Abstract
The myeloproliferative neoplasms (MPNs) are characterized by hematopoietic stem/progenitor cell (HSPC) expansion and overproduction of mature blood cells. The JAK2V617F mutation is present in hematopoietic cells in a majority of patients with MPNs, but the mechanism(s) responsible for MPN stem cell expansion remain incomplete. One hallmark feature of the marrow in patients with MPNs is megakaryocyte (MK) hyperplasia. We report here that mice bearing a human JAK2V617F gene restricted exclusively to the MK lineage develop many of the features of a MPN. Specifically, these mice exhibit thrombocytosis, splenomegaly, increased numbers of marrow and splenic hematopoietic progenitors and a substantial expansion of HSPCs. In addition, wild-type mice transplanted with cells from JAK2V617F-bearing MK marrow develop a myeloproliferative syndrome with thrombocytosis and erythrocytosis as well as pan-hematopoietic progenitor and stem cell expansion. As marrow histology in this murine model of myeloproliferation reveals a preferentially perivascular localization of JAK2V617F-mutant MKs and an increased marrow sinusoid vascular density, it adds to accumulating data that MKs are an important component of the marrow HSPC niche, and that MK expansion might indirectly contribute to the critical role of the thrombopoietin/c-Mpl signaling pathway in HSPC maintenance and expansion.
Similar content being viewed by others
Introduction
The marrow consists of the hematopoietic cells and non-hematopoietic stromal cells, including fibroblasts, reticular cells, endothelial cells (ECs), macrophages, adipocytes and osteoblasts. The hematopoietic stem/progenitor cell (HSPC) niche is a complex marrow microenvironment that maintains and regulates HSPCs throughout life. Currently, controversy surrounds the anatomical location of the HSPC niche which has been identified in the sinusoidal vascular areas (the ‘perivascular niche’) and/or at the endosteal surface (the ‘osteoblastic niche’).1, 2, 3, 4, 5, 6, 7, 8 It has been postulated that different niches may have different roles in HSPC physiology during normal and stress hematopoiesis.5, 9 In addition to its role in normal HSPC biology, an altered microenvironment is an important contributor to the development of hematologic malignancies.10, 11, 12 In a reciprocal fashion, myeloid malignancies also affect the function of the marrow microenvironment to impair normal hematopoiesis while favoring malignant stem cell expansion.13, 14
The cellular composition of the hematopoietic niche includes both marrow stromal cells and hematopoietic cells.5, 15, 16, 17 Megakaryocytes (MK) are rare polyploid marrow cells that give rise to blood platelets. They are often located adjacent to marrow sinusoids, an anatomy required in order for the cells to issue platelets by the forces generated by flowing sinusoidal blood.18 Very recent evidence also implicated MKs in regulating HSPC activity by the many cytokines and extracellular matrix components produced by these cells.19, 20, 21, 22, 23 Therefore, it is not surprising that HSPCs are frequently (~20%) located adjacent to MKs in vivo and transplanted HSPCs preferentially co-localize with mature MKs in the marrow.19, 20, 23
The chronic Philadelphia chromosome (Ph1) negative myeloproliferative neoplasms (MPNs), including polycythemia vera, essential thrombocythemia and primary myelofibrosis, are clonal stem cell disorders characterized by HSPC expansion and overproduction of blood cells. The acquired signaling kinase mutation JAK2V617F has a central role in the pathogenesis of MPN, but our understanding of the stem cell expansion that characterizes MPNs remains incomplete. Although the etiology of dysregulated hematopoiesis has been mainly attributed to the molecular alterations within the HSPCs, abnormalities of the marrow microenvironment are beginning to be recognized as an important factor in the development of MPNs.10, 14, 24, 25
Allogeneic stem cell transplantation is the only curative treatment for patients with MPNs. However, its utility is often limited by poor engraftment, which contributes to treatment-related morbidity and mortality.26 Since the diseased MPN HSPC niche could impair normal hematopoiesis following stem cell transplantation, and favor the residual MPN stem cells,14 studies of the complex interactions between MPN stem cells and their marrow microenvironment could provide new insights into disease pathophysiology and, potentially, to new opportunities for treatment of these disorders.
MK hyperplasia is a hallmark feature of all three chronic Ph1 negative MPNs.27 In the present study, we hypothesized that the presence of the JAK2V617F mutation in MKs affects the marrow microenvironment and could, in so doing, contribute to MPN stem cell expansion and its transformation. To test this hypothesis, we crossed mice that bear a Cre-inducible human JAK2V617F gene (FF1) with mice that express Cre specifically in the MK lineage (Pf4-Cre) to express JAK2V617F restricted to MK lineage.28, 29, 30, 31 This model has provided us with the unique ability to study the effect of JAK2V617F-bearing MKs on MPN disease development in vivo. Using this model, we found that MKs affect HSPC expansion, establishing that MKs form an important part of the marrow HSPC niche.
Materials and methods
Experimental mice
JAK2V617F FF1 mice (which carry a Cre-inducible human JAK2V617F gene driven by the human JAK2 promoter) and Pf4-Cre mice (which express Cre under the promoter of platelet factor 4, a MK-specific gene) were provided by Radek Skoda (University Hospital Basal, Switzerland). FF1 mice were crossed with Pf4-Cre mice to generate MK cell lineage-specific human JAK2V617F knock-in mouse lines (Pf4/FF1) as we previously described.32 All mice used were bred onto a C57BL/6 background and housed and expanded in a pathogen-free mouse facility at Stony Brook University. CD45.1+ congenic mice (B6.SJL) were purchased from Taconic Inc (Albany, NY, USA). Animal experiments were performed in accordance with the guidelines provided by the Institutional Animal Care and Use Committee at Stony Brook University.
Complete blood counts and colony assays
Complete blood counts and hematopoietic colony formation assays were performed as we previously described.32 Detailed information is provided in the Supplementary Information.
