Abstract
Introduction
Preterm births are increasing in number and while the rates of cerebral palsy have declined, there are increasing numbers of infants who survive with handicaps. In some studies, up to 50 % of children will have morbidity when followed up to school age.
Methods
A review of current literature was conducted to determine the validity of routine cranial ultrasound scans (CUS) to predict neurodevelopmental outcomes, including motor and cognitive deficits. We also reviewed the additional benefit offered by including MRI scans in scanning protocols to enhance the reliability in predicting the neurodevelopmental sequelae of prematurity.
Results
CUS is valuable as a screening tool to determine significant brain injury when conducted regularly over the first weeks of life in preterm infants. Subtle changes on CUS are difficult to interpret and more precise information is offered by performing MRI scans. These are most often carried out at term equivalent age but earlier scans may be just as useful in predicting neurocognitive outcomes. When MRI scans are either normal or seriously abnormal, there is a very clear correlation with outcome to 2 years of age. Mild and moderate degrees of injury defined on MRI need more sophisticated scanning sequences to determine the likelihood of associated sequelae. Follow-up to school age is essential to diagnose more subtle cognitive delays.
Conclusion
CUS provides a good screening tool to detect serious brain injury resulting in motor handicaps but MRI scans are complementary and necessary to accurately predict the outcomes of preterm infants, especially cognitive delays.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Preterm births are increasing and with that, there is an absolute increase in the number of infants surviving with handicaps. Handicaps are particularly common in those who are less than 25 weeks gestational age [8]. In this population, despite an improvement in survival over the past two decades, morbidity rates have not changed and equal to 50 % in those infants born at the lowest gestational ages. Morbidities are generally cognitive with many behavioral and learning difficulties not appearing until early childhood or school age, and these are challenging to predict early in the infant's course [7, 25]. Cerebral palsy (CP) is relatively infrequent occurring in less than 10 % of survivors. Recent papers would suggest that the incidence of CP has dropped as low as 2–3 %, possibly as a result of reduced rates of cystic periventricular leukomalacia [25].
Cerebral palsy can usually be predicted on neuroimaging using cranial ultrasound scans (CUS) [5, 18, 27]. However, cognitive and behavioral difficulties usually need more sophisticated imaging techniques such as magnetic resonance imaging (MRI) [15] and they are often impossible to predict even with current MRI technology. The uncertainty regarding prognosis is the subject of this review. In many neonatal units, MRI technology is unavailable or its use is severely restricted. MRI techniques and interpretation are also limited to expert radiologists in the field while CUS has been widely used by neonatologists as well as radiologists, and requires less expertise for most interpretations.
What can we infer from CUS in preterm infants and when should we resort to more sophisticated scanning using MRI technology? The following case histories illustrate some of the challenges which we face in dealing with prognostication for high-risk newborns especially those born preterm.
Case 1 was a 26-week gestational age (GA) male who weighed 1 Kg at delivery and required CPR due to cord prolapse. In his first week of life, he underwent surgery for a bowel malrotation and required prolonged ventilation due to frequent apnea and desaturation spells. He developed bilateral grade 3 hemorrhages with unilateral extension into the white matter on the left side (Fig. 1). Both CUS and MRI demonstrated significant lesions on brain scans, but the posterior limb of the internal capsule on MRI imaging was well preserved providing us with some reassurance that his sequelae would be minimal. Historically, we would have counseled the family that our expectations were for at least a right-sided hemiparesis with the possibility of cognitive impairment at later follow-up. Follow up MRI at 11 months (Fig. 2) demonstrated some delay in myelin maturation, ventriculomegaly and posterior cortical thinning. However, the child had advanced socio-adaptive and language skills at 3 years of age and had been walking skillfully since 12 months of age, indicating the importance of advanced imaging in predicting outcomes.
