Abstract
Objective:
To investigate the accuracy of electrical cardiometry (EC) to measure stroke volume (SV) and cardiac output (CO) and to provide gestational age (GA) and birth weight (BW)-based reference data for SV and CO in hemodynamically stable preterm neonates.
Study Design:
Prospective observational blinded study. Paired measurements of SV and CO on stable preterm infants without any hemodynamic compromise were carried out using EC (SVEC) and echocardiography (SVECHO).
Results:
Seventy-nine preterm neonates (mean GA: 31±3.2 weeks) were enrolled. A good correlation was found for SV (r=0.743; P<0.0001) and CO (r=0.7; P<0.0001) measured by EC and echocardiography. These correlations remained significant after adjusting for GA, patent ductus arteriosus and type of respiratory support (SV: St.β=0.48, P<0.0001 and CO: St.β=0.69, P<0.0001). Mean biases (and variabilities) were −1.1 (from 0.7 to −2.9) ml and −0.21 (from 0.15 to −0.55) l min−1 for SV and CO, respectively. Local regression shows a tendency for EC to overestimate SV and CO especially at higher values (at about >2 ml and >0.4 l min−1, respectively). Coefficient of variation of SV was 48.9% and 52%, for EC and echocardiography. SV and CO rose with increasing GA and BW following an exponential equation (R2>0.8).
Conclusion:
Measuring SV and CO with EC in hemodynamically stable preterm infants shows good correlation and variability similar to that of echocardiography. A trend to overestimation exists at highest values, but it is unlikely to be clinically significant. Reference GA and BW-based nomograms for SV and CO are provided.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Hemodynamic monitoring is a cornerstone of critical care, but it is still very difficult for preterm infants in neonatal intensive care units (NICU). In fact, the techniques available for this purpose are either unreliable and operator-dependent or intermittent (echography) and invasive (thermodilution). Thus hemodynamic assessment is generally based only on blood pressure measurement, echocardiography and indirect signs of tissue perfusion, as other techniques to measure left cardiac output (CO) are unavailable for the smallest patients.
CO estimation by bioimpedance was originally developed for astronautics in 1964 by Kubicek et al.1 The technique was based on the assumption that impedance variation is correlated to intra-thoracic vessel volume change. The introduction of Osypka–Bernstein equation allowed the evolution towards a new technique called electrical cardiometry (EC).2, 3 EC (Aesculon, Osypka Medical, Berlin, Germany) correlates impedance changes with erythrocyte orientation and peak flow velocity in the ascending aorta. EC has been validated against invasive methods of CO measurement obtaining good correlations in animals,4 human adults and children with5, 6, 7, 8, 9, 10 and without11, 12, 13, 14 heart diseases. Actually, EC represents the only available tool that might continuously monitor hemodynamics in preterm neonates. However, limited data are available about EC in neonates,14, 15, 16, 17 and only a small series16 analysed its use in preterm babies. Moreover, no studies deeply investigated the influence of demographic or clinical variables such as gestational age, weight and ductus arteriosus on the EC accuracy. We designed a prospective study with the primary aim to investigate the accuracy of EC in left stroke volume (SV) and CO measurement in hemodynamically stable preterm neonates. Our secondary purpose was to provide reference data for SV and CO according to gestational age and birth weight: specific nomograms will be drawn to facilitate EC use at the bedside.
Methods
This was a prospective observational study performed in a tertiary referral NICU. Local ethical committee approval was granted, and oral informed consent was obtained from parents. All preterm neonates (aged <37 weeks gestation) admitted to the NICU during the first week of life were considered eligible for the study. All pregnancies received full prenatal care. As it is not clear the influence of patent ductus arteriosus (PDA) over EC reliability,15 we included subjects with PDA irrespectively of the hemodynamic significance of ductal shunting. Similarly, we included patients with or without respiratory support. Studying babies with PDA and/or any type of respiratory support was a pragmatic choice, as PDA and ventilatory support are often present in preterm babies and they should not influence an ideal monitoring technique.