Histology and marrow sinusoid vascular density
Tissues were fixed in 10% (vol/vol) formalin and paraffin sections (5 μm thickness) were stained with hematoxylin and eosin. Two control mice and three Pf4/FF1 mice were used for marrow vascular density analysis. A total of 12 and 26 high-quality non-overlapping areas at × 200 magnification were imaged for the control mice and Pf4/FF1 mice, respectively. Histomorphometric analysis of marrow vasculature was performed manually using the grid method with ImageJ software (National Institute of Health, Bethesda, MD, USA) on saved marrow images. Additional details are provided in Supplementary Information.
Flow cytometry
Marrow and spleen cell flow cytometric sorting was performed on a FACSAriaTM III (BD, San Jose, CA, USA). CD45 (Clone 104) (Biolegend, San Diego, CA, USA), EPCR (CD201) (Clone eBio1560, eBioscience, San Diego, CA, USA), CD48 (Clone HM48-1, Biolegend) and CD150 (Clone mShad150, eBioscience) antibodies were used to enumerate CD45+EPCR(CD201)+CD48−CD150+ (E-SLAM) cells. MKs were sorted on the basis of CD41 expression (Biolegend) and cell size.20 Additional details are provided in Supplementary Information.
Bone marrow transplantation assays
Hematopoietic stem cell transplantation was performed on CD45.1+ mice as wild-type recipients and Pf4/FF1 or age-matched littermate controls (CD45.2) as bone marrow donor. Additional details are provided in Supplementary Information.
Isolation of murine lung endothelial cells
Primary murine lung EC isolation was performed using a protocol modified from previous published protocols.32, 33, 34 Details are provided in the Supplementary Information.
In vitro cultures
E-SLAM HSPCs were sorted and cultured in StemSpan serum-free expansion medium (SFEM) (Stem Cell Technologies, Vancouver, BC, Canada) containing recombinant mouse SCF (300 ng/ml) and recombinant mouse IL-11 (20 ng/ml) (Stem Cell Technologies). CD41+ MK cells were cultured in SFEM with 25ng/ml recombinant mouse SCF and 25 ng/ml recombinant human thrombopoietin (TPO) (Stem Cell Technologies). MK-conditioned media (MKCM) was collected from MK cells after 48–72 h of culture. ECs were cultivated in advanced DMEM/F12 medium supplemented with 20% fetal bovine serum, 50 μg/ml EC growth supplement (Alfa Aesar, Ward Hill, MA, USA), 10 ng/ml recombinant mouse vascular endothelial growth factor and 20 ng/ml recombinant human fibroblast growth factor 2 (FGF2) (both from PeproTech, Rocky Hill, NJ, USA). Details are provided in Supplementary Information.
Assays to examine endothelial cell in vitro angiogenesis and cell migration
EC tube formation assay was performed on Matrigel matrix (Corning Inc., Corning, NY, USA) and the number of branches and nodes were quantified using ImageJ software (National Institute of Health) as a measure of in vitro angiogenesis. Scratch assay was used to assess EC migration in vitro. Details are provided in the Supplementary Information.
Statistical analysis
Statistical analyses were performed using Student’s unpaired, two-tailed t-tests using Excel software (Microsoft, Bellevue, WA, USA). A P-value <0.05 was considered significant. For all bar graphs, data are represented as mean±s.e.m.
Results
Pf4/FF1 Mice develop an essential thrombocythemia phenotype
To induce the expression of human JAK2V617F exclusively in MKs, we crossed the JAK2V617F FF1 mice with the Pf4-Cre mice. In Pf4-Cre mice, the Cre cDNA is under the control of the platelet factor 4 (Pf4) promoter, which is activated exclusively in the MK lineage.28, 30, 32 Levels of the human JAK2V617F expression in MKs isolated from the Pf4/FF1 mice was ~10% of that of the endogenous murine JAK2, which is close to what expected in blood cells of patients with MPNs expressing a single copy of the mutant JAK2 kinase.32
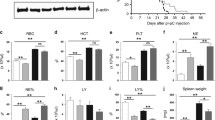
Modest thrombocytosis was observed in Pf4/FF1 mice at 16 weeks of age. At 28 weeks of age, Pf4/FF1 mice showed a significant increase in platelet count (1406 vs 808 × 109/l, P=0.00087) but normal hemoglobin and white blood cell counts, resembling the blood profile of patients with essential thrombocythemia. Blood cell counts over time for the Pf4/FF1 mice and age-matched littermate control mice are shown in Figure 1a. Spleens collected from 28-week-old mice showed moderate splenomegaly in the Pf4/FF1 mice compared with the control mice (spleen weight 101 mg vs 70 mg, P=0.040) (Figure 1b). The number of marrow hematopoietic progenitors in Pf4/FF1 mice was assessed using colony formation assays, and revealed significant increases in colony-forming unit-MK (CFU-MK)-committed progenitors (1.9-fold, P=0.00009) (Figure 1c), burst forming unit-erythroid (BFU-e) (1.8-fold, P=0.011), colony-forming unit-granulocyte/macrophage (CFU-GM) (1.8-fold, P=0.017), and total hematopoietic progenitor cells (1.8-fold, P=0.007) (Figures 1d–f) compared with control mice.
Pf4/FF1 mice develop an essential thrombocythemia phenotype. (a) We found a significant increase in platelet count but no change in hemoglobin or white blood cell count in Pf4/FF1 mice compared with age-matched littermate controls (n=4–7 mice per group and time-point). On the basis of the two-sample t-test, a sample size of five mice per group will have 80% power to detect a difference of 2.1 s.d. between two experiment groups. (b) Spleens collected from 28-week-old mice revealed splenomegaly in the Pf4/FF1 mice with a significant increase in spleen weight compared with controls. (c) Colony assays from isolated marrow cells showed a significant increase (1.9-fold, P=0.00009) in the MK progenitors (CFU-MK) in Pf4/FF1 mice compared with littermate controls. (d–f) Pf4/FF1 mice also exhibited significant increases in BFU-e (1.8-fold, P=0.011), CFU-GM (1.8-fold, P=0.017), and total hematopoietic colonies (1.8-fold, P=0.007) compared with littermate controls.
