L, Liu Y, Ren Y, Xie R, Fan H et al. Adoptive therapy by transfusing expanded donor murine natural killer T cells can suppress acute graft-versus-host disease in allogeneic bone marrow transplantation. Transfusion 2009; 50: 407–417.
Pillai A, Teo P, George T, Mukhopadhyay A, Dejbakhsh-Jones S, Strober S . Alloantigen recognition is critical for CD8 T cell-mediated graft anti-tumor activity against murine BCL1 lymphoma after myeloablative bone marrow transplantation. Bone Marrow Transplant 2007; 40: 487–497.
Leveson-Gower DB, Olson JA, Sega EI, Luong RH, Baker J, Zeiser R et al. Low doses of natural killer T cells provide protection from acute graft-versus-host disease via an IL-4-dependent mechanism. Blood 2011; 117: 3220–3229.
Kuns RD, Morris ES, Macdonald KP, Markey KA, Morris HM, Raffelt NC et al. Invariant natural killer T cell-natural killer cell interactions dictate transplantation outcome after alpha-galactosylceramide administration. Blood 2009; 113: 5999–6010.
Magenau JM, Qin X, Tawara I, Rogers CE, Kitko C, Schlough M et al. Frequency of CD4(+)CD25(hi)FOXP3(+) regulatory T cells has diagnostic and prognostic value as a biomarker for acute graft-versus-host-disease. Biol Blood Marrow Transplant 2010; 16: 907–914.
Ukena SN, Velaga S, Geffers R, Grosse J, Baron U, Buchholz S et al. Human regulatory T cells in allogeneic stem cell transplantation. Blood 2011; 118: e82–e92.
Kawano Y, Kim HT, Matsuoka K, Bascug G, McDonough S, Ho VT et al. Low telomerase activity in CD4+ regulatory T cells in patients with severe chronic GVHD after hematopoietic stem cell transplantation. Blood 2011; 118: 5021–5030.
Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood 2005; 106: 2903–2911.
Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 2011; 117: 3921–3928.
Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP 3rd et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med 2011; 365: 2055–2066.
de Lalla C, Rinaldi A, Montagna D, Azzimonti L, Bernardo ME, Sangalli LM et al. Invariant NKT cell reconstitution in pediatric leukemia patients given HLA-haploidentical stem cell transplantation defines distinct CD4+ and CD4- subset dynamics and correlates with remission state. J Immunol 2010; 186: 4490–4499.
Beziat V, Nguyen S, Exley M, Achour A, Simon T, Chevallier P et al. Shaping of iNKT cell repertoire after unrelated cord blood transplantation. Clin Immunol 2010; 135: 364–373.
Rubio MT, Moreira-Teixeira L, Bachy E, Bouillie M, Milpied P, Coman T et al. Early posttransplantation donor-derived invariant natural killer T-cell recovery predicts the occurrence of acute graft-versus-host disease and overall survival. Blood 2012; 120: 2144–2154.
Rosenzwajg M, Dhedin N, Maury S, Bensimon G, Landau DA, Norol F et al. Regulatory T cell content in the bone marrow graft does not predict the occurrence of acute GVHD. Biol Blood Marrow Transplant 2010; 17: 265–269.
Chaidos A, Patterson S, Szydlo R, Chaudhry MS, Dazzi F, Kanfer E et al. Graft invariant natural killer T-cell dose predicts risk of acute graft-versus-host disease in allogeneic hematopoietic stem cell transplantation. Blood 2012; 119: 5030–5036.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med 1980; 69: 204–217.
Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009; 30: 899–911.
Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol 2009; 7: e54.
R Development Core Team. R: A Language and Environment for Statistical Computing. 2006 [cited ISBN 3-900051-07-0, Available at: http://www.R-project.org)].
Therneau TM, Grambsch PM . Modeling Survival Data: Extending the Cox Model. Springer: New York, 2000.
Tosteson TD, Buonaccorsi JP, Demidenko E, Wells WA . Measurement error and confidence intervals for ROC curves. Biom J 2005; 47: 409–416.
Sing T, Sander O, Beerenwinkel N, Lengauer T . ROCR: visualizing classifier performance in R. Bioinformatics 2005; 21: 3940–3941.
Schmid C, Schleuning M, Ledderose G, Tischer J, Kolb HJ . Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol 2005; 23: 5675–5687.
Malard F, Labopin M, Chevallier P, Guillaume T, Duquesne A, Rialland F et al. Larger number of invariant natural killer T cells in PBSC allografts correlates with improved GVHD-free and progression-free survival. Blood 2016; 127: 1828–1835.
Pillai AB, George TI, Dutt S, Teo P, Strober S, Host NKT . cells can prevent graft-versus-host disease and permit graft antitumor activity after bone marrow transplantation. J Immunol 2007; 178: 6242–6251.
