Abstract
Objective:
To compare information obtained from preterm magnetic resonance imaging (MRI; 31–34 weeks) brain scan to that done at term equivalent age.
Study design:
Prospective observational study of premature infants with evidence or suspicion of parenchymal brain injury on cranial ultrasound. Brain injury on two scans scored using a scoring system and analyzed.
Results:
Fourteen infants with a median (range) gestation at birth of 28 (25–29) weeks and birth weight of 1254 (680–1557) grams were studied. There was a strong correlation between the brain injury scores for the two scans (Spearman ρ=0.87, P=0.001) with excellent agreement between two radiologists (interclass correlation coefficient 0.9–0.94). There was also a high level of agreement between the preterm and term MRI two scores (Intraclass correlation coefficient, 0.79 (0.53–0.94)).
Conclusions:
Preterm MRI is a feasible option for the assessment of preterm brain injury and analysis of data obtained from scan at preterm age is comparable to that obtained at term equivalent age.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Most preterm infants born at or above 24 weeks of gestation now survive, although a high rate of neonatal morbidity is observed in survivors.1 Preterm infants, especially those born at extremes of gestation, are also at risk of long-term neuromorbidity and developmental disorders. Follow-up studies have revealed high rates of neurodevelopmental disability among very preterm infants who survive, with 5 to 15% having cerebral palsy, severe neurosensory impairment or both and 25 to 50% having cognitive, behavioral and social difficulties that impede progress in school and require special educational support.2, 3
Cranial ultrasound is routinely used in neonatal units to identify major brain pathologies because it is accessible, inexpensive, non-invasive, and can be performed by a variety of trained professionals.4 Outcomes after major destructive lesions, such as hemorrhagic parenchymal infarction or cystic periventricular leukomalacia, are well documented. However, most infants with neurocognitive deficits do not exhibit these patterns of injury and the sensitivity of abnormalities detected by ultrasound to predict developmental outcome is poor.5 Fortunately, magnetic resonance imaging (MRI) is superior to ultrasound at detecting both subtle brain abnormalities and diffuse white matter damage.6, 7 Measurements of the size, volumes and growth rates of many regions of the brain, such as the corpus callosum, ventricular system, cortex, deep gray matter and cerebellum, which may be altered following preterm birth, are also better studied by MRI.8 In very preterm infants, abnormal MRI findings at term equivalent age strongly predict adverse neurodevelopmental outcomes at 2 years of age.9, 10
However, MRI is not as accessible as ultrasound.11 Neonates must be moved to a scanner separate from the neonatal intensive care unit or to a different hospital. This can be dangerous for unstable neonates. Many infants return to a tertiary facility after discharge for their term MRI scans. There is paucity of literature exploring the role of MRI in the mid-late preterm age. In this prospective observational study, we have tried to compare the information obtained from preterm MRI brain scan to term equivalent age MRI in relation to preterm brain injury. We hypothesized that the information obtained will be comparable between the two scans.
Methods
Premature infants born below 30 weeks were recruited into this prospective study if cranial ultrasounds showed evidence or suspicion of parenchymal injury. All preterm infants born <30 weeks were screened for brain injury using the unit protocol, which included a routine cranial ultrasound (done using an iU22 Ultrasound machine, Philips, Best, The Netherlands) within the first 3 days of life, at the start of the second week, 28 days and 42 days after birth. More frequent scans (weekly or twice weekly) scans were undertaken if there was any evidence of brain injury on the scans. Pediatric radiologists reported the ultrasound scans.
Ethics
The institutional Human Research Ethics Committee approved the study. Written signed informed parental consent was obtained prior to enrolment in the study.
Setting
This prospective study was conducted in the neonatal unit of Monash Children’s Hospital, Clayton in suburban Melbourne, Victoria, Australia, over a period of 18 months (August 2013–January 2015). This is a large tertiary perinatal unit attached to a high-risk obstetric service delivering close to 4000 infants an year. Most infants are inborn but the unit also receives a small number of outborn infants every year.
Inclusion criteria
Infants born at <30 weeks gestation with evidence or suspicion of parenchymal brain injury. These may include ultrasound findings of hemorrhagic parenchymal infarction (associated with intraventricular hemorrhage), persistent periventricular echodensities/echogenicities (defined as those present on two scans separated by at least 7 days) or cystic evolution of periventricular leukomalacia. The study lead author (AM) determined eligibility of infants by reviewing ultrasound scan images and radiologist reports.
