Abstract
The JAK2V617F mutation commonly found in myeloproliferative neoplasms (MPNs) induces constitutive phosphorylation/activation of the signal transducer and activator of transcription 3 (Stat3). However, the contribution of Stat3 in MPN evoked by JAK2V617F remains unknown. To determine the role of Stat3 in JAK2V617F-induced MPN, we generated Stat3-deficient Jak2V617F-expressing mice. Whereas expression of Jak2V617F resulted in a polycythemia vera-like disease characterized by increased red blood cells (RBCs), hematocrit, neutrophils and platelets in the peripheral blood of Jak2V617F knock-in mice, deletion of Stat3 slightly reduced RBC and hematocrit parameters and modestly increased platelet numbers in Jak2V617F knock-in mice. Moreover, deletion of Stat3 significantly increased the neutrophil counts/percentages and markedly reduced the survival of mice expressing Jak2V617F. These phenotypic manifestations were reproduced upon bone marrow (BM) transplantation into wild-type animals. Flow cytometric analysis showed increased hematopoietic stem cell and granulocyte-macrophage progenitor populations in the BM and spleens of Stat3-deficient Jak2V617F mice. Stat3 deficiency also caused a marked expansion of Gr-1+/Mac-1+ myeloid cells in Jak2V617F knock-in mice. Histopathologic analysis revealed marked increase in granulocytes in the BM, spleens and livers of Stat3-deficient Jak2V617F-expressing mice. Together, these results suggest that deletion of Stat3 increases the severity of MPN induced by Jak2V617F.
Similar content being viewed by others
Introduction
The JAK2V617F mutation has been found in most patients with Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs) including polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF).1, 2, 3, 4, 5 Expression of JAK2V617F results in constitutive activation of the JAK2 tyrosine kinase and its downstream signaling pathways,1, 2, 6 and confers cytokine hypersensitivity to hematopoietic cells and progenitors.1, 2 Several studies using bone marrow (BM) transplantation, transgenic and knock-in mouse models of Jak2V617F have confirmed a pathogenic role for JAK2V617F mutation in MPNs,6, 7, 8, 9, 10, 11, 12, 13 but how JAK2V617F mediates hematopoietic transformation/MPNs remains poorly understood. Unraveling the contribution of signaling pathways downstream of JAK2V617F in MPNs will improve our understanding of the pathogenesis of MPNs and lead to new therapeutic strategies for MPNs.
Several members of the signal transducer and activator of transcription (Stat) family proteins including Stat1, Stat3 and Stat5 are constitutively phosphorylated/activated in MPN cells expressing JAK2V617F.1, 2, 14, 15 We and other groups have shown previously that Stat5 is critical for Jak2V617F-evoked PV disease in mice.16, 17 Recently, it has been shown that Stat1 promotes megakaryopoiesis and has a role in induction of ET-like phenotype in a mouse model of Jak2V617F.18 However, the role of Stat3 in MPN mediated by Jak2V617F remains unknown.
Stat3 is an important signaling molecule that is phosphorylated/activated in response to a variety of cytokines including interleukin-6 (IL-6), IL-11 and granulocyte-colony stimulating factor (G-CSF).19 It has been suggested that Stat3 serves as a negative regulator of granulopoiesis and positive regulator of B lymphopoiesis.20, 21 It also has been reported that Stat3-deficient mice exhibit mitochondrial dysfunction and defects in hematopoietic stem cell (HSC) and progenitor function.22 Constitutive activation of Stat3 has been observed in various human malignancies.23 Although Stat3 has a tumor-promoting role in some malignancies, such as in pancreatic tumorigenesis and myeloid neoplasms induced by KrasG12D24, 25 and in gastric cancer caused by the Helicobacter pylori infection,26 other studies also have found a tumor suppressive function of Stat3 in certain malignancies. For instance, Stat3 negatively regulates BRAFV600E-induced thyroid tumorigenesis27 or suppresses PTEN loss-induced glioblastoma.28 Thus, Stat3 can positively or negatively regulate cell growth and tumor progression. Here, we sought to determine the role of Stat3 in JAK2V617F-evoked MPN using conditional Stat3 knockout (Stat3 floxed) and Jak2V617F knock-in mice. Our results show that deletion of Stat3 increases neutrophil counts in the peripheral blood and enhances expansion of hematopoietic progenitors and myeloid precursor cells in the BM and spleens of Jak2V617F knock-in mice. Furthermore, deletion of Stat3 markedly reduces the survival in a Jak2V617F knock-in mouse model of MPN. Taken together, these results suggest a negative role for Stat3 in Jak2V617F-induced MPN.
Materials and methods
Mice
Conditional Jak2V617F knock-in6 and floxed Stat3 (Stat3fl/fl) mice29 have been described previously. MxCre mice30 (purchased from the Jackson Laboratory, Bar Harbor, ME, USA) were crossed with Jak2V617F and Stat3fl/fl mice to generate Jak2V617F-expressing (MxCre;V617F/+), Stat3-deficient (MxCre;Stat3fl/fl) and Stat3-deficient Jak2V617F-expressing (MxCre;V617F/+;Stat3fl/fl) mice. Mice were administrated with two doses of 200 μg polyinosine–polycytosine (pI–pC, GE Healthcare, Piscataway, NJ, USA) by intraperitoneal injection to induce Cre expression. All animal studies were approved by the Institutional Animal Care and Use Committee of SUNY Upstate Medical University.
Blood and tissue analyses
Blood was collected from tail vein and complete blood counts were measured on Hemavet 950FS (Drew Scientific, Dallas, TX, USA). Mouse tissue specimens (femurs, spleens and livers) were fixed in 10% neutral buffered formalin and embedded in paraffin. Tissue sections (4 μm) were then stained with hematoxylin and eosin.
Flow cytometry
Single-cell suspensions from BM and spleens were prepared and stained for 20 min on ice with either allophycocyanin- or phycoerythrin-conjugated monoclonal antibodies against Ter119, CD71, CD41, CD61, Mac-1, Gr-1, B220 or Thy-1. For stem cell/progenitor analysis, BM or spleen cells were stained with antibodies against lineage (Lin) markers (CD3, CD4, CD8α, CD19, B220, Gr-1, Ter119 and IL-7R (CD127) and c-Kit, Sca1, Flk2 (CD135), CD34, CD16/32 (FcγRII/III) for 60 min on ice. All flow cytometry antibodies were purchased from eBioscience or BioLegend (San Diego, CA, USA). For cell cycle analysis, Lin-negative cells were first enriched by magnetic-activated cell sorting (Miltenyi Biotech, Auburn, CA, USA) and then stained with monoclonal antibodies against Lin markers, c-Kit and Sca1 along with Hoechst 33342 (Sigma-Aldrich, St. Louis, MO, USA) and Pyronin Y (Sigma-Aldrich). Flow cytometry was performed with an LSRII flow cytometer (BD Biosciences, San Diego, CA, USA) and analyzed using FlowJo software (TreeStar, Ashland, OR, USA).