Exclusion criteria were: (1) any heart disease or malformation other than PDA or patent foramen ovale; (2) need for hemodynamic support (any inotrope or volume resuscitation); (3) severe respiratory failure, defined as the need for high frequency oscillatory ventilation (HFOV): HFOV was started when babies needed peak pressure or a tidal volume >25 cm H2O and >6 ml Kg−1, respectively; (4) persistent pulmonary hypertension, defined by the presence of echocardiographic signs of persistent pulmonary hypertension and oxygenation index ⩾20; (5) suspected or proven sepsis;18 (6) necrotizing enterocolitis; (7) neonatal asphyxia, defined as 5-min Apgar score <7; and (8) air leaks. These conditions were excluded because they theoretically can affect SV or CO and we wanted to provide reference data in normal conditions.
CO and SV were assessed by EC, whose principles of operation have been described elsewhere.3, 13 Briefly, EC releases a high frequency and low amperage current through the thorax by two electrodes (placed on head and thigh). Two others electrodes (on the neck and thorax) received this signal modified by thoracic impedance. Changes in the impedance are related to the electrocardiography captured at the same time. Electrodes for conventional NICU monitoring were placed as far as possible from EC electrodes, following the manufacturer’s recommendations.
Paired measurements of SV were carried out using EC (SVEC) and echocardiography (SVECHO). All measurements were performed on supine, quiet neonates in stable conditions; special care was taken to reduce stressful triggers (noise, lights, discomfort) as more as possible. SVEC values were recorded at 6 time points before and 6 time points (12 measurements per patient) after the echocardiography, which was performed within 15 min. EC measurements were considered when an optimal quality signal was achieved, according to the manufacturer’s recommendations. In detail, optimal signal was characterized by the presence of signal quality index at least >80 (after 1 min from the electrodes placement) and of contemporary electrocardiographic trace without any artefact. SVEC values were masked to the investigator performing the echocardiography. SVECHO measurements have been obtained using an 8-MHz curved array microprobe (GE Logiq 8C,GE Healthcare, Little Chalfont, UK), and all measurements have been performed by the same investigator, who is board-certified in paediatric echocardiography, to avoid inter-operator variability. Ultrasound probe was placed behind the aortic valve, in line with the blood stream ejected from left ventricle to aorta in five cavities—apical view to measure velocity time integral (VTI) in low angle and best alignment as possible. VTI data were averaged over three measurements per patient. Each measurement lasted 10 s on average. During this time, blood velocity at the aortic valve was also constantly monitored with pulsed wave Doppler. When Doppler tracings have similar shape and size, a still frame was acquired and measurements were performed averaging data obtained over five cardiac cycles during the corresponding 10 s. Cross-sectional area of the aortic valve (CSA) was measured three times per patient in the long-axis view using the following formula: CSA=d2 × π/4 where d is aortic annulus diameter. SVECHO was finally calculated as SVECHO=CSA × VTI. These echocardiographic calculations were carried out some days after the recruitment by the investigator blinded to the SVEC values. Heart rate and the presence of a PDA were also recorded. SVEC and SVECHO multiplied by heart rate yielded COEC and COECHO, respectively.
Statistics
Data are expressed as mean±s.d. The coefficient of variation (CV) was calculated to verify the consistency of measurements. Pearson correlation coefficients and linear regressions were performed between EC and echocardiographic measurements. Multivariate linear regressions were then performed to adjust for factors potentially influencing the relationship between EC and ultrasound measurements. Factors included in the models were gestational age, presence of PDA and the type of respiratory support, based on available literature15 or on physiopathology. The type of respiratory support was defined as follows: 0=no respiratory support; 1=continuous positive airway pressure; and 2=conventional ventilation. As the covariates have different measure units, we considered a standardized coefficient (St.β) for every independent variable. For a covariate, the closer the St.β coefficient will be to 1, the higher will be its association with the independent variable. Model goodness of fit was evaluated with adjusted R2 values (adjR2).20
As Pearson’s coefficient alone can be a poor indicator for estimating the agreement between two diagnostic tests,19 we used a modified Bland–Altman graph to assess SVEC and COEC bias and variability: as echocardiographic measurement is not the gold standard for SV and CO, biases were compared against the averaged SV and CO values (measured with both techniques). Bias was defined as the difference (SVECHO−SVEC) or (COEC−COECHO) and variability was defined as ±1.96 × s.d. of the mean bias. To study trends in the Bland–Altman graph, a curve was plotted with local (smoother) regression method21, 22 using Epanechnikov’s kernel and fitting at least 80% of data.