Case 1, day 15 MRI. Hemosiderin staining of the brainstem (short white arrow) and vermis and blood clot within the third and fourth ventricles (long white arrows), the aqueduct of Sylvius, the supravermian cistern, and the vallecula of the cisterna magna (short black arrow) are shown on T2W sagittal image (a). Layering of blood is present within both posterior horns and blood clots are present within the germinal matrix of bilateral caudo-thalamic grooves (short white arrows) and the adjacent foramina of Monro and third ventricle on T2W (b) and T1W (c) images. There is a blood clot in the choroid plexus on the left where there is also extension into the adjacent peritrigonal brain. Axial ADC (d) confirms lack of diffusion restriction in adjacent left posterior limb of internal capsule (PLIC) (black arrow)
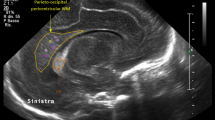
Case 2 was a 28-week GA female born via emergency cesarean section for a non reassuring fetal heart rate tracing. The Apgar scores were 7 and 8, at 1 and 5 min, and birth weight was 1 Kg. The infant had multiple episodes of rebound hyperbilirubinemia requiring prolonged phototherapy. While early CUS showed mild periventricular white matter echogenicity on days 1 and day 8 (Fig. 3), MRI showed definite periatrial linear signal change with diffusion restriction (Fig. 4). The term equivalent age (TEA), MRI which was performed demonstrated bilateral parieto-occipital and peri-trigonal white matter volume loss with thinning of the corpus callosum seen on subsequent images (Fig. 5). When seen for a Bayley-III assessment at 18 months of age, the child had a small head circumference and significant limitations in all domains of development. Once again, MRI provided the most information helping us to more appropriately counsel the family.
Case 1, 11-month MRI. Aqueductal stenosis and callosal thinning (white arrow) are demonstrated on sagittal T1W image (a). Delay in myelin maturation is revealed on T1W axial image (b). Posterior cortical mantle thinning, more severe on the left, is shown on T1W (b), T2W (c), and FLAIR (d) axial images. The PLIC (short white arrow) is normal bilaterally on all axial images (b, c, d), despite adjacent white matter deficiency
Case 2, MRI 13 days old. Periatrial and parietal white matter injury in the region of increased echogenicity seen on the same day (Figure 3b, b1) is identified as linear bright signal (arrows) on T1W axial images (a, d) and linear decreased signal on T2W images (b, e). Diffusion restriction is shown (arrows) on ADC maps (c, f)
Case 2, follow-up MRI at term. Marked thinning of the corpus callosum (arrow) is seen on T2W sagittal image (a). Peritrigonal white matter gliosis is present (arrow) on T1W axial image (b). T2W image (c) shows diminished amount of peritrigonal white matter with sulci nearly approximating the ependymal. Classic “angular” appearance (arrow) of the margin of the trigone of the lateral ventricle is seen on T2W axial image (c)
The most pressing reason to understand the probability of a poor outcome is to aid in the selection of patients to undergo potentially risky therapeutic interventions where the benefits outweigh the risks, such as therapeutic hypothermia or randomized trials of erythropoietin. Perhaps, even more relevant for families is the opportunity to make choices about the appropriate use of technology to maintain the life of their baby. A redirection of care to comfort measures only may be more appropriate than the continuation of intensive care when the prognosis for intact survival is extremely poor. Preparing all families for what lies ahead is also extremely important, especially since neurobehavioral interventions require their full compliance with treatments to be effective. Some would argue that providing information based on TEA MRI scans may only serve to worry parents if the scans are not completely normal, but the majority of families forced to deal with the reality of a high-risk preterm delivery will worry, regardless of MRI findings. In these cases, the scans may serve to reassure parents who would otherwise worry based on population statistics alone, and may identify those children in need of intensive early infant intervention with physiotherapy and occupational therapy.
Prognostic reliability of brain scanning is thus of utmost importance and the use of both modalities of CUS and MRI will be discussed. Computed tomogram (CT) is rarely used today especially in preterm infants as the radiation involved is considerable, equivalent to approximately 200 chest radiographs. MRI gives considerably better information about the brain substance and development than does CT.
What can cranial ultra sound scans tell us?
The most severe forms of brain injury, grades III or IV intraventricular hemorrhage (IVH), or cystic periventricular leukomalacia (PVL), and cerebellar hemorrhages (CBH) are considered to be predictive of poor outcomes [5]. These lesions are obvious on CUS and usually significantly associated with poor outcomes. Children with CP will have an abnormal CUS in 83 % of cases. In cases of cystic PVL, more than 90 % develop CP. On the other hand, when the CUS is normal, it is generally a good predictor of normal motor function and outcome. Figure 6 demonstrates examples of grades I–IV IVH as seen on CUS.