Having demonstrated that Pf4/FF1 mice developed thrombocytosis and expanded hematopoietic progenitor cells, we undertook a quantitative evaluation of the marrow and spleen HSPC compartment in Pf4/FF1 and control mice. Consistent with the thrombocytosis phenotype, CD41+ MK cell frequency was increased 2.9-fold (2.9 vs 1.0% of total marrow cells, P=0.032, n=4) in Pf4/FF1 mice compared with control mice (Figures 2a and b). Although the hemoglobin and white blood cell counts were normal in Pf4/FF1 mice, the expanded hematopoietic progenitor cells (Figures 1d–f) suggested that the HSPCs may be involved in the disease process. Therefore, we assessed the numbers of CD45+EPCR+CD48−CD150+ (E-SLAM) cells, which is a highly purified long-term repopulating HSPC population.4 In Pf4/FF1 mice, marrow E-SLAM cell frequency was increased 3.2-fold (0.102 vs 0.032% of total marrow cells, P=0.028, n=4) compared with control mice (Figures 2c and d). Considering that the total marrow cells were increased 1.44-fold (P=0.013) in Pf4/FF1 mice (Figure 2e), compared with control animals there were 4.2-fold and 4.6-fold increases in absolute MK cell numbers and E-SLAM cell numbers in Pf4/FF1 mice, respectively. E-SLAM cells and MKs also significantly increased in Pf4/FF1 mice spleen compared with wild-type (WT) controls (data not shown). Lineage analysis showed increased myeloid (Mac-1+Gr-1+) cells and decreased erythroid (CD71+) cells in Pf4/FF1 mice marrow and spleen compared with controls (data not shown). No significant difference in B220+ lymphoid cells was observed between Pf4/FF1 and control mice.
Pf4/FF1 mice have HSPC expansion. (a) Representative flow cytometry plots for the isolation of marrow CD41+ MKs. (b) In Pf4/FF1 mice marrow, MK frequency was increased 2.9-fold (P=0.032, n=4) compared with littermate controls. (c) Representative flow cytometry plots for the isolation of marrow CD45+/EPCR+/CD48-/CD150+ (E-SLAM) cells. Live cells were first gated for CD45+CD201+ then subsequently gated for CD48-CD150+. (d) In Pf4/FF1 mice marrow, E-SLAM cell frequency was increased 3.2-fold (P=0.028, n=4) compared with littermate controls. (e) Total marrow cell numbers were increased in Pf4/FF1 mice (1.44-fold, P=0.013) compared with control mice which was set as ‘1’. (f) As determined by reverse transcription polymerase chain reaction (RT-PCR) for FF1, human JAK2V617F was expressed in MK cells from Pf4/FF1 mice, but not in E-SLAM cells. (g) No JAK2V617F expression was detected in plucked CFU-GM colony cells from Pf4/FF1 mice. (top) FF1 RT-PCR; (bottom) mouse actin RT-PCR. (h) A serial dilution test using a mix of Pf4/FF1 MK and control MK samples demonstrated that our FF1 RT-PCR assay was able to detect positive JAK2 gene expression after 1:48 dilution of the Pf4/FF1 samples.
To be certain that JAK2V617F did not directly influence the numbers of E-SLAM cells because the Pf4 promoter was ‘leaky’,35, 36 we tested purified cells by FF1 RT-PCR.32, 37 Consistent with previous reports,19, 28, 30 human JAK2 gene expression was detected in MKs but not in E-SLAM cells. (Figure 2f) Furthermore, no JAK2V617F expression was detected in plucked CFU-GM colonies from Pf4/FF1 mice. (Figure 2g) To be certain we did not miss small populations of recombined cells,35 we examined the sensitivity of our PCR assay. A serial dilution test using a mix of Pf4/FF1 MK and control MK samples demonstrated that our PCR assay was able to detect positive JAK2 gene expression after a 1:48 dilution of the Pf4/FF1 samples. (Figure 2h).
To test whether the phenotype of the Pf4/FF1 mice is intrinsic to hematopoietic cells, we transplanted marrow cells from 28-week-old Pf4/FF1 mice or controls (CD45.2) into lethally irradiated congenic CD45.1 recipients (n=5 in each group). Eight weeks post transplant, recipients of Pf4/FF1 marrow displayed a myeloproliferative syndrome (MPS), with significantly elevated platelet count and mild elevation of Hb compared with mice transplanted with control marrow cells, suggesting the Pf4/FF1 phenotype was intrinsic to the hematopoietic cells. The time course of blood cell counts in the transplant recipient mice is shown in Figure 3. JAK2V617F expression was not detected in BFUe colonies from recipient of the Pf4/FF1 marrow (data not shown), suggesting that the observed erythrocytosis does not represent activation of the JAK2V617F at the HSPC or committed erythroid progenitor cell level during marrow reconstitution.
Recipients of Pf4/FF1 marrow displayed a MPS phenotype. Marrow cells from 28-week-old Pf4/FF1 mice or controls (CD45.2) were transplanted into lethally irradiated congenic CD45.1 recipients (n=5 in each group). Shown here are CD45.2 donor chimerism (a), white blood cell (WBC) (b), red blood cell (RBC) (c), and platelet (d) counts following transplantation. Eight weeks post transplant, recipients of Pf4/FF1 marrow displayed a MPS phenotype, with significantly elevated platelet count and mild elevation of Hb compared with mice transplanted with control marrow cells. *P<0.05.