Exclusion criteria
Infants with known malformations of the central nervous system, or those being considered for redirection of intensive care due to neurological or coexistent morbidities.
MRI protocol
Infants underwent two MRI brain scans: one at preterm age (31 to 34 weeks) and other at term equivalent age (TEA) (38 to 48 weeks). The preterm age MRI was conducted using an magnetic resonance-compatible incubator with a dedicated head coil (Lammers Medical Technology, Lubeck, Germany) on a Siemens 1.5 Tesla MRI scanner (Siemens, Erlangen, Germany). Infants were sedated with chloral hydrate (10 to 25 mg kg−1) given 30 min before transport to the MRI facility. Continuous pulse oximetry was carried out using a portable Masimo Radical 7 monitor (Masimo Corporation, Irvine, CA, USA) and infant’s temperature was also monitored regularly. The MRI scan protocol included isotropic T1- and T2-weighted scans, diffusion-weighted imaging and apparent diffusion coefficient maps. Analysis of the scans included assessment of white matter volume and ventricular size, signal abnormality in the white matter, deep gray matter and cortical gray matter, and assessment of the white matter tracts for maturity and myelination.
Scoring of brain injury on MRI
Scans were reported based on the patterns of abnormality as previously defined and described for Cerebral Palsy by the lead study radiologist (MD).12 For the purposes of this study, the following domains were used: white matter volume, ventricular dilatation, periventricular cysts, T1 and T2 signal change, intraventricular or periventricular hemorrhage, and any other lesions observed (Table 1). Two radiologists blinded to the findings of the cranial ultrasound and previous MRI, scored the images independently.
Statistical analysis
The individual patient characteristics were tabulated. Descriptive statistics were then calculated for the scores given by the lead radiologist involved (MD). The inter-observer agreement between the radiologists was determined by interclass correlation coefficient. The agreement between the preterm and term overall MRI score was determined by computing the Spearman correlation coefficient, the intraclass correlation coefficient and Bland–Altman limits of agreement.13 Agreement between the individual components of the MRI score on the preterm and term MRI was also determined by calculating the weighted Kappa scores and applying Altman’s method14 to describe the level of agreement qualitatively. All statistical analyses were undertaken using Stata (I/C) version 12 (StataCorp, College Station, TX, USA).
Results
Two hundred and twenty-four infants were born at <30 weeks gestation during the study period. Twenty-six infants had evidence or suspicion of parenchymal brain injury on cranial ultrasound out of which 14 preterm infants were recruited into the study after parental consent (Table 2). All infants were inborn, the median gestation at birth was 28 weeks (range, 25 to 29) and the median birth weight was 1254 g (680 to 1557). Eleven (78.5%) infants were male. The median 5-min Apgar score was 8 (2 to 9). All infants developed respiratory distress syndrome requiring respiratory support early in their neonatal course, whereas eight (57%) later developed chronic lung disease. Five (35.7%) infants suffered a significant episode of sepsis or meningitis, whereas two (14.2%) infants developed necrotizing enterocolitis.
Cranial ultrasound findings leading to eligibility to the study included periventricular parenchymal echogenicities (either periventricular echodensities/echogenicities or hemorrhagic parenchymal infarction) (10/14, 71.4%) or cystic periventricular leukomalacia (4/14, 28.5%). Ten infants (71.4%) also had coexistent intraventricular hemorrhage. The preterm MRI brain was done at a median postnatal age of 39 days (19 to 65) and a median corrected gestation (postconceptional age) of 33.5 weeks (31 to 34). The median weight of the infant at the time of scan was 1762 g (1247 to 2507). Six out of the fourteen infants were on non-invasive pressure support (continuous positive airway pressure or humidified high-flow nasal cannula) at the time of the scan and one infant required low-flow oxygen via nasal cannula. The median oxygen requirement for those on pressure support was 21%. The rest of the infants (7/14) were self-ventilating in air. Chloral hydrate was used as a sedative in 12/14 infants with a dose range of 10 to 25 mg kg−1 with no adverse events. Infant’s body temperature was closely monitored during the transport and MRI scan, and was consistently maintained between 36.5 and 36.7 °C for all infants. Continuous pulse oximetry was normal in all infants during the course of the scan and transport. There were no adverse events during the MR or transport to or from the nursery. The term MRI was done at a median corrected age of 38 weeks (37 to 45) by which age most infants had been discharged to another hospital or home.