IVH grading. Grade 1 unilateral germinal matrix bleed (arrow) is identified on coronal US (a). Bilateral grade 2 bleeds (arrows) are seen on coronal US (b). Grade 3 bleed (arrow) is present on coronal US (c).Grade 4 bleed is identified on coronal US (d). There is ventricular dilatation, choroid plexus clot (arrow), and periatrial extension of bleed with cavitary change (arrowheads)
When CUS scans are normal, disabling impairment occurs in 4 % of infants born <32 weeks GA. However, with grades I–II (Fig. 6a, b) hemorrhages, 6–8 % will develop CP [1, 17] which is more relevant if periventricular echogenicity (PVE) is also seen [11]. Grade III (Fig. 6c) hemorrhages result in 12–28 % CP rate especially if post hemorrhagic hydrocephalus should occur [21]. Grade IV (Fig. 6d) hemorrhage is a problem, because we are dealing with two potential different etiologies, IVH extension or that of a hemorrhagic parenchyma infarct, or a venous infarct due to impairment of medullary veins, both of which can evolve into porencephalic cysts or multiple small cysts over a period of 2–6 weeks [13]. Grade IV IVH is uncommon and usually unilateral. For these lesions, the outcome will be determined by the site and size of the lesions. Damage to parietal white matter involving trigone is more often linked to motor dysfunction. Overall, 50 % will typically develop unilateral spastic hemiplegia [4].
Cerebellar hemorrhage (CBH) has an incidence of 1.3–9 %, more commonly seen in extremely low gestational age infants especially those less than 750-g birth weight. The CBH may be large and severe, often with associated supratentorial lesions, or they may be isolated lesions which are worse if the cerebellar vermis is involved. Smaller punctate lesions in the cerebellum cannot be seen on CUS, due to the inherently poor acoustic window to evaluate the cerebellum. Even when identifiable on CUS, MRI may clarify the findings (Fig. 7).
Cerebellar bleed. Brain sonogram (a) in a 1-week-old 29-week gestational age infant with NEC and DIC shows multifocal areas of abnormal echogenicity (arrows) in the cerebellum on coronal sonography (a). Findings are clarified on MRI performed within 48 h. Bright signal on T1W image (b) corresponds to a right cerebellar bleed (arrow). Note periatrial foci in this patient with bilateral periatrial white matter bleeds. Multifocal low signal intensity bleeds (arrow) are shown on coronal (c) and axial (d) T2W images
In one case control study of isolated CBH by Limperopoulos et al., the outcomes were extremely poor [12]. Motor disability occurred in 48 %; expressive language delay in 42 %; receptive language impairment in 37 %, and cognitive disability in 40 % of those with CBH compared to the controls. In addition, 37 % were diagnosed with autism. Behavioral problems were seen in 34 vs. 9 % of control infants.
PVE is much more commonly seen on CUS than all other lesions and more difficult to interpret or offer prognostication. The interpretation of PVE is subjective and therefore challenging. If seen in isolation, it is necessary for it to be identified on repeated scans before the information can be interpreted. The greater the duration of the PVE or when the PVE is inhomogeneous, the more likely that the PVE reflects white matter damage (WMD). Bilateral spastic CP (diplegia) is seen in 4–10 % of surviving infants with these findings [19]. Periventricular white matter damage on CUS is best defined by an MRI carried out at TEA [5]. It is more relevant to poor outcome if associated with ventricular dilatation. Ventricular dilatation due to WMD must be distinguished from post hemorrhagic dilatation. Thus, IVH associated with abnormal-sized ventricles suggesting associated white matter damage predicts poor outcome at 2 years with a positive predictive value (PPV) of 34 % and negative predictive value (NPV) of 94 %. In van Wezel-Meijler's study, all other abnormal findings on CUS had a PPV of 14–17 % and NPV of 88–93 % [26].