Megakaryocyte hyperplasia is accompanied by changes in the vascular niche in Pf4/FF1 mice
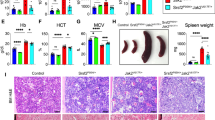
Histological analysis of marrow hematoxylin/eosin sections revealed markedly increased numbers of MKs in the Pf4-Cre/FF1 mice compared with controls. (Figure 4a) In addition, we noticed dilated marrow sinusoids in the Pf4/FF1 mice and MKs were preferentially located near sinusoid vessels (Figure 4b). Quantitative analysis revealed increased marrow sinusoid vascular density in Pf4/FF1 mice marrow compared with controls (2.7-fold, P=0.010) (Figure 4b). As has been previously described, MKs reside in close contact with the marrow ECs in vivo and ECs have an important role in the regulation of MK maturation and release of platelets.4, 19, 38, 39, 40, 41 In contrast, few studies have examined the functional role of MKs in the regulation of marrow vasculature niche function, despite MKs representing an important reservoir of bioactive hematopoietic and angiogenic factors. To study the effects of JAK2V617F MKs on EC function, tube formation assay (as a measure of in vitro angiogenesis) and scratch assay (as a measure of in vitro EC migration) were performed in primary murine lung ECs isolated from wild-type C57BL/6 mice (Supplementary Figure 1). Pf4/FF1 MKCM significantly stimulated EC tube formation and EC migration in vitro compared with WT MKCM did. (Figure 5) These findings suggest that JAK2V617F-mutant MKs may expand the sinusoidal vascular niche,42 which in turn could contribute to the thrombocytosis and pan-hematopoietic expansion phenotype displayed by the Pf4/FF1 mice. These findings also resemble those seen in human MPNs, characterized by increased marrow MKs, increased hematopoietic progenitors of all lineages, and enhanced angiogenesis compared with normal marrow.43, 44, 45 It should be noted, however, that in contrast to human MPNs and many murine models of MPN, the Pf4/FF1 mice did not display marrow or splenic fibrosis, at least by 28 week of age.
Hematoxylin and eosin sections of bone marrow revealed MK hyperplasia and increased marrow sinusoid density. (a) There was markedly increased numbers of MK in Pf4-Cre/FF1 mice (right) compared with controls (left). (b) Red blood cell-engorged dilated central sinus (*) was observed in 3/3 Pf4/FF1 mice marrow and MKs were preferentially located near sinusoids (S) (top). Quantitative analysis revealed increased marrow sinusoid vascular density in Pf4/FF1 mice (n=3) marrow compared with controls (n=2) (2.7-fold, P=0.010) (bottom).
Pf4/FF1 MKCM promoted EC in vitro angiogenesis and cell migration. (a) Pf4/FF1 MKCM stimulated EC tube formation. Primary murine lung ECs (6 × 104) were seeded in Matrigel matrix and incubated in the presence of SFEM, control MKCM or Pf4/FF1 MKCM. The effect of MKCM on EC tube formation was observed after a 4 h incubation. A representative picture is shown. Magnification: × 100 (b) Quantification of Pf4/FF1 MKCM-mediated tube formation stimulation. Images of tube formation were taken at × 40 magnification and quantification was done by counting the number of nodes (or branch points) and tubes in four non-overlapping fields. Results are expressed as the mean±s.e.m. (n=4). Data are from one of two independent experiments that gave similar results. (c) Pf4/FF1 MKCM promoted EC migration. A lesion was produced across the primary murine EC monolayer. Cells were cultured in the presence of SFEM, control MKCM or Pf4/FF1 MKCM for 24 h and then labeled with 10 ug/ml of DiI-Ac-LDL at 37 °C for 4 hours before photographed. A representative picture is shown. Magnification: 40 × (d). Quantification of recovery of the scratched wound after MKCM treatment. The distances from one side of the wound to the other side were measured using ImageJ software (National Institute of Health) at 6–12 different locations in two duplicate wells for each culture condition. The distance of wound closure at time 16, 24 and 40 h was compared with the distance at time 0 h which was set as 1. The results were expressed as the mean±s.e.m. (n=6-12). Data are from one of two independent experiments that gave similar results.
JAK2V617F-mutant megakaryocytes affect hematopoietic stem/progenitor cell function in Pf4/FF1 mice
Previous studies have demonstrated that MKs regulate HSPC function by both direct and indirect effects on the marrow niche. Our findings of altered marrow vascular niche in association with increased MK numbers and E-SLAM cell numbers in Pf4/FF1 mice prompted us to study how JAK2V617F-bearing MK cells might affect E-SLAM cell numbers and function as a component of the stem cell niche.
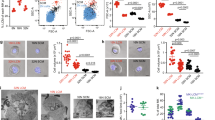
We purified MKs using FACS on the basis of CD41 expression and cell size.20 Consistent with the increased megakaryopoiesis in vivo, the JAK2V617F-mutant Pf4/FF1 MKs had a higher proliferation rate in vitro than did control MKs (1.7-fold, P=0.003) (Figure 6a). In contrast, there was no significant difference in cell proliferation between the Pf4/FF1 E-SLAM cells and control E-SLAM cells (Figure 6b). MKs secrete a broad array of cytokines to regulate hematopoiesis.46 To begin to understand the MK signals responsible for E-SLAM expansion in Pf4/FF1 mice, we measured the expression levels of FGF1, Pf4, Transforming growth factor beta 1 (TGFb-1), Chemokine (C-X-C motif) ligand 12 (CXCL12), VEGFa, chemokine (C-X-C motif) receptor 4 (CXCR4) in Pf4/FF1 (n=4) and control MKs (n=3) using quantitative PCR (qPCR). We confirmed that there was upregulation of FGF1 (twofold, P=0.004) in Pf4/FF1 MKs compared with control MKs (Figure 6c). MKs are the major source for FGF1 in marrow which positively expand MKs and facilitate HSPC expansion during stress hematopoiesis.19, 22, 47 Thus, increased FGF1 signaling in Pf4/FF1 MKs may contribute to the HSPC expansion we observed.