Colony-forming assays
BM (2 × 104) or spleen (1 × 105) cells were plated in duplicate in methylcellulose medium M3434 (Stem Cell Technologies, Vancouver, BC, Canada) containing cytokines. Burst‐forming units-erythroid, colony-forming units-granulocyte‐macrophage and CFU-granulocyte, erythrocyte, macrophage, and megakaryocyte colonies were scored on day 7. To detect Erythropoietin (Epo)-dependent/independent colony-forming unit-erythroid (CFU-E) colonies, BM or spleen cells (1 × 105) were seeded in duplicate in methylcellulose medium M3234 (Stem Cell Technologies) in the presence or absence of Epo (3 U/ml). CFU-E colonies were counted after 2 days by staining with benzidine solution (Sigma-Aldrich).
BM transplantation assay
Total BM cells from control, MxCre;V617F/+ or MxCre;V617F/+;Stat3fl/fl mice were harvested 2 weeks after pI–pC induction. BM cells (1 × 106) were transplanted by retro-orbital injection into lethally irradiated (2 × 550 cGy) C57BL/6 recipient mice. Mice were maintained on acidified water.
Quantitative real-time PCR
Total RNA was extracted from multi-potent progenitors (MPP) from control, MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice using RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and reverse transcribed by using QuantiTect Reverse Transcription kit (Qiagen). Quantitative real‐time PCR was carried out with SYBR Green PCR master mix (Roche Diagnostics, Indianapolis, IN, USA) on a LightCycler 480 (Roche Applied Science, Indianapolis, IN, USA) using the following primers:
5′-AACGACCTGCAGCAATACCA-3′ and 5′-AAGGTGATCAGGTGCAGCTC-3′ for Stat3, 5′-AGCTCCAAAAGCGAGTACCA-3′ and 5′-TGACGCTCAACGTGAAGAAG-3′ for SOCS3, 5′-CCTGTGCCAGTATGATGTGG-3′ and 5′-CAGGGTCACACTCGTCAATG-3′ for Notch1, 5′-ATCGCTGCAGCTTCCTATGT-3′ and 5′-AGTCATGCTTTCCCGTGTTC-3′ for C/EBP-β, 5′-CGCCGCTAGAGGTGAAATTC-3′ and 5′-TTGGCAAATGCTTTCGCTC-3′ for 18S. The data were normalized to 18S and fold change in gene expression was determined by the ΔΔCt method.
Statistical analysis
Results are presented as mean ±s.e.m., and statistical significance was determined by one-way analysis of variance or Student's t-test using Prism Version 5 software (GraphPad, San Diego, CA, USA). P<0.05 was considered statistically significant.
Results
To determine the role of Stat3 in Jak2V617F-induced MPNs, we crossed our conditional Jak2V617F knock-in mice6 with Stat3-floxed (Stat3fl/fl) mice29 and MxCre transgenic mice,30 and generated the MxCre;Stat3fl/fl, MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice. The expression of Jak2V617F and the deletion of Stat3 were simultaneously induced in hematopoietic progenitors of these mice at 4 weeks after birth by intraperitoneal injection of two doses of 200 μg pI–pC. Four groups of mice were analyzed: control (Stat3fl/fl); Stat3-deleted (MxCre;Stat3fl/fl), Jak2V617F-expressing (MxCre;V617F/+) and Stat3-deleted Jak2V617F-expressing (MxCre;V617F/+;Stat3fl/fl) mice. Immunoblot analysis showed that Stat3 was almost completely deleted in MxCre;Stat3fl/fl and MxCre;V617F/+;Stat3fl/fl mice upon induction with pI–pC (Figure 1a). Interestingly, all MxCre;V617F/+;Stat3fl/fl mice died within 4 weeks after pI–pC injection with a median survival of 18 days, whereas no death was observed in control and MxCre;V617F/+ groups during this time period (Figure 1b). Due to the early deaths in Stat3-deleted Jak2V617F-expressing (MxCre;V617F/+;Stat3fl/fl) mice, they were assessed at 2–3 weeks after pI–pC injection unless otherwise specified. MxCre;Stat3fl/fl mice died within 6 weeks after pI–pC injection with a median survival of 21 days (Figure 1b). MxCre;Stat3fl/fl mice were analyzed at 3–4 weeks after pI–pC injection.
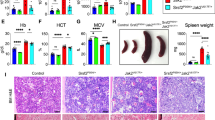
Deletion of Stat3 leads to neutrophilia and early deaths in mice expressing Jak2V617F. (a) Immunoblot analysis of Stat3 protein expression in the BM of control (Stat3fl/fl), Stat3-deleted (MxCre;Stat3fl/fl), Jak2V617F (MxCre;V617F/+) and Stat3-deleted Jak2V617F-expressing (MxCre;V617F/+;Stat3fl/fl) mice. β-actin was used as a loading control. (b) Kaplan–Meier survival analysis of control, MxCre;Stat3fl/fl, MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice (n=19) after pI–pC induction. Both MxCre;Stat3fl/fl and MxCre;V617F/+;Stat3fl/fl mice exhibited significantly decreased survival compared with control and MxCre;V617F/+ mice; **P<0.005, log‐rank test. Peripheral blood red blood cells (c), hematocrit (d), platelets (e), neutrophils (f), neutrophil percentage (g), lymphocytes (h) and lymphocyte percentage (i) were analyzed at 2–3 weeks (for control, MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice) or 3–4 weeks (for MxCre;Stat3fl/fl mice) after induction with pI–pC (n=10–12). Spleen weight (j) was significantly increased in MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice compared with controls; however, compared with MxCre;V617F/+ mice, spleen weight was significantly decreased in MxCre;V617F/+;Stat3fl/fl mice (at 2–3 weeks after pI–pC injection; n=10). Data are presented as mean±s.e.m. One-way ANOVA was used for comparisons of all four groups of mice, and Student's t-test was used to compare between two groups of mice (*P<0.05; **P<0.005).