The details of the MRI scans at the preterm and TEA age are shown in Table 3. There was excellent inter-observer agreement between the two radiologists for both preterm (0.9) and term scans (0.94). The median score of the preterm MRI scan was 5 (IQR 3 to 8) and TEA scan was 6 (IQR 3 to 7). The pairwise correlation coefficient for the two MRIs was 0.87 (P<0.001; Figure 1). The intraclass correlation coefficient between the two MRIs was 0.79 (0.53 to 0.94). The Bland–Altman limits of agreement plot (Figure 2) showed a mean difference (term vs preterm) between scores of −0.46 (95% confidence intervals 3.97 to −4.89). The limits of agreement plot also shows that when the average score was <6, the preterm scores tended to be smaller than the term scores; however, for scores over 6, the preterm score was larger than the term score.
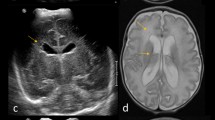
In regards to the specific components of the score, besides evidence of white matter injury (seen in 13 out of 14 infants), findings observed on MRI scans included coexistent intraventricular hemorrhage (64.2%), isolated T1 signal intensity change in the periventricular region (28.5%) and other abnormalities (cerebellar injury/hemorrhage (28.5%), extraxial blood (14.3%) and basal ganglia volume loss (7.1%)). Myelination of posterior limb of internal capsule was seen in none of the preterm scans and in almost half (57%) of the TEA scans. Only one scan (term age) required repeat due to motion artifacts resulting in poor image quality. Individual and total scores in each of the domains for WMI are described. An example of severe brain injury on T2 image of preterm and TEA scan is shown in Figure 3. One infant who suffered a major cardiorespiratory collapse in the interval period between the two scans was excluded from the final analysis. Analysis of agreement of individual components of injury score showed that the best agreement existed for T1 foci (weighted kappa=0.91), and other brain injury (weighted kappa=0.81), whereas white matter volume (weighted kappa=0.32) and T2 signal intensity (weighted Kappa=0.45) had an average agreement using the Bland classification14 (Table 4).
Discussion
Term equivalent age MRI is a well-established clinical imaging tool used to identify infants at high for adverse neurodevelopment.15 The present study demonstrated that a preterm MRI brain undertaken ~31 to 34 weeks post menstrual age showed comparable information to TEA MRI brain. Furthermore, preterm MRI scans were practical and feasible without any adverse events. An objective comparison between the two scans was done with good inter-rater reliability and reproducibility.
White matter injury was the commonest type of injury seen and is known to have clear MRI determinants, which have been previously studied at term age.6 TEA MRI is routinely used to prognosticate neurodevelopmental outcomes. This study compared MRI information obtained from term and preterm scans with the hypothesis that if the information from the scans is comparable, then the long-term outcomes may be similarly predicted on the basis of the preterm scan. A number of studies have examined the utility of an early age MRI brain in the preterm population. The studies included in a systematic review by Plaiser et al.16 did not provide clear evidence on the optimal timing of MR imaging, with either sequential data (from preterm to term age)17, 18, 19, 20 or a single preterm study undertaken.21 Debate continues around transient brain lesions that may have disappeared by the TEA scan.22 Although an increasing number of reports have demonstrated MR evidence of brain abnormalities during early preterm life, few have linked these findings to outcome.20, 23 Miller et al.17 who found that MRI findings at 32 weeks and TEA gestation both reliably predicted neurodevelopment. Their finding suggests that predictive MRI may be performed well before term equivalent age provided it is after the first week of life. We believe that quantifying the severity and extent of injury through a scoring system adds weight to the value of the preterm MRI (32 weeks onwards) for prediction of adverse neurodevelopment.
A variety of MRI scoring systems have been developed to classify and grade brain injury seen on MRI. These systems rely on dividing the information obtained from MRI into various areas of the brain and then scoring them depending on specific lesions seen.24, 25 We used a semi-quantitative grading system a variation of which was previously used in studies on cerebral palsy,26, 27 and found good correlation across the two scans, suggesting that the information obtained from the scans was comparable. The Bland–Altman limits of agreement revealed interesting information. The mean difference of 0.46 between scans, although small could be relevant especially for low scores and needs to be further evaluated with 2-year neurodevelopmental results. There appears to be a systematic bias in those infants with high average (preterm and term) scores where the preterm score is higher than the term score, whereas in infants with low average scores, there seems to be very little bias with preterm scores being generally lower. This may be related to resolution in brain injury markers such as intraventricular hemorrhage over time. The high scores also contribute to the relatively large confidence intervals in a small sample set. Similarly, there was a variation of the individual weighted kappa, being relatively weak correlation between preterm and term scans for white matter volume changes that may evolve over time.