Pillai AB, George TI, Dutt S, Strober S . Host natural killer T cells induce an interleukin-4-dependent expansion of donor CD4+CD25+Foxp3+ T regulatory cells that protects against graft-versus-host disease. Blood 2009; 113: 4458–4467.
Lowsky R, Takahashi T, Liu YP, Dejbakhsh-Jones S, Grumet FC, Shizuru JA et al. Protective conditioning for acute graft-versus-host disease. N Engl J Med 2005; 353: 1321–1331.
Morris ES, MacDonald KP, Hill GR . Stem cell mobilization with G-CSF analogs: a rational approach to separate GVHD and GVL? Blood 2006; 107: 3430–3435.
Baev DV, Peng XH, Song L, Barnhart JR, Crooks GM, Weinberg KI et al. Distinct homeostatic requirements of CD4+ and CD4- subsets of Valpha24-invariant natural killer T cells in humans. Blood 2004; 104: 4150–4156.
Jing Y, Gravenstein S, Chaganty NR, Chen N, Lyerly KH, Joyce S et al. Aging is associated with a rapid decline in frequency, alterations in subset composition, and enhanced Th2 response in CD1d-restricted NKT cells from human peripheral blood. Exp Gerontol 2007; 42: 719–732.
Croudace JE, Curbishley SM, Mura M, Willcox CR, Illarionov PA, Besra GS et al. Identification of distinct human invariant natural killer T-cell response phenotypes to alpha-galactosylceramide. BMC Immunol 2008; 9: 71.
Jukes JP, Wood KJ, Jones ND . Bystander activation of iNKT cells occurs during conventional T-cell alloresponses. Am J Transplant 2012; 12: 590–599.
Liu TY, Uemura Y, Suzuki M, Narita Y, Hirata S, Ohyama H et al. Distinct subsets of human invariant NKT cells differentially regulate T helper responses via dendritic cells. Eur J Immunol 2008; 38: 1012–1023.
Schneidawind D, Pierini A, Alvarez M, Pan Y, Baker J, Buechele C et al. CD4+ invariant natural killer T cells protect from murine GVHD lethality through expansion of donor CD4+CD25+FoxP3+ regulatory T cells. Blood 2014; 124: 3320–3328.
Acknowledgements
We are grateful to the NIH Tetramer Core Facility for providing CD1d tetramer reagents, the clinical investigation center (CIC) of Necker Hospital for their participation in clinical studies, the ‘Ligue Nationale contre le Cancer’, the APHP (Assistance Publique des Hopitaux de Paris), the ‘Cancéropole d’Ile de France’, and the ‘Institut National du Cancer’ for supportive grants. MM thanks Prof JV Melo (Adelaide, Australia) for critical reading of the manuscript. MM also acknowledges the educational grants received from the ‘Association for Training, Education and Research in Hematology, Immunology and Transplantation’ (ATERHIT). The Saint-Antoine hospital group (MTR, MM) is supported by several grants from the national Hospital Clinical Research Program (‘PHRC’) from the French National Cancer Institute.
Author contributions
MTR, MB and OH collected the data. MTR, FS, AG, DS, AB, EP, SNK, MM and OH provided the patients’ data. HTN, MC and OL provided materials and expertise in cell phenotyping. MTR, MB, NB, SU and OH analyzed the data. MTR, MB, MM, NB, SU and OH wrote and commented on the manuscript. All authors approve the final manuscript.
Key points
(1) Graft CD4− iNKT cell ex vivo expansion capacity is the best graft parameter capable of predicting the occurrence of acute GVHD after allogeneic stem cell transplantation from PBSC. (2) Donor CD4− iNKT cell expansion capacity test could be used to select the best donor before performing allogeneic stem cell transplantation with PBSC.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Rubio, MT., Bouillié, M., Bouazza, N. et al. Pre-transplant donor CD4− invariant NKT cell expansion capacity predicts the occurrence of acute graft-versus-host disease. Leukemia 31, 903–912 (2017). https://doi.org/10.1038/leu.2016.281
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.281
- Springer Nature Limited
This article is cited by
-
‘Off-the-shelf’ allogeneic CAR T cells: development and challenges
Nature Reviews Drug Discovery (2020)
-
Immune regulation in hematopoietic cell transplantation
Bone Marrow Transplantation (2019)
-
CD3+ graft cell count influence on chronic GVHD in haploidentical allogeneic transplantation using post-transplant cyclophosphamide
Bone Marrow Transplantation (2018)
-
Influence of post-transplant mucosal-associated invariant T cell recovery on the development of acute graft-versus-host disease in allogeneic bone marrow transplantation
International Journal of Hematology (2018)