All induced MxCre;V617F/+ mice displayed a PV-like MPN characterized by increased red blood cells (RBC), hematocrit, platelets and neutrophils in their peripheral blood (Figures 1c–f). Deletion of Stat3 significantly increased neutrophil counts and percentage in Jak2V617F knock-in mice (Figures 1f and g). Correspondingly, lymphocyte counts and percentage were proportionally reduced in MxCre;V617F/+;Stat3fl/fl mice compared with control and MxCre;V617F/+ mice (Figures 1h and i). RBC and hematocrit parameters were slightly decreased, whereas platelet counts were modestly increased in MxCre;V617F/+;Stat3fl/fl mice compared with MxCre;V617F/+ mice (Figures 1c–e). MxCre;Stat3fl/fl mice showed significant increase in neutrophil counts but decrease in RBC, hematocrit and platelet counts in their peripheral blood compared with control animals (Figures 1c–f). Splenomegaly was observed in both MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice (Figure 1j). Spleen size/weight in MxCre;V617F/+;Stat3fl/fl mice was smaller than in MxCre;V617F/+ mice, but was significantly larger than in control animals (Figure 1j).
Histopathologic analyses of the BM sections from MxCre;Stat3fl/fl mice showed increased, predominantly mature, granulocytic cells (Figure 2a). BM of MxCre;V617F/+ mice showed trilineage hyperplasia with increased immature erythroid and myeloid precursors and increased megakaryocytes (Figure 2a). BM of MxCre;V617F/+;Stat3fl/fl mice showed markedly increased immature myeloid (granulocytic) precursors and megakaryocytes but less erythroid precursors (Figure 2a). Spleen sections from MxCre;Stat3fl/fl mice showed red pulp with increased immature and mature granulocytes and also maturing erythroid precursors (Figure 2b). Spleen sections from MxCre;V617F/+ mice showed attenuation of white pulp and expansion of red pulp with clusters of immature erythroid precursors and dysplastic megakaryocytes (Figure 2b). Spleen sections from MxCre;V617F/+;Stat3fl/fl mice exhibited diminished white pulp and expanded red pulp with immature and mature granulocytic myeloid cells and megakaryocytes (Figure 2b). Liver sections from MxCre;Stat3fl/fl mice showed histology similar to the control animals (Figure 2c). Livers from MxCre;V617F/+ mice showed relatively normal architecture with some vacuolar degeneration, whereas livers from MxCre;V617F/+;Stat3fl/fl mice exhibited significant infiltration of granulocytes (Figure 2c).
Histopathologic analysis. (a) Hematoxylin and eosin (H&E) staining of the BM sections (500 × ) from MxCre;Stat3fl/fl mice show increased, predominantly mature, granulocytic cells. BM sections from MxCre;V617F/+ mice display hypercellularity with trilineage (megakaryocyte/erythrocyte/granulocyte) hyperplasia. BM sections from MxCre;V617F/+;Stat3fl/fl mice show markedly increased immature granulocytic precursors and megakaryocytes but less erythroid precursors. (b) Spleen sections (H&E staining; 500 × ) from MxCre;Stat3fl/fl mice show expanded red pulp with increased immature and mature granulocytes and also maturing erythroid precursors. Spleen sections from MxCre;V617F/+ mice show attenuation of white pulp and expansion of red pulp with clusters of immature erythroid precursors and dysplastic megakaryocytes. Spleen sections from MxCre;V617F/+;Stat3fl/fl mice exhibit diminished white pulp and expanded red pulp with immature and mature granulocytic myeloid cells and megakaryocytes. (c) Liver sections (H&E staining; 500 × ) from MxCre;Stat3fl/fl mice show histology similar to the control animals. Liver sections from MxCre;V617F/+ mice show relatively normal architecture with little infiltration of myeloid cells, whereas livers from MxCre;V617F/+;Stat3fl/fl mice exhibit significant foci of infiltration by granulocytes.
Flow cytometric analysis revealed significant increase in myeloid (Gr-1+/Mac-1+) precursors in both BM and spleens of Stat3-deficient (MxCre;Stat3fl/fl) mice compared with control animals (Figures 3a and b). The frequency of erythroid precursors (Ter119+CD71+) was reduced in the BM but increased in the spleens of Stat3-deficient (MxCre;Stat3fl/fl) mice compared with control animals (Figures 3a and b). Mice expressing Jak2V617F (MxCre;V617F/+) exhibited significant increase in early (CD71+) and late (CD71+Ter119+) erythroid precursors in the BM and spleens but no significant change in Gr-1+/Mac-1+ populations compared with control animals at 2–3 weeks after pI–pC induction (Figures 3a and b). Deletion of Stat3 in the context of Jak2V617F led to a dramatic expansion of the Gr-1+/Mac-1+ population in the BM and spleens of MxCre;V617F/+;Stat3fl/fl mice (Figures 3a and b). Deletion of Stat3, however, resulted in significant decrease in the late erythroid precursors (CD71+Ter119+) in the BM of Jak2V617F mice as myeloid precursors (Gr-1+/Mac-1+) were dramatically expanded in the BM of MxCre;V617F/+;Stat3fl/fl mice (Figures 3a and b). Although the frequency of late erythroid precursors (CD71+Ter119+) in the spleens of MxCre;V617F/+;Stat3fl/fl mice was reduced compared with MxCre;V617F/+ mice, the CD71+Ter119+ population was still significantly greater (~15-fold) in the spleens of MxCre;V617F/+;Stat3fl/fl mice compared with control animals (Figures 3a and b). Interestingly, CD71+ early erythroid precursors were slightly increased in the BM and spleens of Stat3-deleted Jak2V617F-expressing mice (Figures 3a and b). Megakaryocytic populations (CD41+CD61+) were significantly expanded in the spleens of MxCre;V617F/+;Stat3fl/fl mice (Figures 3a and b). B-cell (B220+) population was proportionately reduced in the BM and spleens of MxCre;Stat3fl/fl, MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice (Figures 3a and b). Together, these results suggest that the deletion of Stat3 enhances granulocytic/megakaryocytic cell expansion, decreases erythropoiesis to some extent and shortens the survival of Jak2V617F mice.