JAK2V617F-mutant MK contribute to the stemness of hematopoietic stem cells in Pf4/FF1 Mice. JAK2V617F-mutant Pf4/FF1 MKs had a higher proliferation rate in vitro than did control MKs (1.7-fold, P=0.003) (a). In contrast, there was no significant difference in cell proliferation between the Pf4/FF1 E-SLAM cells and control E-SLAM cells (b). (c) The expression levels of fibroblast growth factor 1 (FGF1), platelet factor 4 (Pf4), transforming growth factor beta 1 (TGFb-1), chemokine (C-X-C motif) ligand 12 (CXCL12), chemokine (C-X-C motif) receptor 4 (CXCR4) and vascular endothelial growth factor A (VEGFa) in control MKs (n=3) and Pf4/FF1 MKs (n=4) were measured using real-time qPCR. Gene expression is shown as the fold-change compared with the average control MK expression which was set as ‘1’. (d) Pf4/FF1 MKCM suppressed E-SLAM cell proliferation in the serum-free liquid culture medium (2.6-fold, P=0.0008) (left) and decreased progenitor output of the co-cultured E-SLAM cells (0.4-fold, P=0.049) when assayed in methylcellulose medium (right), while control MKCM had no significant effect on E-SLAM cell proliferation and its hematopoietic colony growth. (e) MPL expression levels in MKs (left) and E-SLAM cells (right) were measured using qPCR. There was no difference in MPL expression between Pf4/FF1 MKs and control MKs, while MPL expression was significantly increased in Pf4/FF1 E-SLAM cells compared with control E-SLAM cells (4.2-fold, P=0.012). MPL expressions in Pf4/FF1 cells were shown as the fold-change compared with the control cells which was set as ‘1’.
To further investigate if cytokines secreted by the JAK2V617F-mutant Pf4/FF1 MKs are responsible for the observed expansion in E-SLAM cell numbers, we cultured the Pf4/FF1 E-SLAM cells with prospectively collected MKCM. Pf4/FF1 MKCM suppressed E-SLAM cell proliferation in the serum-free liquid culture medium (2.6-fold, P=0.0008) and decreased progenitor output of the co-cultured E-SLAM cells (0.4-fold, P=0.049) when assayed in methylcellulose medium, while control MKCM had no significant effect on E-SLAM cell proliferation and its hematopoietic colony growth (Figures 6d). Using trypan blue dye exclusion, we did not observe any difference in cell viability between E-SLAM cells cultured alone and cells cultured with the MKCM. Although this was contrary to our expectation and a previous report,23 it was consistent with other recent reports that MKs maintain HSPC quiescence, which is required for long-term HSPC function.19, 20 This result also suggests that in order to achieve the E-SLAM cell expansion observed in Pf4/FF1 mice, certain direct cell–cell interactions involving the stem cells and their niche cells was required, conditions which were not present in the in vitro MKCM experimental setting.
We then assessed the expression of TPO receptor, c-MPL, a key regulator of HSPC quiescence.48, 49, 50, 51, 52, 53 c-MPL is expressed in long-term HSPCs and is associated with both HSPC repopulating activity and HSPC quiescence.51, 52 Previously, we demonstrated that c-MPL expression was essential for the development of thrombocytosis and the increased neoplastic stem cell pool found in a murine model of JAK2V617F-positive MPNs in which JAK2V617F was expressed in all hematopoietic cells.54 Specifically, reducing c-MPL expression attenuated MPN severity with reduced platelet count and E-SLAM cell numbers, suggesting a gene-dosage effect of receptor expression levels on the disease process. Therefore, we checked c-MPL expression levels in MKs and E-SLAM cells by qPCR. We found that, while there was no difference in c-MPL expression between Pf4/FF1 MKs and control MKs, MPL expression was significantly increased in the Pf4/FF1 E-SLAM cells compared with control E-SLAM cells (4.2-fold, P=0.012), suggesting that altered TPO/MPL signaling may be involved in the altered HSPC function in the Pf4/FF1 mice. (Figure 6e).
Discussion
Despite significant advances in our understanding of the development of MPNs, the mechanisms that lead to stem cell expansion that characterizes MPNs remains incomplete. One hallmark feature of the MPNs is MK hyperplasia. By crossing the previously described human JAK2V617F knock-in mice (FF1) with a MK lineage-specific Pf4-Cre, we have been able to highlight the importance of JAK2V617F-mutant MKs in the abnormal hematopoiesis found in a murine model of a MPS.
MKs are rare marrow cells that give rise to blood platelets. Recent studies also implicated MKs in regulating HSPC activity.19, 20, 21, 22, 23, 55, 56 Many hematological malignancies, including MPNs, are associated with aberrant megakaryopoiesis. Although increased MK numbers and/or altered MK-derived factors can increase HSPC cycling, it is not clear whether MKs can contribute to the malignant transformation in these diseases. The crossing of Pf4/Cre mice28 and JAK2V617F ‘Flip-Flop’ mice37 enabled us to express JAK2V617F solely in the MK lineage alone. Our Pf4/FF1 mice developed an essential thrombocythemia-like phenotype with thrombocytosis, splenomegaly, increased numbers of CD41+ MKs and a wide range of hematopoietic progenitors. Importantly, the myeloproliferation seen in Pf4/FF1 mice was transplantable to WT mice, demonstrating the disease is intrinsic to hematopoietic cells. The MPS developed in our Pf4/FF1 model is very different from the classic Ph1 chromosome-negative MPNs in patients, and in virtually every other murine model of MPNs, where the HSPCs harbor the disease-associated mutation. The MK lineage-specific Cre expression in the Pf4-Cre mice was previously demonstrated.28, 30, 32 Although some have raised concerns that the Pf4-Cre activity might ‘leak’ into HSPCs35, 36 we have confirmed that the human JAK2V617F was expressed in MKs but not in E-SLAM cells, CFU-GM progenitors, or BFU-e progenitors in Pf4/FF1 mice or its transplant recipients using a highly sensitive PCR assay. In addition, in contrast to the massive thrombocytosis (platelet count 3000–4000 × 109/l) developed in Vav-Cre/FF1 mice (where FF1 is expressed in all hematopoietic cells) at 10 weeks of age,37 Pf4/FF1 mice maintain a stable moderate thrombocytosis (platelet count ~1400 × 109/l) with normal hemoglobin and white blood cell counts during more than 1-year follow-up (data not shown), further supporting that JAK2V617F did not directly affect E-SLAM cells. Of note, the myeloproliferaitve phenotype (that is, thrombocytosis) in Pf4/FF1 mice did not emerge until at least 16 weeks of age. Some Pf4/FF1 mice had further delayed onset later than 16 weeks of age (data not shown), explaining why we did not observe this phenotype in our previous study.32 This stands in contrast with virtually all other murine MPN models where the HSPCs harbor the disease-causing mutation and the phenotype usually develops by 4–10 weeks of age.32, 37, 57, 58, 59, 60 It is not clear to us why the WT mice transplanted with Pf4/FF1 marrow developed the myeloproliferative phenotype earlier than the primary Pf4/FF1 mice. It is possible that the stress hematopoiesis following irradiation and marrow transplantation could produce results that are different from unperturbed hematopoiesis in the primary Pf4/FF1 mice.