Finally, gestational age and birth weight-based nomograms were drawn both for SVEC and COEC, providing 25th, 50th and 75th percentiles. Percentile data were analysed with multiple curve estimation procedures to find the best-fitting mathematical model,20 and exponential models were chosen to fit the data because they presented the highest R2 values.23 SV and CO were measured several times during the first week of life for the correlation and Bland–Altman analyses, while the measurements were averaged to draw the nomograms. P-values <0.05 were considered to be statistically significant. Excel 2010 (Microsoft, Redmond, WA, USA) and SPSS 15.0 (SPSS, Chicago, IL, USA) were used for the analysis.
Results
Table 1 shows the details of the enrolled population. We enrolled 79 neonates, with a slight prevalence of male neonates. A total of 451 paired measurements were performed. Thirty-five (44%) babies had PDA. Continuous positive airway pressure and conventional mechanical ventilation were used in 33 (41.8%) and 25 (31.6%) patients, respectively. CV was 48.9% and 52% for SVEC and SVECHO, respectively. CV gave similar results for COEC (50%) and COECHO (52%). There was no problem to achieve optimal EC signal and no malfunctioning was observed.
There was a good correlation (Figure 1) between SVEC and SVECHO (r=0.743; P<0.0001; R2=0.55) and between COEC and COECHO (r=0.7; P<0.0001; R2=0.49). These results remained significant after adjustment in multivariate models (St.β=0.48, P<0.0001, adjR2=0.65 for SVEC and St.β=0.69, P<0.0001, adjR2=0.5 for COEC).
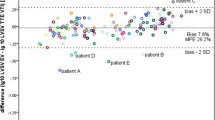
Figure 2 shows Bland–Altman analysis. Mean biases (and variabilities) were −1.1 (from 0.7 to −2.9) ml and −0.21 (from 0.15 to −0.55) l min−1 for SV and CO, respectively. Local regression analysis generates a curve that fits data and shows a tendency for EC to overestimate SV and CO especially at higher values (at about >2 ml and >0.4 l min−1, respectively).
Modified Bland–Altman analysis for (a) left stroke volume (SV) and (b) left cardiac output (CO). Fitting data curves generated by local smoother regression are in bold grey and indicate tendency for electrical cardiometry (EC) to overestimate SV and CO. Dashed bold lines represent mean bias and its variability. Thin black line represents zero. ECHO, echocardiography.
Figure 3 depicts reference data in the form of gestational age and birth weight-based nomograms for SV and CO measured with CE: 25th, 50th and 75th percentiles are shown. Both SV and CO increase with increasing gestational age and birth weight: nomograms follow exponential equations that nicely fit the data (R2 always >0.8).
Reference nomograms for left stroke volume (SV) and left cardiac output (CO) measured by electrical cardiometry (EC) in the first week of life. Panels (a) and (b) represent gestational age-based nomograms, while panels (c) and (d) represent birth weight-based nomograms. Diamonds, squares and triangles represent data points of 25th, 50th and 75th percentile, respectively. Thin lines represent percentiles. Percentiles have been drawn regressing data with exponential equations (R2 always >0.8). For gestational age-based graphs (a and b), regressions prolonged the nomograms down to 24 weeks and up to 37 weeks.