Flow cytometric analysis. (a) Dot plots demonstrate significant increase in myeloid (Gr-1+/Mac-1+) precursors in the BM and spleens of MxCre;Stat3fl/fl and MxCre;V617F/+;Stat3fl/fl mice compared with control and MxCre;V617F/+ mice. (b) Percentages of myeloid (Gr-1+/Mac-1+), erythroid (Ter119+/CD71+) and megakaryocytic (CD61+/CD41+) precursors as well as B cells (B220+) and T cells (Thy-1+) are shown in bar graphs as mean±s.e.m. (for control, MxCre;Stat3fl/fl and MxCre;V617F/+ mice, n=5; for MxCre;V617F/+;Stat3fl/fl mice, n=6). One-way ANOVA was used for comparisons of all four groups of mice, and Student's t-test was used to compare between two groups of mice (*P<0.05; **P<0.005). Note that the deletion of Stat3 leads to marked expansion of myeloid (Gr-1+/Mac-1+) precursors in the BM and spleens of mice expressing Jak2V617F.
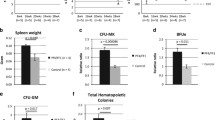
We further determined the effects of Stat3 ablation on HSCs and progenitor compartments in mice expressing Jak2V617F by flow cytometric analysis. Deletion of Stat3 in Jak2V617F knock-in mice significantly increased the HSC-enriched LSK (Lin-Sca1+c-kit+) compartment and its subsets including long-term HSC (Lin-Sca1+c‐kit+CD34-CD135-), short-term HSC (Lin-Sca1+c‐kit+CD34+CD135-), and multi-potent progenitors (MPP; Lin-Sca1+c‐kit+CD34+CD135+) in the BM and spleens. Specifically, the increases in LSK, long-term HSC, short-term HSC and MPP populations were much greater in the spleens than in the BM of Stat3-deleted Jak2V617F mice (Figures 4a and b). Flow cytometric analysis also revealed marked expansion of granulocyte-macrophage progenitors (GMP; Lin-Sca1-c-kit+CD34+FcγRII/IIIhigh) in both BM and spleens upon concomitant deletion of Stat3 in mice expressing Jak2V617F (Figures 4c and d). The megakaryocyte/erythroid progenitor compartment (Lin-Sca1-c-kit+CD34-FcγRII/III-) was reduced in the BM of MxCre;V617F/+;Stat3fl/fl mice compared with MxCre;V617F/+ mice. However, no significant difference in megakaryocyte/erythroid progenitor compartment was observed in the spleens between MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice, both of which had a significantly higher (four- to five-fold) megakaryocyte/erythroid progenitor compartment population than in control mice (Figures 4c and d). LSK, long-term HSC, short-term HSC and MPP populations in the BM of Stat3-deficient (MxCre;Stat3fl/fl) mice were comparable to those in control animals (Supplementary Figure 1a). However, there was a trend of increased LSK, long-term HSC, short-term HSC and MPP populations in the spleens of MxCre;Stat3fl/fl mice (Supplementary Figure 1a). GMP and megakaryocyte/erythroid progenitor compartment populations were also significantly increased in the spleens of MxCre;Stat3fl/fl mice compared with control animals (Supplementary Figure 1b).
Deletion of Stat3 increases hematopoietic stem cell compartment and granulocyte-macrophage progenitors (GMP) in Jak2V617F mice. (a) Flow cytometric analysis of LSK (Lin-Sca1+c-kit+), LT-HSC (Lin-Sca1+c‐kit+CD34-CD135-), ST-HSC (Lin-Sca1+c‐kit+CD34+CD135-) and MPP (Lin-Sca1+c‐kit+CD34+CD135+) in the BM and spleens from control, MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice at 2–3 weeks after pI–pC induction. Representative contour plots from four independent experiments are shown. (b) Percentages of LSK, LT-HSC, ST-HSC and MPP are shown in bar graphs as mean±s.e.m. (n=4). Data are presented as percentage of live cells. (c) Flow cytometric analysis of myeloid progenitors including CMP (Lin-Sca1-c-kit+CD34+FcγRII/IIIlo), GMP (Lin-Sca1-c-kit+CD34+FcγRII/IIIhi) and MEP (Lin-Sca1-c-kit+CD34-FcγRII/III-) in the BM and spleens from control, MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice at 2–3 weeks after pI–pC induction. Representative contour plots from four independent experiments are shown. (d) Percentages of CMP, GMP and MEP are shown in bar graphs as mean±s.e.m. (n=4). (e and f) Hematopoietic progenitor colony assays. BM (2 × 104; e) or spleen cells (1 × 105; f) from control (n=4), MxCre;V617F/+ (n=4) and MxCre;V617F/+;Stat3fl/fl mice (n=5) were plated in complete methylcellulose medium (Methocult M3434) containing cytokines. BFU-E, CFU-GM and CFU-GEMM colonies were scored 7–8 days after plating. (g– i) CFU-E colony assays. BM (g) or Spleen cells (h; 1 × 105) from control, MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice (n=4) were plated in methylcellulose medium (Methocult M3234) in the presence of erythropoietin (Epo; 3 U/ml). (i) Spleen cells (1 × 105) from control, MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice (n=4) were plated in methylcellulose medium (Methocult M3234) in the absence of Epo. CFU-E colonies in the presence or absence of Epo were scored after 2 days. All data are shown as mean±s.e.m. (*P<0.05 and **P<0.005).
Next, we preformed progenitor colony assays to evaluate the effects of Stat3 deletion on hematopoietic progenitor compartments of Jak2V617F mice. Although there was no significant difference in Burst‐forming units-erythroid and colony-forming units-granulocyte‐macrophage colonies in the BM among control, MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice at 2–3 weeks after pI–pC induction (Figure 4e), spleens of MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice exhibited significantly larger numbers of Burst‐forming units-erythroid and colony-forming units-granulocyte‐macrophage colonies compared with control animals (Figure 4f). Noticeably, deletion of Stat3 further increased Burst‐forming units-erythroid and colony-forming units-granulocyte‐macrophage colonies in the spleens of mice expressing Jak2V617F (Figure 4f). BM and spleens of MxCre;V617F/+ mice exhibited significant increase in CFU-E colonies in the presence of Epo (Figures 4g and h). Deletion of Stat3 resulted in marked decrease in CFU-E colonies in the BM of MxCre;V617F/+;Stat3fl/fl mice (Figure 4g). However, the numbers of CFU-E colonies in the presence of Epo in the spleens of MxCre;V617F/+;Stat3fl/fl mice were comparable to that observed in the spleens of MxCre;V617F/+ mice (Figure 4h). Epo-independent CFU-E colonies, a hallmark feature of PV,31 was also observed in the spleens of both MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice (Figure 4i). However, there was a modest (not statistically significant) decrease in Epo-independent CFU-E colony formation in the spleens of MxCre;V617F/+;Stat3fl/fl mice compared with MxCre;V617F/+ mice (Figure 4i). Overall, the deletion of Stat3 in Jak2V617F-expressing mice was associated with an expansion of HSC and GMP compartments and a trend toward decreased erythroid progenitors/precursors.