One of the main concerns of MRI scans at a preterm age has been safety of the infant, feasibility and practical issues around transport from a neonatal unit to the MRI facility.28 An MRI compatible incubator as used in this study allows for uninterrupted care and minimizes handling of the infant during transport and image acquisition. None of the infants in this study had any adverse events during transport or scanning and all infants remained normothermic throughout. A number of studies have highlighted the safety and efficacy of MRI compatible incubators for neonatal use especially in the preterm age.29, 30 Previous studies have also demonstrated that scanning through the incubator with a head coil permits high-quality images.31
There are a number of advantages of early diagnosis or confirmation of a significant brain injury on MRI. The most significant of these would be the avoidance of return to a tertiary MRI facility at term equivalent age after discharge to a step-down nursery or home. In some instances, this could mean several hours of transport and overnight stay at the tertiary institution. In this study, seven infants (50%) had to return to the tertiary facility after discharge for TEA MRI. Early diagnosis of a possible brain injury would also facilitate earlier involvement and management by the developmental care team and appropriate referral to early childhood intervention services. Most importantly, there is a high amount of parental anxiety associated with the diagnosis or suspicion of a brain injury on cranial ultrasound and earlier scanning may allow earlier prognostic information to be given. This information could also open new avenues for early neuroprotective and intervention strategies and therapies to be instituted. Performing an MRI early in the preterm age and at TEA will also help to monitor brain growth and injury over time and use as a biomarker to evaluate the effect of neuroprotective strategies and treatments.
The study does have limitations. This prospective study included a systematic evaluation of information obtained from preterm MRI brain. Only infants with a high likelihood of parenchymal brain injury were included for this preliminary study, so as to validate the scoring system and inter-rater reliability before preterm MRI can be considered in a larger population of high-risk infants with no apparent injury on cranial ultrasound. Ideally, an independent screen of the ultrasound scans to determine eligibility would have been better. The study also could not get a breadth of lesion severity due to its small sample size. Hence, it is difficult to comment whether the preterm MRI brain scan would detect subtle or milder degrees of WMI as well as it detects moderate and severe injury when compared to the TEA MRI. Significant blood stream infection was present in 35.7% infants in the study population and a limitation of an early scan could be that brain injury in some infants may potentially be missed especially those who suffer from a late infection or a similar significant event (prolonged need for mechanical ventilation beyond 32 weeks or necrotizing enterocolitis) during their neonatal admission. This has been highlighted in at least one study previously.18
Conclusions
MRI brain scan done at a preterm age in extremely premature infants is comparable to a term equivalent age MRI brain scan. Larger cohort studies including infants with normal scans are required to evaluate the role of preterm MRI brain scan after 32 weeks in low- and high-risk preterm infants.
References
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010; 126: 443–456.
Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ . Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA 2002; 288: 728–737.
Marlow N, Wolke D, Bracewell MA, Samara M . Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med 2005; 352: 9–19.
Gupta P, Sodhi KS, Saxena AK, Khandelwal N, Singhi P . Neonatal cranial sonography: a concise review for clinicians. J Pediatr Neurosci 2016; 11: 7–13.
Whyte HE, Blaser S . Limitations of routine neuroimaging in predicting outcomes of preterm infants. Neuroradiology 2013; 55 (Suppl 2): 3–11.
Woodward LJ, Clark CA, Bora S, Inder TE . Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PloS ONE 2012; 7: e51879.
de Vries LS, Benders MJ, Groenendaal F . Progress in neonatal neurology with a focus on neuroimaging in the preterm infant. Neuropediatrics 2015; 46: 234–241.
Ment LR, Hirtz D, Huppi PS . Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol 2009; 8: 1042–1055.
Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE . Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 2006; 355: 685–694.
Anderson PJ, Cheong JL, Thompson DK . The predictive validity of neonatal MRI for neurodevelopmental outcome in very preterm children. Semin Perinatol 2015; 39: 147–158.
Plaisier A, Raets MM, Ecury-Goossen GM, Govaert P, Feijen-Roon M, Reiss IK et al. Serial cranial ultrasonography or early MRI for detecting preterm brain injury? Arch Dis Child Fetal Neonatal Ed 2015; 100: F293–F300.