Although a differentiated hematopoietic cell itself, the marrow MK has been shown in recent studies to influence HSPCs and regulate hematopoietic homeostasis by direct cell–cell contacts (with osteoblast, ECs and HSPCs) and by the secretion of soluble factors.19, 20, 21, 22, 23, 46, 55, 56 To investigate how JAK2V617F-mutant MKs affect E-SLAM cell numbers and function, we performed a series of experiments. Although there was upregulation of FGF1, which positively expand MKs and facilitate HSPC expansion during stress hematopoiesis, in Pf4/FF1 MKs compared with control MKs, Pf4/FF1 MKCM suppressed E-SLAM cell proliferation in vitro, suggesting that the sum of the MK humoral influences is not responsible for the expansion of E-SLAM cells witnessed in our studies. (Figures 6c and d) In further investigating the mechanism of the E-SLAM cell expansion seen in Pf4/FF mice, we found that c-MPL expression was markedly enhanced in E-SLAM cells from mutant compared with control mice. Two conclusions can be drawn from these data to guide future investigation. First, our data suggest that the in vivo E-SLAM cell expansion in Pf4/FF1 mice require either direct cell–cell interaction, or other HSPC niche cells that were not present in our in vitro experimental setting. Indeed, histological analysis revealed dilated marrow sinusoids and increased sinusoidal vascular density in Pf4/FF1 mice (Figure 4c). In addition, Pf4/FF1 MKCM stimulated EC angiogenesis and cell migration in vitro (Figure 5). Since the majority of HSPCs reside in the peri-sinusoidal vessels,4, 6, 7, 8, 42 JAK2V617F-mutant MKs may affect the vascular niche, which in turn could contribute to the thrombocytosis and E-SLAM expansion phenotype displayed by the Pf4/FF1 mice.
Second, with very recent reports that implicated MKs in regulating HSPC quiescence and proliferation,19, 20 our data suggested that JAK2V617F-mutant MKs might promote the ‘stemness’ of E-SLAM cells by promoting their quiescence, which is required for long-term HSPC function and expansion. TPO and c-MPL are well known for their role in HSPC maintenance.48, 49, 50, 51, 52, 53 Considering that HSPCs are often located adjacent to MKs in vivo,19, 20, 23 the increase of c-MPL expression on E-SLAM cells derived from Pf4/FF1 mice further supports an important role for MKs within the HSPC ‘niche’ that preserves the ‘stemness’ of HSPCs to allow their continued expansion. Moreover, our observation that the expression of c-MPL was significantly increased in E-SLAM cells derived from Pf4/FF1 mice suggests a possible mechanism for the MPS that developed in Pf4/FF1 mice and their transplant recipients. We and others have shown that c-MPL is essential for the development of thrombocytosis and an increased neoplastic stem cell pool in JAK2V617F-positive MPNs,54 and the Souyri laboratory has demonstrated that enhanced expression of c-MPL leads to a MPN in mice. Thus, our data and that from these report suggest that JAK2V617F-mutant MKs enhance c-MPL expression on marrow E-SLAM cells in Pf4/FF mice, enhancing stem cell expansion. The exact mechanism by which abnormal JAK2 signaling in MKs affect c-MPL expression in HSPC is not clear. Since c-MPL is expressed in long-term HSPCs and is associated with HSPC quiescence, we hypothesize that JAK2V617F-bearing MKs induce HSPC quiescence which contributes to the increased MPL expression.51, 52, 53 Competitive repopulation assays would provide a definitive answer as to whether JAK2V617F-bearing MKs have enhanced stem cell function in Pf4/FF1 mice.
In summary, our study established for the first time that JAK2V617F-bearing MKs form an important part of the marrow HSPC niche and can contribute to abnormal stem cell expansion in a murine model. We showed that MKs form an important part of the marrow HSPC niche and that expression of JAK2V617F in MKs leads to a pan-hematopoietic expansion. Further, we showed that HSPCs, as represented by the E-SLAM population, are substantially expanded by the presence of JAK2V617F-expressing MKs, and that c-MPL expression is substantially enhanced on these E-SLAM cells, consistent with prior work indicating that exogenous expression of c-MPL in murine marrow cells leads to myeloproliferation. Further work is required to fully understand the mechanism of how JAK2V617F MKs affect HSPCs. For example, what is the mechanism by which abnormal JAK2 signaling in MKs affect c-MPL expression in HSC? Is it simply an expanded number of niche-forming MKs, or is the ‘quality’ of the JAK2V617F MKs somehow changed by the mutant kinase? We believe both the number and the quality of MKs regulate its niche function and the JAK2V617F mutation not only increases MK numbers (4.2-fold) but also alters MK quality (for example, increased FGF1 mRNA expression and proangiogenic effect on EC tube formation and cell migration). Also, considering that both JAK2 wild-type clones and JAK2V617F mutant clones coexist in most patients with MPNs, how does the JAK2V617F MKs interact with normal MKs and HSPCs in vivo to contribute to the mutant clone expansion? Understanding the complex interactions between neoplasia and its microenvironment may reveal important therapeutic targets, potentially providing new treatment opportunities for these disorders.