Current recommendations for all infants born at less than 30 weeks GA are for CUS using a mechanical sector scanner with high resolution transducer, with a wide-angle insonation using anterior and posterior fontanel, as well as the mastoid window. Infants require sequential scans at least every 2–6 weeks, especially between 7 and 14 days and at 36–40 weeks corrected age. The inter-rater reliability for significant brain lesions using this technology is >0.75 [9]. Difficulties arise when we attempt to predict the outcomes of an individual infant based on CUS and what we know from cohort studies of specific brain lesions (Fig. 5). De Vries et al. would recommend even more frequent CUS to enhance prognostic validity of CUS [4]. In some centers where MRI is available, a TEA scan is recommended to try to enhance the predictions. Earlier MRI scans are still the subject of clinical trials.
When and why should an MRI be carried out?
MRI remains a gold standard for diagnosis of brain lesions [14, 20]. MRIs on preterm infants have most often been completed at TEA, although Miller et al. showed early MRI scans at 31–33 weeks were equivalent to TEA for predicting motor outcomes [16].
De Vries et al.'s MRI work focused on evaluating the myelination of the posterior limb of internal capsule to predict motor outcomes [3]. Asymmetric vs. symmetric abnormal myelination predicted unilateral or bilateral spastic cerebral palsy, while normal myelination resulted in normal motor outcomes. Other studies demonstrate that WMD is associated with a reduced maturational score on MRI at term equivalent age and is associated with volume loss in central gray nuclei as well [23].
Kidokoro et al. found that combining the measures of injury and impaired growth, moderate to severe abnormalities were most commonly seen in white matter and cerebellum but still notable in the gray and deep gray matter. WMD was associated with reduced deep gray matter area but not cerebellar abnormality [10]. Cerebellar hemorrhages are more easily seen on MRI than on CUS as demonstrated by Tam et al. where they were seen in 10 % preterm births [24] (Fig. 7). These infants had a fivefold increase in motor problems but no increase in cognitive impairment.
Moderate and severe white matter damage is best seen on MRI. Subtle white matter lesions are also often seen on MRI and are most difficult to interpret or offer prognostication [6]. This is especially true of the punctate white matter lesions seen on MRI, which have been interpreted as benign in the study by Cornette (Fig. 8) [2].
Woodward et al. looked at white matter abnormalities on MRI in preterm infants and described a PPV of 31 % (95 % CI 17–49) [28]. More recently, her studies demonstrated that 21 % of preterm infants had moderate to severe WMD while 49 % had gray matter damage; 97 % of those with gray matter damage also had WMD. The outcomes of her studies are best summarized in Table 1 [29]. However, a substantial proportion of children with moderate to severe white matter damage were free of handicap at 2 years of age.
This demonstrates only too well the importance of not over interpreting the findings on MRI which provides so much more information than the CUS. Findings on CUS tend to show only the “tip of the iceberg” and as such, will miss a large amount of brain damage. However, there is also a danger that MRI findings will be over interpreted, as even subtle lesions may be seen as distinct changes on MRI (Fig. 8). Sie et al. would argue that the findings on MRI at both ends of the spectrum, normal and seriously abnormal, would accurately predict neurodevelopmental outcomes at 18 months corrected age [22]. There remains a critical need for greater understanding of the impact of subtle lesions, as they may help predict the later cognitive dysfunction that is not necessarily evident as brain injury, either on CUS or routine clinical MRI scans. In addition, injury and growth impairment in both gray and white matter may be best characterized by an MRI scoring system. This would provide a more comprehensive and objective classification of the nature and extent of abnormalities than existing measures [10].
Summary
CUS images are limited in the information that they can provide, but remain useful in rapid follow-up of evolving ventriculomegaly and IVH. MRI is an important adjunctive imaging technique which should be included in the routine protocol for imaging premature brains especially when CUS look normal; they should be considered as complementary examinations. Both white and gray matter lesions, especially those which are more subtle, are better visualized on MRI. Neuro-imaging is not always predictive of outcomes and the optimal timing of scans is still under debate although most clinicians will advise a scan to be completed at term corrected age. Quantitative multimodal MRI techniques are preferable to better predict cognitive outcomes.
Outcomes in preterm infants at 2 years of age are limited by our ability to diagnose cognitive delays this early. In fact, school age follow-up is essential to characterize the true extent of the sequelae of being born prematurely.