Discussion
To the best of our knowledge, this is the largest study evaluating EC accuracy in NICU patients. We focused on a homogeneous population of preterm infants, as they represent the majority of NICU patients: our findings show on overall a good performance for EC and we also provide reference nomograms for evaluating SV and CO measured by EC. We have chosen to focus on infants without hemodynamic troubles in order to provide normative data as a reference, which is a necessary tool to use a technique at the bedside. Conversely, EC is already known to have a good correlation with echocardiography in mixed general neonatal and paediatric populations.13, 14, 15, 16, 17
Our study confirms that EC has good correlation with echocardiography. Grollmuss and Gonzalez16 reported similar results in a smaller cohort of 28 preterm infants. Our correlations remained significant after adjustment for gestational age, PDA and type of respiratory support. This is relevant, as gestational age is widely varying among NICU patients and PDA is often present in this population. Respiratory support is also commonly given to preterm infants: Grollmuss and Gonzalez16 hypothesized that mechanically ventilated and sedated babies would have more accurate EC measurements, owing to the absence of spontaneous movements and artefacts. Our larger cohort shows that the effect of ventilation, if any exists, does not significantly influence the relationship between EC measurements and echocardiography. Moreover, it is unlikely that this issue would be clinically relevant, as critically ill babies needing hemodynamic monitoring are almost invariably under mechanical ventilation. We excluded babies under HFOV, as we focussed on normal preterm neonates and so we cannot draw any conclusion about the accuracy of EC in this particular population; other authors have suggested a reduced EC accuracy under HFOV.24 Our study shows similar results both for CO and SV: this latter had never been studied so far. As heart rate does not change significantly across gestational age, the accuracy of EC is the same for SV and CO. Thus any of the two might be used for clinical monitoring.
Our findings show EC variability to be roughly similar to that reported in previous studies.15 Interestingly, EC tends to overestimate echocardiography as previously reported:15, 16 our local regression analysis showed this tendency to be more relevant for values >2 ml and 0.4 l min−1 for SV and CO, respectively. This happens for about 25% of our measurements, but it is unlikely to be clinically relevant, as babies with these high SV and CO do not have any hemodynamic impairment. Conversely, it is important to note that EC does not have relevant overestimation at low SV and CO values.
As we used echocardiography as reference method, its intrinsic imprecision could have affected these results and led to a significant underestimation of the EC precision.25 Echocardiography is not the gold standard for hemodynamic monitoring but it is the commonest technique used in NICU for this purpose. As cardiac catheterization is not feasible in preterm patients, we designed a pragmatic study comparing EC to echocardiography and using a modified Bland–Altman analysis. Echocardiography is not considered as gold standard for hemodynamic measurements for several reasons. For instance, SV evaluation using spectral Doppler is not always realized in the same way by each clinician and sometimes it is based on non-validated geometrical assumptions. To reduce these problems, in our study all echocardiographic measurements have been performed at the same site and by the same experienced operator (blinded to the EC values) and all ultrasound data have been averaged over three measurements per neonate. Importantly, Norozi et al.5 and Noori et al.15 performed the measurements in a similar way (expert operator, blindness) and reported precision of EC to be similar to that of echocardiography: consistently, we found CV values almost identical for the two techniques. Of note, EC has shown promising results also when compared with invasive monitoring techniques. In detail, EC showed excellent agreement with the thermodilution method both in piglets4 and humans.11 Moreover, EC showed excellent correlation, bias and variability also when compared against Fick’s method in patients with congenital heart disease.5 Nonetheless, our findings show a quite relevant variability that prevents EC to be an accurate measuring technique, although it may be an useful tool to monitoring hemodynamic variations over time.
EC is interesting because it provides a continuous, operator-independent and non-invasive hemodynamic monitoring to detect early changes and allow a more tailored therapy. EC goal would be to become a bedside point-of-care tool giving the trend of hemodynamic parameter and not their 'true value'. Ours was a 'pragmatic' study aiming to provide normative data to help the EC use at the bedside. In fact, reference data are necessary to introduce a diagnostic technique in the daily NICU care: they will guide clinicians in differentiate between physiological and pathological situations. Reference data have been provided in the form of a nomogram, which is an usual graphic tool that paediatricians commonly use to distinguish between normal and pathological conditions. Nomograms also allows comparing serial measurements repeated over time. Our nomograms represent the 'normal' SV and CO in preterm infants unaffected by any hemodynamic trouble: these nomograms could be used, together with other clinical data, to decide whether hemodynamic support is needed and to monitor the effect of the different therapeutics. However, decision-making strategies, including the nomogram use, will have to be tested in appropriate randomized clinical trial. Our findings clearly show an increment of SV and CO with both increasing birth weight and gestational age. This seems logical and a significant linear correlation between SV and birth weight has been already reported.16 However, our findings show that the best model describing relationship between SV, CO and birth weight or gestational age is exponential. Our cohort included patients from 26 to 36 weeks, but nomograms have been regressed (Figure 3) down to 24 weeks and the exponential model nicely fitted the data, thus these graphs may be used at the bedside to evaluate SV and CO in the smallest preterm babies.