References
Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003; 425: 841–846.
Zhang J, Niu C, Ye L, Huang H, He X, Tong WG et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003; 425: 836–841.
Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 2004; 118: 149–161.
Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ . SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005; 121: 1109–1121.
Morrison SJ, Scadden DT . The bone marrow niche for haematopoietic stem cells. Nature 2014; 505: 327–334.
Sipkins DA, Wei X, Wu JW, Runnels JM, Cote D, Means TK et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 2005; 435: 969–973.
Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 2013; 502: 637–643.
Tamplin OJ, Durand EM, Carr LA, Childs SJ, Hagedorn EJ, Li P et al. Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell 2015; 160: 241–252.
Yin T, Li L . The stem cell niches in bone. J Clin Invest 2006; 116: 1195–1201.
Walkley CR, Olsen GH, Dworkin S, Fabb SA, Swann J, McArthur GA et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell 2007; 129: 1097–1110.
Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 2010; 464: 852–857.
Geyh S, Oz S, Cadeddu RP, Frobel J, Bruckner B, Kundgen A et al. Insufficient stromal support in MDS results from molecular and functional deficits of mesenchymal stromal cells. Leukemia 2013; 27: 1841–1851.
Zhang B, Ho YW, Huang Q, Maeda T, Lin A, Lee SU et al. Altered microenvironmental regulation of leukemic and normal stem cells in chronic myelogenous leukemia. Cancer Cell 2012; 21: 577–592.
Schepers K, Pietras EM, Reynaud D, Flach J, Binnewies M, Garg T et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell 2013; 13: 285–299.
Ludin A, Itkin T, Gur-Cohen S, Mildner A, Shezen E, Golan K et al. Monocytes-macrophages that express alpha-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nat Immunol 2012; 13: 1072–1082.
Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med 2011; 208: 261–271.
Li JY, Adams J, Calvi LM, Lane TF, DiPaolo R, Weitzmann MN et al. PTH expands short-term murine hemopoietic stem cells through T cells. Blood 2012; 120: 4352–4362.
Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A et al. Dynamic visualization of thrombopoiesis within bone marrow. Science 2007; 317: 1767–1770.
Zhao M, Perry JM, Marshall H, Venkatraman A, Qian P, He XC et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med 2014; 20: 1321–1326.
Bruns I, Lucas D, Pinho S, Ahmed J, Lambert MP, Kunisaki Y et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med 2014; 20: 1315–1320.
Malara A, Currao M, Gruppi C, Celesti G, Viarengo G, Buracchi C et al. Megakaryocytes contribute to the bone marrow-matrix environment by expressing fibronectin, type IV collagen, and laminin. Stem Cells 2014; 32: 926–937.
Zhao M, Ross JT, Itkin T, Perry JM, Venkatraman A, Haug JS et al. FGF signaling facilitates postinjury recovery of mouse hematopoietic system. Blood 2012; 120: 1831–1842.
Heazlewood SY, Neaves RJ, Williams B, Haylock DN, Adams TE, Nilsson SK . Megakaryocytes co-localise with hemopoietic stem cells and release cytokines that up-regulate stem cell proliferation. Stem Cell Res 2013; 11: 782–792.
Arranz L, Sanchez-Aguilera A, Martin-Perez D, Isern J, Langa X, Tzankov A et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature 2014; 512: 78–81.
Mager LF, Riether C, Schurch CM, Banz Y, Wasmer MH, Stuber R et al. IL-33 signaling contributes to the pathogenesis of myeloproliferative neoplasms. J Clin Invest 2015; 125: 2579–2591.
Rondelli D, Goldberg JD, Isola L, Price LS, Shore TB, Boyer M et al. MPD-RC 101 prospective study of reduced-intensity allogeneic hematopoietic stem cell transplantation in patients with myelofibrosis. Blood 2014; 124: 1183–1191.
Ciurea SO, Merchant D, Mahmud N, Ishii T, Zhao Y, Hu W et al. Pivotal contributions of megakaryocytes to the biology of idiopathic myelofibrosis. Blood 2007; 110: 986–993.
Tiedt R, Schomber T, Hao-Shen H, Skoda RC . Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood 2007; 109: 1503–1506.
Hitchcock IS, Fox NE, Prevost N, Sear K, Shattil SJ, Kaushansky K . Roles of focal adhesion kinase (FAK) in megakaryopoiesis and platelet function: studies using a megakaryocyte lineage specific FAK knockout. Blood 2008; 111: 596–604.
Ng AP, Kauppi M, Metcalf D, Hyland CD, Josefsson EC, Lebois M et al. Mpl expression on megakaryocytes and platelets is dispensable for thrombopoiesis but essential to prevent myeloproliferation. Proc Natl Acad Sci USA 2014; 111: 5884–5889.
Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 2010; 13: 133–140.
Etheridge SL, Roh ME, Cosgrove ME, Sangkhae V, Fox NE, Chen J et al. JAK2V617F-positive endothelial cells contribute to clotting abnormalities in myeloproliferative neoplasms. Proc Natl Acad Sci USA 2014; 111: 2295–2300.
Fang S, Wei J, Pentinmikko N, Leinonen H, Salven P . Generation of functional blood vessels from a single c-kit+ adult vascular endothelial stem cell. PLoS Biol 2012; 10: e1001407.
van Beijnum JR, Rousch M, Castermans K, van der Linden E, Griffioen AW . Isolation of endothelial cells from fresh tissues. Nat Protoc 2008; 3: 1085–1091.
Chagraoui H, Kassouf M, Banerjee S, Goardon N, Clark K, Atzberger A et al. SCL-mediated regulation of the cell-cycle regulator p21 is critical for murine megakaryopoiesis. Blood 2011; 118: 723–735.