To address why deletion of Stat3 causes expansion of HSC compartment in mice expressing Jak2V617F, we performed cell cycle analysis on BM LSK cells from control, MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice using Pyronin Y/Hoechst 33342 staining. Compared with control LSK cells, significantly fewer numbers of Jak2V617F-expressing LSK cells were in the quiescent G0 phase, with a higher proportion in the G1 and S/G2/M phases (Figures 5a and b). Concurrent deletion of Stat3 further enhanced Jak2V617F-evoked cell cycle entry of LSK cells (Figures 5a and b). These results suggest that deletion of Stat3 leads to increased proliferation of HSC pool in mice expressing Jak2V617F.
Deletion of Stat3 increases cell cycle entry of LSK cells in mice expressing Jak2V617F. (a) Flow cytometric analysis of Hoechst 33342 and Pyronin Y staining on LSK cells from control, MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice BM. Representative contour plots from three independent experiments are shown. (b) The percentages of LSK cells in G0, G1 and S/G2/M phases of the cell cycle are presented in bar graph as mean±s.e.m. (*P<0.05 and **P<0.005).
To assess whether the observed effects of Stat3 deficiency on Jak2V617F-induced MPN were cell autonomous, we transplanted BM cells from pI–pC-induced control, MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice into lethally irradiated C57BL/6 wild-type recipients as outlined in Figure 6a. Recipient animals were maintained for 8 weeks after transplantation. Similar to the primary Stat3-deficient Jak2V617F mice, recipients of the BM cells from MxCre;V617F/+;Stat3fl/fl mice exhibited significantly increased percentage of neutrophils and decreased percentage of lymphocytes in the peripheral blood (Figure 6b), and markedly elevated Gr-1+/Mac-1+ populations in both BM and spleens (Figures 6c and d). Compared with control BM recipients, transplanted animals receiving BM cells from either MxCre;V617F/+ or MxCre;V617F/+;Stat3fl/fl mice exhibited increased numbers of RBC in their peripheral blood and increased percentage of erythroid precursors (CD71+Ter119+, CD71+) in their BM and/or spleens (Figures 6b and d), indicating the presence of PV-like MPN in mice reconstituted with MxCre;V617F/+ or MxCre;V617F/+;Stat3fl/fl BM cells. However, the RBC counts were slightly lower and the platelet counts were slightly higher in the recipients of MxCre;V617F/+;Stat3fl/fl BM cells compared with the transplanted animals receiving MxCre;V617F/+ BM cells (Figure 6b). Recipients of MxCre;V617F/+;Stat3fl/fl mice BM cells also exhibited a significantly increased GMP population and a trend of increased LSK cells in the BM (Figure 6e). Together, these results suggest that the effects of Stat3 deficiency on Jak2V617F-induced MPN are cell autonomous.
The effects of Stat3 deficiency on MPN induced by Jak2V617F are cell autonomous. (a) Experimental design for cell autonomous BM transplantation assay. (b) Peripheral blood RBC and platelet counts and percentages of neutrophils and lymphocytes from mice receiving control (n=3), MxCre;V617F/+ (n=3) and MxCre;V617F/+;Stat3fl/fl (n=4) BM at 8 weeks after BM transplantation. (c) Flow cytometric analysis of BM and spleens from recipient mice transplanted with control, MxCre;V617F/+, or MxCre;V617F/+;Stat3fl/fl BM. Representative dot plots are presented. (d) The percentages of myeloid (Gr-1+/Mac-1+), erythroid (Ter119+/CD71+) and megakaryocytic (CD61+/CD41+) precursors as well as B cells (B220+) and T cells (Thy-1+) are shown in bar graphs (n=3). (e) GMP population was significantly increased in mice receiving MxCre;V617F/+;Stat3fl/fl BM compared with those receiving control or MxCre;V617F/+ BM. Representative contour plots are shown on the left panel, and the percentages of LSK, CMP, GMP and MEP are presented in bar graphs on the right panel (n=3). Data are presented as mean±s.e.m. (*P<0.05 and **P<0.005).
As homozygous Stat3 deletion led to early deaths in mice expressing Jak2V617F, we also assessed the effects of heterozygous Stat3 deletion on Jak2V617F-induced MPN. As expected, the expression of Stat3 in the BM of MxCre;V617F/+;Stat3fl/+ mice was almost half of those in control or MxCre;V617F/+ mice after pI–pC induction (Supplementary Figure 2a). No early death was found in induced MxCre;V617F/+;Stat3fl/+ mice, which had a similar lifespan as Jak2V617F knock-in mice (data not shown). All MxCre;V617F/+;Stat3fl/+ mice developed a PV-like MPN with comparable blood parameters and splenomegaly to those observed in Jak2V617F knock-in mice after pI–pC injection (Supplementary Figures 2b–g). There was also no significant difference in splenic Epo-independent CFU-E formation between MxCre;V617F/+;Stat3fl/+ and MxCre;V617F/+ mice (Supplementary Figure 2h). Furthermore, flow cytometric analysis showed no significant differences in myeloid, erythroid and megakaryocytic precursors, as well as B-cell and T-cell populations between MxCre;V617F/+;Stat3fl/+ and MxCre;V617F/+ mice (Supplementary Figures 3a and b). Altogether, these data indicate that heterozygous deletion of Stat3 has no significant effect on Jak2V617F-induced MPN.
To determine whether the deficiency of Stat3 had any effect on signaling by other Stat proteins, we assessed the phosphorylation and expression of Stat5 and Stat1 proteins in the BM of control, MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice by immunoblotting. Deletion of Stat3 did not cause any significant change in phosphorylation or expression of Stat5 in Jak2V617F knock-in mice BM (Figure 7a). However, phosphorylation and expression of Stat1 protein was significantly increased upon deletion of Stat3 in Jak2V617F knock-in mice BM (Figure 7a). We also assessed the expression levels of suppressor of cytokine signaling 3 (SOCS3) by immunoblotting since SOCS3 was found upregulated in JAK2V617F-positive MPN patient cells32 and SOCS3 was shown to inversely regulate the activation of Stat1 and Stat3 in response to G-CSF, interferon-γ and IL-6.20, 33, 34 We observed increased SOCS3 protein expression in the BM of MxCre;V617F/+ mice (Figure 7a). Deletion of Stat3 significantly inhibited the SOCS3 protein expression in the BM of Jak2V617F mice (Figure 7a).