References
Ancel PY, Livinec F, Larroque B, Marret S, Arnaud C, Pierrat V, Dehan M, N'Guyen S, Escande B, Burguet A, Thiriez G, Picaud JC, André M, Bréart G, Kaminski M, EPIPAGE Study Group (2006) Cerebral palsy among very preterm children in relation to gestational age and neonatal ultrasound abnormalities: the EPIPAGE cohort study. Pediatrics 117:828–835
Cornette LG, Tanner SF, Ramenghi LA, Miall LS, Childs AM, Arthur RJ, Martinez D, Levene MI (2002) Magnetic resonance imaging of the infant brain: anatomical chacteristics and clinical significance of punctuate lesions. Arch Dis Child Fetal Neonatal Ed 86:F171–F177
De Vries LS, Radenmaker KJ, Groenendaal F, Eken P (1999) Assymetric myelination of the posterior limb of the internal capsule in infants with periventricular hemorrhagic infarction: an early predictor of hemiplegia. Neuropediatrics 30:314–319
De Vries LS, Van Hastert IL, Rademaker KJ, Koopman C, Groenendaal F (2004) Ultrasound abnormalities preceding cerebral palsy in high-risk preterm infants. J Pediatr 144:815–820
De Vries LS, Van Haastert IC, Benders MJ, Groenendaal F (2011) Myth: cerebral palsy cannot be predicted by neonatal brain imaging. Semin Fetal Neonatal Med 16:279–287
Dyet LE, Kennea N, Cell SJ, Maaloof E, Ajayi-Obi M, Duggan P, Harrisson M, Allsop J, Hajnal J, Herlihy A, Edwards B, Laroche S, Cowan F, Rutherford F, Edwards D (2006) Natural history of brain lesions in extremely preterm infants studies with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics 118:536–548
Hack M, Costello DW (2008) Trends in the rates of cerebral palsy associated with neonatal intensive care of preterm children. Clin Obstet Gynecol 51(4):763–774. doi:10.1097/GRF.0b013e3181870922.Review
Hintz SR, Kendrick DE, Vohr BR, Poole WK, Higgins RD (2005) Changes in neurodevelopmental outcomes at 18–24 months corrected age among infants of less than 25 weeks gestational age born in 1993–1995. Pediatrics 115:1645–1651
Hintz S, O'Shea M (2007) Neuroimaging and Neurodevelopmental outcomes in preterm infants. Semin Perinatol 32:11–19
Kidokoro H, Neil JJ, Inder T (2013) New MR imaging assessment tool to define brain abnormalities in very preterm infants at birth. AJNR. doi:10.3174/ajnr.A3521
Kuban KC, Allred EN, O’Shea TM, Paneth N, Pagano M, Dammann O, Leviton A, Du Plessis A, Westra SJ, Miller CR, Bassan H, Krishnamoorthy K, Junewick J, Olomu N, Romano E, Seibert J, Engelke S, Karna P, Batton D, O'Connor SE, Keller CE, ELGAN study investigators (2009) Cranial ultrasound lesions in the NICU predict cerebral palsy at age 2 years in children born at extremely low gestational age. J Child Neurol 24:63–72
Limperopoulos C, Bassan H, Gauvreau K, Robertson RL Jr, Sullivan NR, Benson CB, Avery L, Stewart J, Soul JS, Ringer SA, Volpe JJ, duPlessis AJ (2007) Does cerebellar injury in premature infants contribute to the high prevalence of long term cognitive, learning and behavioral disability in survivors? Pediatrics 120:584–593
Maitre NL, Marshall DD, Price WA, Slaughter JC, O'Shea TM, Maxfield C, Goldstein RF (2009) Neurodevelopmental outcome of infants with unilateral or bilateral periventricular hemorrhagic infarction. Pediatrics 124:e1153–e1160
Mathur AM, Neil JJ, Inder TE (2010) Understanding brain injury and neurodevelopmental disabilities in the preterm infant: the evolving role of advanced magnetic resonance imaging. Semin Perinatol 34:57–66
Myers E, Ment LR (2009) Long-term outcome of preterm infants and the role of neuroimaging. Clin Perinatol 36(4):773–789. doi:10.1016/j.clp.2009.07.008. Review, vi
Miller SP, Ferreiro DM, Leonard C, Piecuch R, Glidden DV, Partridge JC, Perez M, Mukherjee P, Vigneron DB, Barkovich AJ (2005) Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr 147:609–616