Calaminus SD, Guitart AV, Sinclair A, Schachtner H, Watson SP, Holyoake TL et al. Lineage tracing of Pf4-Cre marks hematopoietic stem cells and their progeny. PloS One 2012; 7: e51361.
Tiedt R, Hao-Shen H, Sobas MA, Looser R, Dirnhofer S, Schwaller J et al. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood 2008; 111: 3931–3940.
Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med 2004; 10: 64–71.
Hamada T, Mohle R, Hesselgesser J, Hoxie J, Nachman RL, Moore MA et al. Transendothelial migration of megakaryocytes in response to stromal cell-derived factor 1 (SDF-1) enhances platelet formation. J Exp Med 1998; 188: 539–548.
Rafii S, Shapiro F, Pettengell R, Ferris B, Nachman RL, Moore MA et al. Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood 1995; 86: 3353–3363.
Kong Y, Hu Y, Zhang XH, Wang YZ, Mo XD, Zhang YY et al. Association between an impaired bone marrow vascular microenvironment and prolonged isolated thrombocytopenia after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2014; 20: 1190–1197.
Acar M, Kocherlakota KS, Murphy MM, Peyer JG, Oguro H, Inra CN et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 2015; 526: 126–130.
Medinger M, Skoda R, Gratwohl A, Theocharides A, Buser A, Heim D et al. Angiogenesis and vascular endothelial growth factor-/receptor expression in myeloproliferative neoplasms: correlation with clinical parameters and JAK2-V617F mutational status. Br J Haematol 2009; 146: 150–157.
Boveri E, Passamonti F, Rumi E, Pietra D, Elena C, Arcaini L et al. Bone marrow microvessel density in chronic myeloproliferative disorders: a study of 115 patients with clinicopathological and molecular correlations. Br J Haematol 2008; 140: 162–168.
Gianelli U, Vener C, Raviele PR, Savi F, Somalvico F, Calori R et al. VEGF expression correlates with microvessel density in Philadelphia chromosome-negative chronic myeloproliferative disorders. Am J Clin Pathol 2007; 128: 966–973.
Malara A, Abbonante V, Di Buduo CA, Tozzi L, Currao M, Balduini A . The secret life of a megakaryocyte: emerging roles in bone marrow homeostasis control. Cell Mol Life Sci 2015; 72: 1517–1536.
deHaan G, Weersing E, Dontje B, van Os R, Bystrykh LV, Vellenga E et al. In vitro generation of long-term repopulating hematopoietic stem cells by fibroblast growth factor-1. Dev Cell 2003; 4: 241–251.
Kaushansky K, Lok S, Holly RD, Broudy VC, Lin N, Bailey MC et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature 1994; 369: 568–571.
Sitnicka E, Lin N, Priestley GV, Fox N, Broudy VC, Wolf NS et al. The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells. Blood 1996; 87: 4998–5005.
Kimura S, Roberts AW, Metcalf D, Alexander WS . Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci USA 1998; 95: 1195–1200.
Solar GP, Kerr WG, Zeigler FC, Hess D, Donahue C, de Sauvage FJ et al. Role of c-mpl in early hematopoiesis. Blood 1998; 92: 4–10.
Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 2007; 1: 685–697.
Qian H, Buza-Vidas N, Hyland CD, Jensen CT, Antonchuk J, Mansson R et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell 2007; 1: 671–684.
Sangkhae V, Etheridge SL, Kaushansky K, Hitchcock IS . The thrombopoietin receptor, MPL, is critical for development of a JAK2V617F-induced myeloproliferative neoplasm. Blood 2014; 124: 3956–3963.
Dominici M, Rasini V, Bussolari R, Chen X, Hofmann TJ, Spano C et al. Restoration and reversible expansion of the osteoblastic hematopoietic stem cell niche after marrow radioablation. Blood 2009; 114: 2333–2343.
Olson TS, Caselli A, Otsuru S, Hofmann TJ, Williams R, Paolucci P et al. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood 2013; 121: 5238–5249.
Mullally A, Lane SW, Ball B, Megerdichian C, Okabe R, Al-Shahrour F et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer cell 2010; 17: 584–596.
Xing S, Wanting TH, Zhao W, Ma J, Wang S, Xu X et al. Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood 2008; 111: 5109–5117.
Akada H, Yan D, Zou H, Fiering S, Hutchison RE, Mohi MG . Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood 2010; 115: 3589–3597.
Li J, Spensberger D, Ahn JS, Anand S, Beer PA, Ghevaert C et al. JAK2 V617F impairs hematopoietic stem cell function in a conditional knock-in mouse model of JAK2 V617F-positive essential thrombocythemia. Blood 2010; 116: 1528–1538.
Acknowledgements
The authors thank Dr Ian Hitchcock (University of York, UK) for his scientific consultation and Ms. Laurie Levine (Stony Brook University, NY, USA) for her assistance with the animal work. We thank Dr Radek Skoda (University Hospital Basal, Switzerland) for furnishing the mice (FF1 and Pf4-Cre) and for many fruitful discussions. We thank Drs Stanley Zucker and Hussein Foda(Northport VA Medical Center) for their continuing support throughout this work. This research was supported by the Veterans Affairs Career Development Award CDA210959632 (HZ) and National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK049855 (KK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Zhan, H., Ma, Y., Lin, C. et al. JAK2V617F-mutant megakaryocytes contribute to hematopoietic stem/progenitor cell expansion in a model of murine myeloproliferation. Leukemia 30, 2332–2341 (2016). https://doi.org/10.1038/leu.2016.114
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.114
- Springer Nature Limited
This article is cited by
-
JAK2 inhibitor persistence in MPN: uncovering a central role of ERK activation
Blood Cancer Journal (2022)
-
The Rationale for Immunotherapy in Myeloproliferative Neoplasms
Current Hematologic Malignancy Reports (2019)
-
The JAK2V617F-bearing vascular niche promotes clonal expansion in myeloproliferative neoplasms
Leukemia (2018)