Effects of Stat3 deletion on cell signaling and gene expression in hematopoietic cells and progenitors of Jak2V617F mice. (a) BM cell lysates from control, MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice were immunoblotted with phospho-specific antibodies against Stat3, Stat5 or Stat1, and total antibodies against Stat3, Stat5, Stat1 or SOCS3. β-actin was used as a loading control. (b) Relative expression of Stat3, Notch1, SOCS3 and C/EBP-β mRNA in the multi-potent progenitors (MPP) from control, MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice was determined by quantitative real‐time PCR and normalized with 18S expression. Data are presented as mean±s.e.m. (n=3–4 in each group, *P<0.05 and **P<0.005).
To gain further insights into the mechanism by which Stat3 deficiency increases the myeloid cell expansion and severity of MPN phenotype in Jak2V617F knock-in mice, we analyzed the expression levels of specific genes in MPP from control, MxCre;V617F/+ and MxCre;V617F/+;Stat3fl/fl mice using quantitative real-time PCR. The expression of Stat3 mRNA was almost undetectable in the MPP populations of MxCre;V617F/+;Stat3fl/fl mice, suggesting efficient deletion of Stat3 in the hematopoietic progenitors of these mice (Figure 7b). The expression of Notch1 was significantly downregulated in MPP populations from MxCre;V617F/+;Stat3fl/fl mice compared with those in control and MxCre;V617F/+ mice (Figure 7b). Similar to SOCS3 protein expression, SOCS3 mRNA expression was also significantly reduced in MPP from MxCre;V617F/+;Stat3fl/fl mice compared with those in MxCre;V617F/+ mice (Figure 7b). In contrast, expression of C/EBP-β was found significantly elevated in MPP populations from MxCre;V617F/+;Stat3fl/fl mice compared with those in control and MxCre;V617F/+ mice (Figure 7b). There was also a modest increase in C/EBP-α expression in MPP from MxCre;V617F/+;Stat3fl/fl mice (data not shown). These results suggest that decreased expression of Notch1 and SOCS3 and increased expression of C/EBP-β induced by Stat3 deletion may contribute to the myeloid cell expansion in Stat3-deficient Jak2V617F knock-in mice.
Discussion
Constitutive phosphorylation/activation of Stat3 has been frequently observed in MPNs.15, 35 However, the precise role of Stat3 in the pathogenesis of MPNs remains unclear. In this study, we examined the role of Stat3 in the development of MPN using conditional Jak2V617F knock-in and Stat3-deficient mice. We found that ablation of Stat3 does not prevent the development of MPN, rather augments the myeloid cell expansion and decreases the survival in a Jak2V617F knock-in mouse model of MPN.
Stat3 can be activated by oncogenic tyrosine kinases (such as JAK2V617F),15, 35, 36 by autocrine production of IL-637 or through activating somatic Stat3 mutations.38 Persistent activation of Stat3 has been observed in a variety of human malignancies. In some cases, such as in KrasG12D-induced pancreatic and myeloid malignancies, Stat3 promotes tumorigenesis.24, 25 In other malignancies, such as BRAFV600E-induced thyroid tumorigenesis and PTEN loss-induced glioblastomas, Stat3 functions as a tumor suppressor.27, 28 In this study, we have found that Stat3 is not essential for development of PV-like MPN in mice induced by Jak2V617F. We have observed that deletion of Stat3 rather enhances the severity of MPN induced by Jak2V617F, suggesting a negative role of Stat3 in Jak2V617F-induced PV. Stat3 is also found to be constitutively phosphorylated/activated in MF.15, 35 Since all Stat3-deleted Jak2V617F (MxCre;V617F/+;Stat3fl/fl) mice died within 4 weeks after pI–pC induction (Figure 1b), we were unable to assess the contribution of Stat3 in the development of MF evoked by Jak2V617F. Future studies will determine whether Stat3 is required for the pathogenesis of MF and whether targeting of Stat3 could be beneficial for treatment of MF.
Previous reports have shown that Stat3 positively regulates B-cell development,21 and negatively regulates granulopoiesis.20 Indeed, we observed significantly increased numbers of neutrophils in the peripheral blood and increased percentages of Gr-1+/Mac-1+ myeloid precursors in the BM and spleens of Stat3-deficient Jak2V617F knock-in mice (Figures 1f, 3a and b). We also observed significantly increased percentages of GMP populations in the BM and spleens of Stat3-deficient Jak2V617F mice compared with control or Jak2V617F mice (Figures 4c and d). Furthermore, the HSC compartment was significantly expanded in the BM and spleens of Stat3-deficient Jak2V617F mice (Figures 4a and b). Deficiency of Stat3 alone did not cause a significant expansion of HSC and GMP in the BM but splenic HSC and GMP populations were expanded upon Stat3 deletion (Supplementary Figures 1a and b). However, the expansion of HSC and GMP in the BM and spleens of Stat3-deficient Jak2V617F mice was significantly greater than that observed in mice with Jak2V617F expression or Stat3 deletion alone (Figures 4b and d and Supplementary Figures 1a and b). Cell cycle analysis revealed that Jak2V617F expression increased the cycling of LSK cells (Figures 5a and b). Deletion of Stat3 further enhanced the cycling of Jak2V617F-expressing LSK cells (Figures 5a and b). Thus, increased number of HSC/progenitors in Stat3-deficient Jak2V617F mice could be due to increased proliferation of HSCs induced by Stat3 deficiency. The phenotypic changes observed in primary Stat3-deficient Jak2V617F mice were reproduced in recipient animals that were transplanted with BM from Stat3-deficient Jak2V617F mice (Figure 6). This indicates that the observed effects of Stat3 deficiency in Jak2V617F knock-in mice were cell autonomous.