We acknowledge some study limitations. Our population is relatively small but it is homogeneous, as it only consists of preterm infants and it is the largest among the other neonatal studies published so far.15, 16, 17 In the future, it would be very interesting to draw reference data for a larger population of only extremely preterm babies (that is, <28 weeks gestation), as this represents the population who could benefit the more from EC use. Our study is based on a cohort of preterm infants without any cardiovascular compromise: a specific study focused only on more critically ill babies will clarify whether EC accuracy is affected under these conditions.
In conclusion, EC is an interesting point-of-care technique that may monitor SV and CO in preterm infants with a similar accuracy as compared with echocardiography. EC has the advantage of being non-invasive, operator-independent and continuous. A trend to overestimation exists at highest values, but it is unlikely to be clinical significant. Reference nomograms are provided for EC use at the bedside. Further studies are needed to evaluate the accuracy of EC in hemodynamically instable or extremely preterm patients.
References
Kubicek WG, Karnegis JN, Patterson RP, Witsoe DA, Mattson RH . Development and evaluation of an impedance cardiac output system. Aerosp Med 1966; 37: 1208–1212.
Osypka MJ, Bernstein DP . Electrophysiologic principles and theory of stroke volume determination by thoracic electrical bioimpedance. AACN Clin Issues 1999; 10: 385–399.
Bernstein DP, Lemmens HJ . Stroke volume equation for impedance cardiography. Med Biol Eng Comput 2005; 43: 443–450.
Osthaus WA, Huber D, Beck C, Winterhalter M, Boethig D, Wessel A et al. Comparison of electrical velocimetry and transpulmonary thermodilution for measuring cardiac output in piglets. Paediatr Anaesth 2007; 17: 749–755.
Norozi K, Beck C, Osthaus WA, Wille I, Wessel A, Bertram H . Electrical velocimetry for measuring cardiac output in children with congenital heart disease. Br J Anaesth 2008; 100: 88–94.
Schubert S, Schmitz T, Weiss M, Nagdyman N, Huebler M, Alexi-Meskishvili V et al. Continuous, non-invasive techniques to determine cardiac output in children after cardiac surgery: evaluation of transesophageal Doppler and electric velocimetry. J Clin Monit Comput 2008; 22: 299–307.
Tomaske M, Knirsch W, Kretschmar O, Balmer C, Woitzek K, Schmitz A et al. Evaluation of the Aesculon cardiac output monitor by subxiphoidal Doppler flow measurement in children with congenital heart defects. Eur J Anaesthesiol 2009; 26: 412–415.
Mekis D, Kamenik M, Starc V, Jeretin S . Cardiac output measurements with electrical velocimetry in patients undergoing CABG surgery: a comparison with intermittent thermodilution. Eur J Anaesthesiol 2008; 25: 237–242.
Schmidt C, Theilmeier G, Van Aken H, Korsmeier P, Wirtz SP, Berendes E et al. Comparison of electrical velocimetry and transoesophageal Doppler echocardiography for measuring stroke volume and cardiac output. Br J Anaesth 2005; 95: 603–610.
Grollmuss O, Demontoux S, Capderou A, Serraf A, Belli E . Electrical velocimetry as a tool for measuring cardiac output in small infants after heart surgery. Intensive Care Med 2012; 38: 1032–1039.