Patra K, Wilson–Costello D, Taylor HG, Mecuri–Minich N, Hack M (2006) Grade I-II intraventricular hemorrhage in extremely low birth weight infants: effects on neurodevelopment. J Pediatr 149:169–173
Pinto-Martin JA, Riolo S, Cnaan A, Holtzman C, Susser MW, Paneth N (2005) Cranial ultrasound prediction of disabling and non disabling cerebral palsy at age two in a low birth weight population. Pediatrics 95:249–254
Resch B, Jammernegg A, Perl E, Riccabona M, Maurer U, Müller WD (2006) Correlation of grading and duration of periventricular echodensities with neurodevelopmental outcome in preterm infants. Pediatr Radiol 36(8):810–815, Epub 2006
Rutherford MA, Supramanian V, Ederies A, Chew A, Bassi L, Groppo M, Anjari M, Counsell S, Ramenghi LA (2010) Magnetic resonance imaging of white matter diseases of prematurity. Neuroradiology 52:505–521
Sherlock RL, Anderson PJ, Doyle LW (2005) Neurodevelopmental sequelae of intraventricular hemorrhage at 8 years of age in a regional cohort of ELBW/very preterm infants. Early Hum Dev 81:909–916
Sie L, Hart A, van Hof J, de Groot L, Lems W, Lafeber H, Valk J, Knaap V (2005) Predictive value of neonatal MRI with respect to late MRI findings and clinical outcome. A study in infants with periventricular densities on neonatal ultrasound. Neuropediatrics 36:78–89
Srinivasan L, Dutta R, Counsell SJ, Allsop JM, Boardman JP, Rutherford MA, Edwards AD (2007) Quantification of deep gray matter in preterm infants at term-equivalent age using manual volumetry of 3-tesla magnetic resonance images. Pediatrics 119:759–765
Tam EW, Rosenbluth G, Rogers EE, Ferriero DM, Glidden D, Goldstein RB, Glass HC, Piecuch RE, Barkovich AJ (2011) Cerebellar hemorrhage on magnetic resonance imaging in preterm newborns associated with abnormal neurologic outcome. J Pediatr 158(2):245–250. doi:10.1016/j.jpeds.2010.07.049, Epub 2010 Sep 15
van Haastert IC, Groenendaal F, Uiterwaal CS, Termote JU, van der Heide-Jalving M, Eijsermans MJ, Gorter JW, Helders PJ, Jongmans MJ, de Vries LS (2011) Decreasing incidence and severity of cerebral palsy in prematurely born children. J Pediatr 159(1):86–91.e1. doi:10.1016/j.jpeds.2010.12.053, Epub 2011 Mar 2
van Wezel-Meijler G (2011) Ultrasound detection of white matter injury in very preterm neonates: practical implications. Dev Med Child Neurol 53(suppl4):29–34
Whitaker AH, Feldman JF, Van Rossem R, Whitaker AH, Feldman JF, Van Rossem R, Schonfeld IS, Pinto-Martin JA, Torre C, Blumenthal SR, Paneth NS (1996) Neonatal cranial ultrasound abnormalities in low birthweight infants: relation to cognitive outcomes at six years of age. Pediatrics 98:719–729
Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE (2006) Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Eng J Med 355:685–694
Woodward LJ, Clark CA, Pritchard VE, Anderson PJ, Inder TE (2011) Neonatal white matter abnormalities predict global executive function impairment in children born very preterm. Dev Neuropsychol 36(1):22–41. doi:10.1080/87565641.2011.540530
Acknowledgments
We thank Dr Charles Raybaud, Dr. Margot Taylor, PhD, and Dr Steven Miller for their guidance in writing and reviewing the manuscript.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the special supplement “The Premature Brain”—Guest Editor: Charles Raybaud
Rights and permissions
About this article
Cite this article
Whyte, H.E.A., Blaser, S. Limitations of routine neuroimaging in predicting outcomes of preterm infants. Neuroradiology 55 (Suppl 2), 3–11 (2013). https://doi.org/10.1007/s00234-013-1238-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-013-1238-6