We observed a compensatory increase in phosphorylation of Stat1 but not Stat5 in Stat3-deficient Jak2V617F mice BM (Figure 7a), consistent with the previous reports that Stat1 and Stat3 oppose each other’s activation in response to G-CSF, interferon-γ and IL-6.20, 33, 34 Increased activation of Stat1 might be due to the decreased expression of SOCS3, which is a negative regulator of cytokine signaling,39 and/or increased levels of cytokines, such as G-CSF, interferon-γ and IL-6, which can induce phosphorylation of Stat1.19 Increased levels of SOCS3 expression was observed in granulocytes from JAK2V617F-positive MPN patients.32 Moreover, SOCS3 protein was found to be hyperphosphorylated and stabilized by JAK2V617F.40 We observed increased expression of SOCS3 mRNA and protein in Jak2V617F knock-in (MxCre;V617F/+) mice, and deletion of Stat3 significantly reduced the SOCS3 levels in Stat3-deficient Jak2V617F mice (MxCre;V617F/+;Stat3fl/fl; Figures 7a and b). These data suggest that Stat3 is required for induction of SOCS3 in response to Jak2V617F. SOCS3 was shown to negatively regulate G-CSF receptor signaling, and mice deficient in SOCS3 exhibited increased granulopoiesis.41, 42 Thus, it is plausible that decreased expression of SOCS3 in the absence of Stat3 may partially contribute to the increased granulopoiesis and compensatory increase in Stat1 phosphorylation in Stat3-deficient Jak2V617F mice. The loss of Stat1 was shown to reduce megakaryopoiesis and increase erythropoiesis in a transgenic Jak2V617F mouse model of MPN, although Stat1 was not essential for ET phenotype in this model.18 Thus, the trend toward decreased erythropoiesis observed in Stat3-deleted Jak2V617F mice could also be partly due to a compensatory increase in Stat1 activation in these mice. In contrast, Stat5 ablation completely inhibited the PV phenotype in Jak2V617F knock-in and retroviral transduction/transplantation mouse models.16, 17 Therefore, the roles and requirements for Stat1, Stat3 and Stat5 in MPNs are distinct.
We also found that Stat3 deficiency in Jak2V617F knock-in mice MPP resulted in significant reduction in Notch1 expression (Figure 7b). Notch signaling can have both oncogenic and tumor-suppressor functions.43 A recent study has suggested a tumor-suppressor function of Notch1 in myeloid leukemia.44 Deletion of Notch1 resulted in significant expansion of GMP population in mice.44 The increase in myeloid cells and GMP populations observed in Stat3-deficient Jak2V617F mice could be in part due to the reduced Notch1 expression. We also observed marked increase in C/EBP-β and modest increase in C/EBP-α expression in the MPP from Stat3-deficient Jak2V617F mice (Figure 7b and data not shown). C/EBPs have an important role in granulopoiesis.45 Mice deficient in C/EBP-α exhibit defects in granulopoieis,46 whereas ectopic expression of C/EBP-β induces granulocytic differentiation.47 Increased C/EBP-β and C/EBP-α expression induced by Stat3 deficiency may also contribute to the myeloid cell expansion observed in Stat3-deficient Jak2V617F-expresssing mice.
In conclusion, our data show that Stat3 acts as a suppressor of Jak2V617F-induced MPN in mice. Several Stat3 inhibitors have been developed and currently undergoing pre-clinical and clinical testing for various human malignancies. As Stat3 can positively or negatively regulate tumorigenesis, its role in different cancer settings should be carefully examined before utilizing the Stat3 inhibitors. Further studies will determine whether Stat3 has a pathogenic role in human MPNs (PV, ET and MF) and whether inhibition of Stat3 has beneficial or adverse effects in human MPNs.
References
James C, Ugo V, Le Couédic JP, Staerk J, Delhommeau F, Lacout C et al. A unique clonal JAK2 mutation leading to constitutive signaling causes polycythemia vera. Nature 2005; 434: 1144–1148.
Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJP et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005; 7: 387–397.
Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005; 365: 1054–1061.
Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Eng J Med 2005; 352: 1779–1790.
Zhao R, Xing S, Li Z, Fu X, Li Q, Krantz SB et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem 2005; 280: 22788–22792.
Akada H, Yan D, Zou H, Fiering S, Hutchison RE, Mohi MG . Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood 2010; 115: 3589–3597.
Wernig G, Mercher T, Okabe R, Levine RL, Lee BH, Gilliland DG . Expression of V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood 2006; 107: 4274–4281.
Tiedt R, Hao-Shen H, Sobas MA, Looser R, Dirnhofer S, Schwaller J et al. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood 2008; 111: 3931–3940.
Shide K, Shimoda HK, Kumano T, Karube K, Kameda T, Takenaka K et al. Development of ET, primary myelofibrosis and PV in mice expressing JAK2 V617F. Leukemia 2008; 22: 87–95.
Xing S, Wanting TH, Zhao W, Ma J, Wang S, Xu X et al. Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood 2008; 111: 5109–5117.
Marty C, Lacout C, Martin A, Hasan S, Jacquot S, Birling MC et al. Myeloproliferative neoplasm induced by constitutive expression of JAK2V617F in knock-in mice. Blood 2010; 116: 783–787.
Mullally A, Lane SW, Ball B, Megerdichian C, Okabe R, Al-Shahrour F et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell 2010; 17: 584–596.
Li J, Spensberger D, Ahn JS, Anand S, Beer PA, Ghevaert C et al. JAK2 V617F impairs hematopoietic stem cell function in a conditional knock-in mouse model of JAK2 V617F-positive essential thrombocythemia. Blood 2010; 116: 1528–1538.
Chen E, Beer PA, Godfrey AL, Ortmann CA, Li J, Costa-Pereira AP et al. Distinct clinical phenotypes associated with JAK2V617F reflect differential STAT1 signaling. Cancer Cell 2010; 18: 524–535.
Anand S, Stedham F, Gudgin E, Campbell P, Beer P, Green AR et al. Increased basal intracellular signaling patterns do not correlate with JAK2 genotype in human myeloproliferative neoplasms. Blood 2011; 118: 1610–1621.
Yan D, Hutchison RE, Mohi G . Critical requirement for Stat5 in a mouse model of polycythemia vera. Blood 2012; 119: 3539–3549.
Walz C, Ahmed W, Lazarides K, Betancur M, Patel N, Hennighausen L et al. Essential role for Stat5a/b in myeloproliferative neoplasms induced by BCR-ABL1 and JAK2(V617F) in mice. Blood 2012; 119: 3550–3560.
Duek A, Lundberg P, Shimizu T, Grisouard J, Karow A, Kubovcakova L et al. Loss of Stat1 decreases megakaryopoiesis and favors erythropoiesis in a JAK2-V617F-driven mouse model of MPNs. Blood 2014; 123: 3943–3950.
Baker SJ, Rane SG, Reddy EP . Hematopoietic cytokine receptor signaling. Oncogene 2007; 26: 6724–6737.