Zoremba N, Bickenbach J, Krauss B, Rossaint R, Kuhlen R, Schälte G . Comparison of electrical velocimetry and thermodilution techniques for the measurement of cardiac output. Acta Anaesthesiol Scand 2007; 51: 1314–1319.
Archer TL, Conrad BE, Suresh P, Tarsa M . Electrical velocimetry demonstrates the increase in cardiac output and decrease in systemic vascular resistance accompanying cesarean delivery and oxytocin administration. J Clin Anesth 2012; 24: 79–82.
Rauch R, Welisch E, Lansdell N, Burrill E, Jones J, Robinson T et al. Non-invasive measurement of cardiac output in obese children and adolescents: comparison of electrical cardiometry and transthoracic Doppler echocardiography. J Clin Monit Comput 2013; 27: 187–193.
Blohm M, Obrecht D, Hartwich J, Mueller G, Kersten J, Weil J et al. Impedance cardiography (electrical velocimetry) and transthoracic echocardiography for non-invasive cardiac output monitoring in pediatric intensive care patients: a prospective single-center observational study. Crit Care 2014; 18 (6): 603.
Noori S, Drabu B, Soleymani S, Seri I. Continuous non-invasive cardiac output measurements in the neonate by electrical velocimetry: a comparison with echocardiography. Arch Dis Child Fetal Neonatal Ed 2012; 97: F340–F343.
Grollmuss O, Gonzalez P . Non-invasive cardiac output measurement in low and very low birth weight infants: a method comparison. Front Pediatr 2014; 2: 16.
Lien R, Hsu KH, Chu JJ, Chang YS . Hemodynamic alterations recorded by electrical cardiometry during ligation of ductus arteriosus in preterm infants. Eur J Pediatr 2014; 174 (4): 543–550.
Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in paediatrics. Pediatr Crit Care Med 2005; 6: 2–8.
Bland JM, Altman DG . Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–310.
Norusis M . SPSS 13.0 Advanced Statistical Procedures Companion. Prentice Hall Inc.: Upper Saddle River, NJ, USA, 2004.
Cleveland WS . Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 1979; 74: 829–836.
De Luca D, Zecca E, Corsello M, Tiberi E, Semeraro C, Romagnoli C . Attempt to improve transcutaneous bilirubinometry: a double-blind study of Medick BiliMed versus Respironics BiliCheck. Arch Dis Child Fetal Neonatal Ed 2008; 93: F135–F139.
Cameron AC, Windmeijer FAG . An R-squared measure of goodness of fit for some common nonlinear regression models. J Econom 1997; 77: 329–342.
Song R, Rich W, Kim JH, Finer NN, Katheria AC . The use of electrical cardiometry for continuous cardiac output monitoring in preterm neonates: a validation study. Am J Perinatol 2014; 31 (12): 1105–1110.
Cecconi M, Rhodes A, Poloniecki J, Della Rocca G, Grounds RM . Bench-to-bedside review: the importance of the precision of the reference technique in method comparison studies—with specific reference to the measurement of cardiac output. Crit Care 2009; 13 (1): 201.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Boet, A., Jourdain, G., Demontoux, S. et al. Stroke volume and cardiac output evaluation by electrical cardiometry: accuracy and reference nomograms in hemodynamically stable preterm neonates. J Perinatol 36, 748–752 (2016). https://doi.org/10.1038/jp.2016.65
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2016.65
- Springer Nature America, Inc.
This article is cited by
-
Electrical Cardiometry during transition and short-term outcome in very preterm infants: a prospective observational study
European Journal of Pediatrics (2024)
-
How is bioelectrical impedance used in neonatal intensive care? A scoping review
European Journal of Pediatrics (2024)
-
Assessing fluid responsiveness with ultrasound in the neonatal intensive care setting: the mini-fluid challenge
European Journal of Pediatrics (2024)
-
Hemodynamic evaluation in preterm infants using ultrasonic cardiac output monitor (USCOM)
European Journal of Pediatrics (2024)
-
Effect of assessing velocity time integral at different locations across ventricular outflow tracts when calculating cardiac output in neonates
European Journal of Pediatrics (2023)