Ashwal S, Russman BS, Blasco PA, Miller G, Sandler A, Shevell M et al. Practice parameter: diagnostic assessment of the child with cerebral palsy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2004; 62: 851–863.
Bland JM, Altman DG . Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–310.
Altman DG . Practical Statistics for Medical Research. Taylor & Francis: UK, 1990.
Hintz SR, Barnes PD, Bulas D, Slovis TL, Finer NN, Wrage LA et al. Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics 2015; 135: e32–e42.
Plaisier A, Govaert P, Lequin MH, Dudink J . Optimal timing of cerebral MRI in preterm infants to predict long-term neurodevelopmental outcome: a systematic review. AJNR Am J Neuroradiol 2014; 35: 841–847.
Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge JC et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr 2005; 147: 609–616.
Glass HC, Bonifacio SL, Chau V, Glidden D, Poskitt K, Barkovich AJ et al. Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics 2008; 122: 299–305.
Rademaker KJ, Uiterwaal CS, Beek FJ, van Haastert IC, Lieftink AF, Groenendaal F et al. Neonatal cranial ultrasound versus MRI and neurodevelopmental outcome at school age in children born preterm. Arch Dis Child Fetal Neonatal Ed 2005; 90: F489–F493.
Dyet LE, Kennea N, Counsell SJ, Maalouf EF, Ajayi-Obe M, Duggan PJ et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics 2006; 118: 536–548.
Badr LK, Bookheimer S, Purdy I, Deeb M . Predictors of neurodevelopmental outcome for preterm infants with brain injury: MRI, medical and environmental factors. Early Hum Dev 2009; 85: 279–284.
De Vries LS, Benders MJ, Groenendaal F . Should early cranial MRI of preterm infants become routine? Arch Dis Child Fetal Neonatal Ed 2015; 100: F284–F285.
Doria V, Arichi T, Edwards DA . Magnetic resonance imaging of the preterm infant brain. Curr Pediatr Rev 2014; 10: 48–55.
Kidokoro H, Neil JJ, Inder TE . New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol 2013; 34: 2208–2214.
Shiran SI, Weinstein M, Sirota-Cohen C, Myers V, Ben Bashat D, Fattal-Valevski A et al. MRI-based radiologic scoring system for extent of brain injury in children with hemiplegia. AJNR Am J Neuroradiol 2014; 35: 2388–2396.
Reid SM, Dagia CD, Ditchfield MR, Carlin JB, Meehan EM, Reddihough DS . An Australian population study of factors associated with MRI patterns in cerebral palsy. Dev Med Child Neurol 2014; 56: 178–184.
Robinson MN, Peake LJ, Ditchfield MR, Reid SM, Lanigan A, Reddihough DS . Magnetic resonance imaging findings in a population-based cohort of children with cerebral palsy. Dev Med Child Neurol 2009; 51: 39–45.
Stokowski LA . Ensuring safety for infants undergoing magnetic resonance imaging. Adv Neonatal Care 2005; 5: 14–27, quiz 52-4.
Lane A, Chuk LM, Colditz PB, Coulthard A . The MRI-compatible neonatal incubator in practice. J Paediatr Child Health 2013; 49: E377–E380.
Tocchio S, Kline-Fath B, Kanal E, Schmithorst VJ, Panigrahy A . MRI evaluation and safety in the developing brain. Semin Perinatol 2015; 39: 73–104.
O'Regan K, Filan P, Pandit N, Maher M, Fanning N . Image quality associated with the use of an MR-compatible incubator in neonatal neuroimaging. Br J Radiol 2012; 85: 363–367.
Acknowledgements
The study was supported by a Southern Health Senior Medical Staff Association Award and the Victorian Government’s Operational Infrastructure Program. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards (HREC approval number 11219B). Informed consent was obtained from all individual participants included in the study.
Author contributions
AM designed the study, recruited infants, collected data and wrote the first draft of the manuscript. MF helped in the design of the study and contributed to the manuscript submission. MDT provide statistical support in the design and report of the study; FW and EC contributed to the design of the study and are involved in the follow up of the infants and manuscript submission. GW and MD were the radiologists involved in reporting and scoring all the MRI scans and contributed to the manuscript. All authors approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Malhotra, A., Fahey, M., Davies-Tuck, M. et al. Comparison of preterm and term equivalent age MRI for the evaluation of preterm brain injury. J Perinatol 37, 864–868 (2017). https://doi.org/10.1038/jp.2017.39
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2017.39
- Springer Nature America, Inc.