Lee CK, Raz R, Gimeno R, Gertner R, Wistinghausen B, Takeshita K et al. STAT3 is a negative regulator of granulopoiesis but is not required for G-CSF-dependent differentiation. Immunity 2002; 17: 63–72.
Chou WC, Levy D, Lee CK . STAT3 positively regulates an early step in B-cell development. Blood 2006; 108: 3005–3011.
Mantel C, Messina-Graham S, Moh A, Cooper S, Hangoc G, Fu XY et al. Mouse hematopoietic cell-targeted STAT3 deletion: stem/progenitor cell defects, mitochondrial dysfunction, ROS overproduction, and a rapid aging-like phenotype. Blood 2012; 120: 2589–2599.
Yu H, Pardoll D, Jove R . STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009; 9: 798–809.
Corcoran RB, Contino G, Deshpande V, Tzatsos A, Conrad C, Benes CH et al. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res 2011; 71: 5020–5029.
Gough DJ, Marié IJ, Lobry C, Aifantis I, Levy DE . STAT3 supports experimental K-RasG12D-induced murine myeloproliferative neoplasms dependent on serine phosphorylation. Blood 2014; 124: 2252–2261.
Bronte-Tinkew DM, Terebiznik M, Franco A, Ang M, Ahn D, Mimuro H et al. Helicobacter pylori cytotoxin-associated gene A activates the signal transducer and activator of transcription 3 pathway in vitro and in vivo. Cancer Res 2009; 69: 632–639.
Couto JP, Daly L, Almeida A, Knauf JA, Fagin JA, Sobrinho-Simões M et al. STAT3 negatively regulates thyroid tumorigenesis. Proc Natl Acad Sci USA 2012; 109: E2361–E2370.
de la Iglesia N, Konopka G, Puram SV, Chan JA, Bachoo RM, You MJ et al. Identification of a PTEN-regulated STAT3 brain tumor suppressor pathway. Genes Dev 2008; 22: 449–462.
Alonzi T, Maritano D, Gorgoni B, Rizzuto G, Libert C, Poli V . Essential role of STAT3 in the control of the acute-phase response as revealed by inducible gene activation in the liver. Mol Cell Biol 2001; 21: 1621–1632.
Kühn R, Schwenk F, Aguet M, Rajewsky K . Inducible gene targeting in mice. Science 1995; 269: 1427–1429.
Prchal JF, Axelrad AA . Bone-marrow responses in polycythemia vera. N Engl J Med 1974; 290: 1382.
Fourouclas N, Li J, Gilby DC, Campbell PJ, Beer PA, Boyd EM et al. Methylation of the suppressor of cytokine signaling 3 gene (SOCS3) in myeloproliferative disorders. Haematologica 2008; 93: 1635–1644.
Qing Y, Stark GR . Alternative activation of STAT1 and STAT3 in response to interferon-gamma. J Biol Chem 2004; 279: 41679–41685.
Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol 2003; 4: 540–545.
Vainchenker W, Constantinescu SN . JAK/STAT signaling in hematological malignancies. Oncogene 2012; 32: 2601–2613.
Vainchenker W, Delhommeau F, Constantinescu SN, Bernard OA . New mutations and pathogenesis of myeloproliferative neoplasms. Blood 2011; 118: 1723–1735.
Schuringa JJ, Wierenga AT, Kruijer W, Vellenga E . Constitutive Stat3, Tyr705, and Ser727 phosphorylation in acute myeloid leukemia cells caused by the autocrine secretion of interleukin-6. Blood 2000; 95: 3765–3770.
Koskela HL, Eldfors S, Ellonen P, van Adrichem AJ, Kuusanmäki H, Andersson EI et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med 2012; 366: 1905–1913.
White CA, Nicola N . SOCS3: an essential physiological inhibitor of signaling by interleukin-6 and G-CSF family cytokines. JAKSTAT 2013; 2: e25045.
Hookham MB, Elliott J, Suessmuth Y, Staerk J, Ward AC, Vainchenker W et al. The myeloproliferative disorder-associated JAK2 V617F mutant escapes negative regulation by suppressor of cytokine signaling 3. Blood 2007; 109: 4924–4929.
Croker BA, Mstcalf D, Robb L, Wei W, Mifsud S, DiRago L et al. SOCS3 is a critical physiological negative regulator of G-CSF signaling and emergency granulopoiesis. Immunity 2004; 20: 153–165.
Kimura A, Kinjyo I, Matsumura Y, Mori H, Mashima R, Harada M et al. SOCS3 is a physiological negative regulator for granulopoiesis and granulocyte colony-stimulating factor receptor signaling. J Biol Chem 2004; 279: 6905–6910.
Lobry C, Oh P, Mansour MR, Look AT, Aifantis I . Notch signaling: switching an oncogene to a tumor suppressor. Blood 2014; 123: 2451–2459.
Klinakis A, Lobry C, Abdel-Wahab O, Oh P, Haeno H, Buonamici S et al. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature 2011; 473: 230–233.
Friedman AD . Transcriptional control of granulocyte and monocyte development. Oncogene 2007; 26: 6816–6828.
Zhang DE, Zang P, Wang ND, Hetherington CJ, Darlington GJ, Tenen DG . Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proc Natl Acad Sci USA 1997; 94: 569–574.
Wang QF, Friedman AD . CCAAT/enhancer-binding proteins are required for granulopoiesis independent of their induction of the granulocyte colony-stimulating factor receptor. Blood 2002; 99: 2776–2785.
Acknowledgements
We thank Dr Valeria Poli (University of Turin, Italy) for providing the Stat3-floxed mouse. This work was supported by the grants from the Leukemia and Lymphoma Society and US National Institute of Health (NIH; R01 HL095685) awarded to GM. GM is a scholar of the Leukemia and Lymphoma Society.
Author Contributions
DY performed research, analyzed data and wrote the manuscript; FJ performed research; REH performed histopathologic analysis and revised the manuscript; GM designed the research, analyzed data and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Yan, D., Jobe, F., Hutchison, R. et al. Deletion of Stat3 enhances myeloid cell expansion and increases the severity of myeloproliferative neoplasms in Jak2V617F knock-in mice. Leukemia 29, 2050–2061 (2015). https://doi.org/10.1038/leu.2015.116
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2015.116
- Springer Nature Limited
This article is cited by
-
Progress in elucidation of molecular pathophysiology of myeloproliferative neoplasms and its application to therapeutic decisions
International Journal of Hematology (2020)
-
CD300f:IL-5 cross-talk inhibits adipose tissue eosinophil homing and subsequent IL-4 production
Scientific Reports (2017)